Abstract
Purpose
MicroRNA-93 (miR-93) usually acts as a promoter of tumor progression in several human carcinomas. It has been found distinctly high in eyes with proliferative diabetic retinopathy (DR). The present study aims to investigate the role of plasma miR-93 in the progression of type 2 diabetic retinopathy (T2DR).
Methods
Our study subjects were made up of 140 type 2 diabetes mellitus (T2DM) patients who were assigned into DR (DR patients, n = 75), NDR (non-DR patients, n = 65), and control (healthy individuals, n = 127) groups. Levels of fasting blood glucose (FBG), fasting plasma glucose (FPG), triglyceride (TG), glycosylated hemoglobin (HbA1c), total cholesterol (TC), blood urea nitrogen (BUN), creatinine (Cr), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and fasting insulin (FIsn) were detected by automatic biochemical analyzer. Enzyme-linked immunosorbent assay (ELISA) was performed for the levels of interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α and vascular endothelial growth factor (VEGF), qRT-PCR for the miR-93 expression in plasma, and mRNA expressions of IL-1, IL-6, TNF-α and VEGF; receiver operating characteristic (ROC) curve for the diagnostic performance of miR-93 to T2DR, Pearson correlation analysis for correlation analysis between miR-93 and other indexes detected before and multivariate logistic regression analyses for the risk factors for T2DR.
Results
The DR and NDR groups exhibited elevated course of disease, and decreased levels of FBG, FPG, TG, HbA1c, TC, BUN, Cr, HDL-C, FIsn, IL-1, IL-6, TNF-α and VEGF but declined LDL-C level as compared to the control group. The course of disease was longer and the levels of FBG, FPG, HbA1c, IL1, IL6 and VEGF were higher in the DR group than those in the NDR group (all P < 0.05). The miR-93 expression and RNA expressions of IL-1, IL-6, TNF-α and VEGF were higher in the DR group than those in the NDR group (P < 0.05). The best cutoff for miR-93 to assess T2DR was 1.31, with a Youden index of 0.63, sensitivity of 73.33%, specificity of 89.24%, and area under the curve (AUC) of 0.866. Pearson correlation analysis indicated that miR-93 expression was positively associated with course of disease, the levels of FPG, HbA1c, TNF-α and VEGF. T2DM patients with longer disease course, higher levels of FBG, HbA1c, VEGF and miR-93 expression were risk factors for developing DR.
Conclusion
Our study demonstrates that plasma miR-93 is associated with the progression of T2DR and it can sever as a diagnostic marker for T2DR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR), one of the common microvascular complications diagnosed in diabetes, has been a primary cause of vision loss among people of a working age [1]. The prevalence of diabetes has doubled between the years 2000 and 2030, with an overall prevalence of any retinopathy among patients with diabetes mellitus (DM) of 40.3% [2]. There are two serious vision-threatening stages recognized in DR, namely proliferative DR (PDR), and diabetic macular edema (DME) [3]. It is pointed out that DME is more commonly seen in type II DM (T2DM) than PDR due to the high occurrence of T2DM and has become a main reason for preventable loss of visual acuity in diabetic patients [4]. General risk factors for the occurrence and progression of DR are diabetic duration, hyperglycemia, hypertension, poor glycemic control and dyslipidemia [5]. Interestingly, laser therapy, pharmacotherapies, as well as surgical therapies have been proved to be of good efficacy for curing DR [6]. However, DR progresses slowly before becoming symptomatic, so the diabetic duration is hard to accurately determine [7]. As early diagnostic biomarkers of diabetes remain challenging to identify, the crucial role of microRNA in chronic metabolic diseases like diabetes and diabetes-induced complications has raised public concern [8].
MicroRNA (miRNA) is made up of a group of small non-coding RNAs that mainly function as gene expression regulators by binding to specific sites of target mRNA to repress their expression, resulting in a decline in target protein production [9]. The miRs were implicated in lots of pathophysiological and physiological processes, such as glucose angiogenesis, homeostasis, and inflammatory response modulation, and played a role in the related macro- and micro-vascular complications and pathogenesis of diabetes [10]. MiRNA-93 (miR-93) usually acts as a promoter of tumor progression in several human carcinomas like breast cancer and glioblastoma by targeting LAST2 and integrin-β8 [11, 12]. Also, it can also be used as a significant negative regulator biomarker in colon cancer [13]. As demonstrated in a previous study, an increase of miR-93 expression was observed in patients with polycystic ovary syndrome (PCOS) and insulin resistance, and the upregulation of miR-93 expression is a leading cause of the occurrence of PCOS by targeting at GLUT4, an important protein in regulating glucose homeostasis [14]. Also, another experiment discovered that miR-93 expression is distinctly high in eyes with PDR, indicating that miR-93 may be associated with angiogenesis and fibrosis [15]. Since miR-93 has been indicated as a significant regulator in DR, the objective of our study is to further investigate the potential role of miR-93 in the development of T2DR, and to explore the mechanisms of miR-93 in DR progression so as to pave the way for DR diagnosis and treatment.
Materials and methods
Study subjects
From April 2013 to April 2015 at Suqian People’s Hospital, Nanjing Drum Tower Hospital, 140 cases of T2DM patients, including 77 males and 63 females aged 25 to 72 years, with a mean course of disease of 8.54 ± 2.92 years were recruited into this study. Of these 140 T2DM patients, there were 75 patients with DR (the DR group) and 65 patients without DR (the NDR group). The DR group had 41 males and 34 females, with a mean age of 48.33 ± 8.58 years and a mean course of disease of 9.33 ± 2.80 years, and the NDR group had 36 males and 29 females, with a mean age of 49.28 ± 8.54 years and a mean course of disease of 7.62 ± 2.81 years. Inclusion criteria: the diagnosis of patients were in accordance with the diagnostic criteria of the World Health Organization (WHO) on T2DM in 1999 [16]; after routine fundus examination and fundus fluorescence angiography examination, T2DM patients suffered from any one of hemangioma, a few small bleeding points, neovascularization, vitreous hemorrhage, or secondary retinal detachment in the retina. Exclusion criteria: patients had acute complications like diabetic ketosis, hyperglycemic coma, severe stress such as recent cardiovascular events, trauma operation, acute or chronic infection, hepatic disease, and other endocrine metabolic disease. Meanwhile, 127 healthy subjects were selected for the control group, among which 66 were male and 61 female, aged from 23 to 76 years, with a mean age of 47.25 ± 9.75 years. None of these healthy subjects were associated with a history or family history of T2DM or other eye diseases. All subjects were measured with their heights and weights, and calculated body mass index (BMI = weight/height2 (kg/m2). Data of history, age, gender, course of disease, and blood glucose control were also analyzed. The study was approved by the Ethical Committee of Suqian People’s Hospital, Nanjing Drum Tower Hospital, and all subjects signed the informed contents.
Plasma separation and detections of indexes
All subjects fasted for 8–12 hours, with approximately 6 mL of venous blood collected into an ethylene diamine tetraacetic acid (EDTA)-containing tube at 6 A.M. The tubes were centrifuged at 3000 rpm/min for 10 minutes at room temperature to obtain the upper plasma, and then the upper plasma was kept in several Eppendorf (Ep) tubes frozen at -80 °C. Fasting blood glucose (FPG) was determined by glucose oxidase method. Triglyceride (TG) was measured using glycerol phosphate oxidase-p-aminophenazone (GPO-PAP) method. Blood urea nitrogen (BUN), creatinine (Cr), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and fasting insulin (FIsn) were detected using a Beckman automatic biochemical analyzer (Roche Ltd., Basel, Switzerland). Glycosylated hemoglobin (HbA1c) was determined using a VARIANT analyzer (Bio-Rad Laboratories, Hercules, CA, USA). Enzyme-linked immunosorbent assay (ELISA) was performed for the levels of interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α and vascular endothelial growth factor (VEGF). All procedures were in strict accordance with the kit instructions.
Quantitative real-time polymerase chain reaction (qRT-PCR)
A total of 300 μL of total RNA in plasma was extracted according to the kit instruction (Qiagen Company, Hilden, Germany). RNA with a concentration of 4∼12 ng/μL, OD260/OD280 of 1.9 and relative high purity detected by ultraviolet spectrophotometry (Nandodrop2000, Thermo Fisher Scientific Inc., Waltham, MA, USA) was selected and preserved at -70 °C for further use. The cDNAs were synthesized according to the kit instruction (Qiagen Company, Hilden, Germany). Premier Primer 5.0 software was used to design premiers based on the gene sequences published in the GenBank and miRBASE, with the following specificity reverse transcription and PCR amplification primers with stem ring structure presented as Table 1. All primers were synthesized by Shanghai Sangon Biological Engineering Co., Ltd (Shanghai, China). The reaction system was 20 μL in total, including 1 μL of cDNA (final concentration of 5 ng), 2.5 μL of Premix type reagent of 2× concentrations, 0.5 μmol/L of forward primer, and 0.5 μmol/L of reverse primer. Three repeated wells were prepared for each sample. The reaction conditions were as follows: initial denaturation at 95 °C for 7 min and then 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 25 s and extension at 75 °C for 25 s. With U6 as an internal reference, the melt curve was used to evaluate the reliability of PCR results and the relative expression of target gene was calculated using the 2-△△Ct method, with the formula of ΔCt = CtmiRNA-CtU6 (the Ct was adapted as the elbow value of amplification curve) and ΔΔCt = [Ct (target gene) - Ct (reference gene)]experimental group-[Ct (target gene) - Ct (reference gene)]control group. These experiments were repeated three times.
Statistical analysis
All data were analyzed by SPSS 21.0 statistical software (SPSS Inc., Chicago, IL, USA). Measurement data were displayed as mean ± standard deviation, comparison between two groups was analyzed by t tests, and comparison among more than two groups was conducted by one-way analysis of variance (ANOVA). Enumeration data were presented as percentage or rate, with comparison between two groups analyzed by chi-square tests. A receiver operating characteristic (ROC) curve was used to evaluate the diagnostic value of miR-93 on T2DR. Pearson correlation analysis was performed for the correlation of miR-93 and various indicators. Binary logistic regression analysis was also performed, with the presence of T2DR as the dependent variable. A P value of <0.05 indicates significant difference.
Results
Baseline characteristics of subjects in the DR, NDR and control groups
Course of disease, FBG, HbA1c, TC, HDL-C, LDL-C, FPG, TG, BUN, FIns, Cr, IL-1, IL-6, TNF-α and VEGF were significantly changed among the DR, NDR and control groups (all P < 0.05). Compared with the control group, the course of disease was lengthened, and the levels of FBG, HbA1c, TC, LDL-C, FPG, TG, BUN, Fins, Cr, IL-1, IL-6, TNF-α and VEGF were increased, but the HDL-C level was decreased in the DR and NDR groups (all P < 0.05). The course of disease was longer and the levels of FBG, HbA1c, FPG, IL-1, IL-6, and VEGF in the DR group were significantly higher than those in the NDR group (all P < 0.05). No significant changes in age, gender, BMI, TC, HDL-C, LDL-C, TG, BUN, FIns, Cr were observed among three groups (all P > 0.05; Table 2).
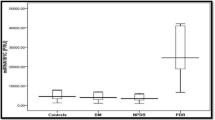
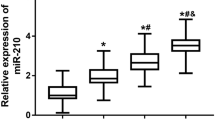
MiR-93 expression and mRNA expressions of IL-1, IL-6, TNF-α and VEGF in the DR, NDR and control groups
The miR-93 expression and mRNA expressions of IL-1, IL-6, TNF-α and VEGF changed significantly among the control, NDR and DR groups (all P < 0.05). Using 2-ΔΔCt relative quantitative method, with relative mRNA expression in the control group as 1, the miR-93 expression and mRNA expressions of IL-1, IL-6, TNF-α and VEGF in the NDR group decreased significantly than those in the DR group (P < 0.05; Fig. 1).
MiR-93 expression and mRNA expressions of IL-1, IL-6, TNF-α and VEGF in the DR, NDR and control groups. Notes: *, P < 0.05, compared with the control group; #, compared with the NDR group; miR-93, microRNA-93; DR group, DR patients; NDR, non-DR patients; DR, diabetic retinopathy; IL, interleukin; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor
Diagnostic value of miR-93 expression for T2DR
The ROC curve showed that the best cut-off of miR-93 for the evaluation of T2DR was 1.31; at this point, the Youden index was 0.63, the sensitivity was 73.33%, the specificity was 89.24%, and the area under the curve (AUC) was 0.866, indicating that miR-93 expression has a diagnostic value in T2DR (Fig. 2).
Pearson correlation analysis between plasma miR-93 and various indexes in the DR group
The result of Pearson correlation analyses showed that miR-93 expression was positively correlated with the course of disease (r = 0.285), HbA1c (r = 0.498), FPG (r = 0.654), TNF-α (r = 0.283) and VEGF (r = 0.305; all P < 0.05), while no significant correlation was found between miR-93 expression and gender, age, BMI, FBG, TC, HDL-C, LDL-C, TG, BUN, FIns, Cr, IL-1 and IL-6 (all P > 0.05; Table 3 and Fig. 3).
Pearson correlation analyses of miR-93 with course of disease, HbA1c, and FPG in the DR group. Notes: a: correlation between miR-93 and course of disease; b: correlation between miR-93 and HbA1c; c: correlation between miR-93 and FPG; miR-93, microRNA-93; d, correlation between miR-93 and TNF-α; e, correlation between miR-93 and VEGF; T2DR, type 2 diabetic retinopathy; HbA1c, glycosylated hemoglobin; FPG, fasting blood-glucose; DR, diabetic retinopathy; IL, interleukin; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor
Binary logistic regression analyses for risk factors of T2DR
Whether T2DR happened was treated as a dependent variable, and the indexes showing significant difference in the NDR and DR groups were selected as independent variables to perform the multivariate logistic regression analyses. And the results showed that the risk factors of T2DR were course of disease EXP (B) = 1.746, 95%CI = 1.041–2.929, P =0.035), FBG (EXP (B) = 3.270, 95%CI = 1.395–7.662, P = 0.006), HbA1c [EXP (B) = 2.641, 95%CI = 1.338–5.214, P = 0.005], VEGF [EXP (B) = 1.388, 95%CI = 1.106–1.742, P = 0.005] and miR-93 [EXP (B) = 35.407, 95%CI = 1.682–745.425, P = 0.022]. T2DM patients with longer course of disease, higher levels of FBG, HbA1c, VEGF and higher expression of miR-93 had an increased risk of developing DR (Table 4).
Discussion
DR, as a frequent microvascular complication of DM, is not only independently related to a lifetime decline in cognitive ability among older men with DM, but also closely linked with visual loss in children [17, 18]. A previous study provided a wealth of evidence that miRNAs played major roles in DR, including the biomarker, miR-93 [19]. Our experiment investigated the association between the expression of plasma miR-93 and the progression of T2DR.
As the initial discovery in our study, course of disease, levels of FBG, FPG, HbA1c, IL-1, IL-6 and VEGF are significantly up-regulated in the DR group comparing with those in the NDR group. Hyperglycemia has been revealed as a major risk factor of DR in cohort studies initiated in earlier decades, and thus intensive glycemic control are applied for DR treatment [6, 20]. Quantitative research investigates the association between FBG level and diabetic mortality, and the results indicates a log-linear relationship between FBG level and diabetic death ratio, and FBG level exceeded the threshold of 100 mg per deciliter (5.6 mmol per liter) [21]. Another study explored the association of HbA1c and FPG levels with DR prevalence, suggesting that when HbA1c surpassed 5.5% and after FPG reached 5.8 mmol/l, DR morbidity started to rise sharply. Also, identification of these changes is valuable for determining the lowest cut-point for DR diagnosis [22]. It was found in a study that VEGF intravitreal concentration was dramatically higher in proliferative diabetic retinopathy (PDR; 798.2 +/- 882.7 pg/mL), suggesting that VEGF exerts effects on the pathogenesis of angiogenesis [23]. Another study demonstrated that aqueous VEGF level was remarkably increased in patients with PDR as compared to the control group, to non-PDR (NPDR) diabetic patients and to NDR diabetic patients [24]. In addition, pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in retinal tissues were very likely to be involved in the DR development, showing in a study that IL-6, IL-1β and TNF-α, as well as their respective receptors, IL-6R, IL-1R, and TNFR1, were all observed elevated in streptozotocin rats, with HIF-1α, IL-6 and TNF-α signaling pathways treated as pharmacological implications for vulnerability and dysfunction related to DR [25]. All results above were in line with our findings; thus, we reached a conclusion that repression of levels of FBG, FPG, HbA1c, IL-1, IL-6 and VEGF could be recommended as a DR treatment of good efficacy.
One of the important findings in our study was that the miR-93 expression and VEGF were evidently elevated in the DR group, indicating that the miR-93 expression and VEGF may be valuable for DR diagnosis. High expression of miR-93 has been detected in a number of human carcinomas like breast cancer and PCOS, and CDKN1A was identified as the target of miR-93 in PCOS, and down-regulation of CDKN1A by miR-93 over-expression is the leading cause of tumor cell growth and proliferation [26]. MiR-93 is also one of the miRNAs that shows substantial increase in DR, and it is a newly identified regulator of VEGF in a hyperglycemic environment, indicating that elevated miR-93 expression may be important in regulating angiogenesis in patients with DR [15, 27]. However, the exact regulation mechanism of miRNAs remains unclear. It is supposed in a study that expression of miRNAs may be regulated by integrating miRNAs into introns of genes which encode protein, as miR-93 is encoded by intron 13 of the MCM7 gene, and thus the expression of glucose-responsive factor on MCM7 may play crucial role in the regulation of miR-93 expression [28]. One study demonstrated that DR was in tight association with VEGF gene -460 T/C polymorphism [29]. Another study suggested that VEGF receptor 1 (VEGFR1) was able to mediate DR complications and target this signaling pathway for the prevention and treatment of DR [30]. Taken together, we can reach the conclusion that miR-93 expression and VEGF were valuable for DR diagnosis. Nevertheless, further researches are still necessary for elucidating the existence of other specific miR-93 promoters and investigating the effect of hyperglycemic conditions on the potential miR-93-specific promoter.
Further analysis demonstrated a positive correlation of miR-93 expression with course of disease, the level of HbA1c and FPG. DM duration is an important risk factor in the development of DM, as long duration (≥8 years) has been reported as a primary cause of the high risk of diabetic complications and total mortality in previous study, and DM duration is highly correlated with age at the onset of disease [31]. In addition, as revealed in our study, HbA1c and FPG are two important glycemic measure elements and are obviously elevated in DR, and suppression of their levels has showed good efficacy in DR treatment. Therefore, miR-93 expression is positively associated with course of disease, the level of HbA1c and FPG in DR.
In conclusion, the course of disease, and levels of FBG, HbA1c and FPG show significant increases in the DR group. Also, miR-93 expression and the level of disease duration, HbA1c and FPG are positively related. Plasma miR-93 is associated with the progression of T2DR and it can serve as a diagnostic marker for T2DR. Collectively, these findings highlight the need to better understand and prevent the multisystem consequences of T2DR.
References
Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet 376:124–136. doi:10.1016/S0140-6736(09)62124-3
Burdon KP, Fogarty RD, Shen W, Abhary S, Kaidonis G, Appukuttan B, Hewitt AW, Sharma S, Daniell M, Essex RW, Chang JH, Klebe S, Lake SR, Pal B, Jenkins A, Govindarjan G, Sundaresan P, Lamoureux EL, Ramasamy K, Pefkianaki M, Hykin PG, Petrovsky N, Brown MA, Gillies MC, Craig JE (2015) Genome-wide association study for sight-threatening diabetic retinopathy reveals association with genetic variation near the GRB2 gene. Diabetologia 58:2288–2297. doi:10.1007/s00125-015-3697-2
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease Study G (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35:556–564. doi:10.2337/dc11-1909
Ding J, Wong TY (2012) Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep 12:346–354. doi:10.1007/s11892-012-0283-6
Yang JK, Liu W, Shi J, Li YB (2010) An association between subclinical hypothyroidism and sight-threatening diabetic retinopathy in type 2 diabetic patients. Diabetes Care 33:1018–1020. doi:10.2337/dc09-1784
Das A, Stroud S, Mehta A, Rangasamy S (2015) New treatments for diabetic retinopathy. Diabetes Obes Metab 17:219–230. doi:10.1111/dom.12384
Kollias AN, Ulbig MW (2010) Diabetic retinopathy: early diagnosis and effective treatment. Dtsch Arztebl Int 107:75–83. doi:10.3238/arztebl.2010.0075, quiz 84
Pandey AK, Agarwal P, Kaur K, Datta M (2009) MicroRNAs in diabetes: tiny players in big disease. Cell Physiol Biochem 23:221–232. doi:10.1159/000218169
Herrera BM, Lockstone HE, Taylor JM, Ria M, Barrett A, Collins S, Kaisaki P, Argoud K, Fernandez C, Travers ME, Grew JP, Randall JC, Gloyn AL, Gauguier D, McCarthy MI, Lindgren CM (2010) Global microRNA expression profiles in insulin target tissues in a spontaneous rat model of type 2 diabetes. Diabetologia 53:1099–1109. doi:10.1007/s00125-010-1667-2
Mastropasqua R, Toto L, Cipollone F, Santovito D, Carpineto P, Mastropasqua L (2014) Role of microRNAs in the modulation of diabetic retinopathy. Prog Retin Eye Res 43:92–107. doi:10.1016/j.preteyeres.2014.07.003
Fang L, Du WW, Yang W, Rutnam ZJ, Peng C, Li H, O’Malley YQ, Askeland RW, Sugg S, Liu M, Mehta T, Deng Z, Yang BB (2012) MiR-93 enhances angiogenesis and metastasis by targeting LATS2. Cell Cycle 11:4352–4365. doi:10.4161/cc.22670
Fang L, Deng Z, Shatseva T, Yang J, Peng C, Du WW, Yee AJ, Ang LC, He C, Shan SW, Yang BB (2011) MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-beta8. Oncogene 30:806–821. doi:10.1038/onc.2010.465
Tang Q, Zou Z, Zou C, Zhang Q, Huang R, Guan X, Li Q, Han Z, Wang D, Wei H, Gao X, Wang X (2015) MicroRNA-93 suppress colorectal cancer development via Wnt/beta-catenin pathway downregulating. Tumour Biol 36:1701–1710. doi:10.1007/s13277-014-2771-6
Chen YH, Heneidi S, Lee JM, Layman LC, Stepp DW, Gamboa GM, Chen BS, Chazenbalk G, Azziz R (2013) miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 62:2278–2286. doi:10.2337/db12-0963
Hirota K, Keino H, Inoue M, Ishida H, Hirakata A (2015) Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 253:335–342. doi:10.1007/s00417-014-2692-5
Daniiarov SB, Zarif’ian AG (1974) Effect of nuredal on frequency of cardiac contractions, catecholamines and tolerance of rats to pressure chamber anoxia under conditions of varied altitude. Sov Zdravookhr Kirg 1:3–10
Ding J, Strachan MW, Reynolds RM, Frier BM, Deary IJ, Fowkes FG, Lee AJ, McKnight J, Halpin P, Swa K, Price JF, Edinburgh Type 2 Diabetes Study I (2010) Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes 59:2883–2889. doi:10.2337/db10-0752
Raczynska D, Zorena K, Urban B, Zalewski D, Skorek A, Malukiewicz G, Sikorski BL (2014) Current trends in the monitoring and treatment of diabetic retinopathy in young adults. Mediat Inflamm 2014:492926. doi:10.1155/2014/492926
Kantharidis P, Wang B, Carew RM, Lan HY (2011) Diabetes complications: the microRNA perspective. Diabetes 60:1832–1837. doi:10.2337/db11-0082
Group AS, Group AES, Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC Jr, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine L (2010) Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 363:233–244. doi:10.1056/NEJMoa1001288
Emerging Risk Factors C, Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 364:829–841. doi:10.1056/NEJMoa1008862
Cheng YJ, Gregg EW, Geiss LS, Imperatore G, Williams DE, Zhang X, Albright AL, Cowie CC, Klein R, Saaddine JB (2009) Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: implications for diabetes diagnostic thresholds. Diabetes Care 32:2027–2032. doi:10.2337/dc09-0440
Izuta H, Chikaraishi Y, Adachi T, Shimazawa M, Sugiyama T, Ikeda T, Hara H (2009) Extracellular SOD and VEGF are increased in vitreous bodies from proliferative diabetic retinopathy patients. Mol Vis 15:2663–2672
Selim KM, Sahan D, Muhittin T, Osman C, Mustafa O (2010) Increased levels of vascular endothelial growth factor in the aqueous humor of patients with diabetic retinopathy. Indian J Ophthalmol 58:375–379. doi:10.4103/0301-4738.67042
Gao X, Li Y, Wang H, Li C, Ding J (2016) Inhibition of HIF-1alpha decreases expression of pro-inflammatory IL-6 and TNF-alpha in diabetic retinopathy. Acta Ophthalmol. doi:10.1111/aos.13096doi
Jiang L, Huang J, Li L, Chen Y, Chen X, Zhao X, Yang D (2015) MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J Clin Endocrinol Metab 100:E729–E738. doi:10.1210/jc.2014-3827
Salam A, Mathew R, Sivaprasad S (2011) Treatment of proliferative diabetic retinopathy with anti-VEGF agents. Acta Ophthalmol 89:405–411. doi:10.1111/j.1755-3768.2010.02079.x
Long J, Wang Y, Wang W, Chang BH, Danesh FR (2010) Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem 285:23457–23465. doi:10.1074/jbc.M110.136168
Gong JY, Sun YH (2013) Association of VEGF gene polymorphisms with diabetic retinopathy: a meta-analysis. PLoS One 8, e84069. doi:10.1371/journal.pone.0084069
He J, Wang H, Liu Y, Li W, Kim D, Huang H (2015) Blockade of vascular endothelial growth factor receptor 1 prevents inflammation and vascular leakage in diabetic retinopathy. J Ophthalmol 2015:605946. doi:10.1155/2015/605946
Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N (2011) Impact of diabetes on cardiovascular disease risk and all-cause mortality in older men: influence of age at onset, diabetes duration, and established and novel risk factors. Arch Intern Med 171:404–410. doi:10.1001/archinternmed.2011.2
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Suqian provided financial support in the form of a grant through the Suqian Science and Technology Support Program (S201415). The sponsor had no role in the design or conduct of this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Yan Wang is regarded as co-first author.
Rights and permissions
About this article
Cite this article
Zou, HL., Wang, Y., Gang, Q. et al. Plasma level of miR-93 is associated with higher risk to develop type 2 diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 255, 1159–1166 (2017). https://doi.org/10.1007/s00417-017-3638-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3638-5