Abstract
Purpose
To evaluate the visual and anatomical outcomes following switching therapy from bevacizumab to aflibercept in patients with persistent diabetic macular edema (DME).
Methods
Patients with DME and central macular thickness (CMT) >300 μm on spectral domain optical coherence tomography (SD-OCT) despite at least 4 intravitreal bevacizumab injections in the prior 6 months were recruited for this prospective, single-armed, single centre, open-label clinical trial. Five loading doses of intravitreal aflibercept were administered every 4 weeks until week 16, at which point the treatment interval was extended to 8 weeks. All participants were reviewed every 4 weeks. At each visit, examination included best-corrected visual acuity (BCVA) measured with an Early Treatment of Diabetic Retinopathy Study chart and CMT measured with SD-OCT. Primary outcome measures were change in CMT and BCVA at week 24 compared with baseline.
Results
A total of 43 eyes from 43 patients were recruited for the study. At enrolment, study eyes had a mean ± standard deviation of 16.6 ± 11.5 previous intravitreal anti-VEGF injections over a period of 26.9 ± 23.8 months. Mean CMT reduced from 417 ± 91 μm at baseline to 380 ± 102 μm at 24 weeks (mean reduction 37 μm, p < 0.01). Mean BCVA improved from 67.8 ± 10.3 letters at baseline to 71.0 ± 10.1 letters at 24 weeks (mean 3.2 letter gain, p < 0.01). Eyes improving by ≥5 letters at 4 weeks following the first injection had improved vision outcomes at 24 weeks (6.8 ± 7.1 letters vs. 1.0 ± 4.7 letters, p < 0.01).
Conclusion
Intravitreal aflibercept was effective in improving anatomical and visual outcomes among patients with incomplete response to intravitreal bevacizumab with 24 weeks of follow up.
Clinical trial registration
ACTRN12614001307695
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic macular edema (DME) is a leading cause of vision loss in people aged 16–64 years [1]. The management of this condition has been revolutionised through the use of drugs targeting vascular endothelial growth factor A (VEGF-A) [2–5]. This class of drugs includes the full-length VEGF-A monoclonal antibody bevacizumab (Avastin, Genentech, Inc., San Francisco, CA), the VEGF-A monoclonal antibody fragment ranibizumab (Lucentis, Genentech, Inc., San Francisco, CA), and aflibercept (Eylea, Regeneron, Tarrytown, NY), a fusion protein that acts as a decoy receptor binding all isoforms of VEGF-A, VEGF-B and placental growth factor (PlGF).
The efficacy of these three anti-VEGF drugs was compared head-to-head in the Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol T study [4]. The 24-month results of this trial have demonstrated efficacy of all three drugs in improving visual acuity and reducing central macular thickness (CMT) [6]. Additionally, in eyes with a lower baseline visual acuity (less than 69 Early Treatment of Diabetic Retinopathy Study (ETDRS) letters) and thicker CMT (greater than 400 μm), aflibercept had superior vision outcomes to bevacizumab.
Despite regular treatment, there are a proportion of patients who incompletely respond to anti-VEGF drugs [7]. In the Protocol T study, treatment failure was defined as persistent central macular thickening identified by OCT and/or a loss of 10 ETDRS letters in vision despite 4-weekly intravitreal injections. Following 24 weeks of therapy, 41% of those patients receiving bevacizumab met these criteria, compared with 27% of those patients in the aflibercept arm [4].
Persistent and chronic macular edema may lead to ultrastructural changes and neuronal damage within the retina, contributing to visual impairment and limiting potential for vision recovery [8]. Due to the differing targets, binding affinities, and clinical efficacy, it has been suggested outcomes in persistent DME may be improved by switching therapy from other anti-VEGF drugs agents to aflibercept [9–12]. In this prospective cohort study, we evaluate the visual and anatomical outcomes in switching therapy from bevacizumab to aflibercept in patients with persistent DME.
Methods
Study design
This study was a prospective, open label, single-armed, clinical trial of patients referred to a tertiary referral retinal clinic in Sydney, Australia. The trial was listed on the Australian and New Zealand Clinical Trials Registry (ACTRN12614001307695). Informed consent was obtained from all individual participants and the study was performed in accordance with the 1964 Declaration of Helsinki.
Participants
One eye from each patient was included in the study. Eligible participants were aged 18 or older, with DME secondary to type 1 or type 2 diabetes mellitus, best corrected visual acuity (BCVA) between 34 and 85 ETDRS letters, retinal thickness greater than 300 μm in the central 1 mm ETDRS field on spectral domain OCT (SD-OCT) and at least 4 previous intravitreal injections of bevacizumab (2.5 mg/0.1 mL) in the 6 months prior to baseline examination. Exclusion criteria included prior intravitreal steroid therapy or vitrectomy surgery in the study eye within 3 months of baseline, cataract surgery or macular laser within 2 months of baseline, pregnancy, active proliferative diabetic retinopathy and uncontrolled diabetes mellitus (HbA1c ≥ 12%).
Study protocol
All participants received 5 loading doses of intravitreal aflibercept (2.0 mg/0.1 mL) administered at 4-week intervals (week 0, week 4, week 8, week 12 and week 16). Further intravitreal aflibercept injections were then given at 8-week intervals, as per product label indication, with a planned total follow-up of 48 weeks. Participants were reviewed at baseline, 1 week after the initial injection, and then every 4 weeks. At each visit ophthalmic examination was undertaken, including BCVA assessed on an ETDRS chart, intraocular pressure (IOP) measured using Goldman applanation tonometry, and central macular thickness (CMT) measured with SD-OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany). In phakic eyes, nuclear, cortical and posterior subcapsular lens opacities were graded according to the Age Related Eye Diseases Study (AREDS) protocol. Fundus fluorescein angiography was performed at baseline to confirm the diagnosis of DME and to exclude other causes of macular edema.
Retinal thickness was defined on OCT as the distance between the inner limiting membrane and Bruch’s membrane. This distance was measured automatically with the inbuilt Heidelberg HRA/OCT software and checked manually to ensure correct segmentation. Segmentation lines were redefined manually if required. CMT values were calculated as the average retinal thickness in the central 1 mm circle of the ETDRS grid. Progression scans utilising eye and landmark tracking were undertaken to ensure accurate measurement of the same anatomical location.
The morphology of DME was analysed and classified on OCT as diffuse, cystoid and/or serous retinal detachment as previously described [13]. The presence or absence of vitreomacular adhesion (VMA), defined as an elevation of the cortical vitreous above the retina surface in the perifoveal area without any changes in foveal contour or retinal morphology, was graded. The inner segment ellipsoid (ISe) band integrity was assessed in the central 1 mm circle of the ETDRS grid with disruption graded from 0 to 2 as previously described [14]. Grade 0 was given for an intact ISe band, Grade 1 for disruption of 200 μm or less, and Grade 2 for greater than 200 μm of disruption. The presence or absence of external limiting membrane (ELM) disruption within the central 1 mm circle of the ETDRS grid was also graded. Disorganisation of the retinal inner layers (DRIL) affecting ≥50% of the 1-mm central retinal zone was graded as previously described [15].
All intravitreal injections were given according to a standardised procedure with strict aseptic technique. The eye was anesthetized using topical oxybuprocaine hydrochloride 0.4% and the conjunctiva was prepared with an antiseptic agent (povidone iodine 5% or chlorhexidine 0.1%). The intravitreal injection was delivered using a 30-gauge needle through the pars plana. Post-procedure topical antibiotic drops were not routinely administered.
Ocular and systemic adverse events were recorded. An increase in lens opacity grading of 2 or more AREDS levels in either nuclear, cortical, or posterior subcapsular cataract, IOP of 25 mmHg or more or a rise in IOP of 10 mmHg or more compared with baseline were considered adverse events.
Statistical analysis
All statistical analyses were performed using IBM SPSS software (version 22; SPSS Inc, Chicago, Illinois, USA). Visual statistical analyses included mean change in BCVA and percentage of patients with a gain or loss of ≥ 5 ETDRS letters at week 24 compared with baseline. Anatomic statistical analyses included mean change in CMT at week 24 compared with baseline and percentage of patients with a decrease or increase in CMT of ≥50 μm compared with baseline. If patients missed a scheduled visit, the previous observation for CMT and BCVA were carried forward and included in the analysis.
Normality of data was confirmed using Shapiro-Wilk tests. Levene’s test for equality of variance was used to assess homogeneity and suitability for subsequent independent samples’ t-tests. Paired t-tests were used to compare differences in means of BCVA and CMT. Independent samples’ t-tests and analysis of variance were performed to compare mean changes in CMT and BCVA, grouping patients by baseline CMT (<400 μm and ≥400 μm) and BCVA (<69 and ≥69 ETDRS letters), BCVA gain ≥5 letters following one injection, prior vitrectomy and OCT morphology. Two multiple regressions were performed to analyze the association of 24 week CMT and BCVA with baseline variables, including gender, age, duration and type of diabetes mellitus, glycated hemoglobin, number of previous intravitreal injections, previous panretinal photocoagulation or macular laser, lens status, presence of hypertension and hypercholesterolemia. For all analyses, a p-value of less than 0.05 was considered to be statistically significant. Only significant potential confounding factors were included in the final model.
Results
Baseline patient characteristics
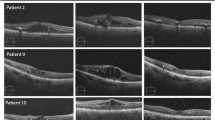
The baseline characteristics of the 43 participants enrolled in the study are summarized in Table 1. Of these, 18 eyes had previous macular laser for DME, 17 had panretinal photocoagulation for proliferative diabetic retinopathy and 5 underwent prior vitrectomy. All eyes received at least 4 bevacizumab injections 6 months prior to switching to aflibercept. One patient withdrew consent from the study after the first injection, and one patient had a retinal detachment after two injections; both of these were excluded from the final analysis. Baseline mean ± standard deviation BCVA was 67.8 ± 10.3 letters, and baseline CMT was 417 ± 91 μm on OCT. Other baseline morphological OCT findings are summarized in Table 2.
Visual and anatomical outcomes
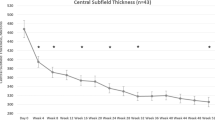
BCVA improved significantly at all follow up visits compared to baseline (p < 0.01) with a mean gain of 3.2 ± 6.3 letters at week 24 (Fig. 1). A significant improvement in CMT was observed at all follow up visits compared to baseline (p < 0.01) (Fig. 2), with a mean reduction of 37 ± 63 μm at 24 weeks. Maximum reduction in CMT occurred prior to extension of the treatment interval to 8 weeks at week 20 (−59 ± 78 μm).
Eyes that improved by 5 or more ETDRS letters at 4 weeks following the first aflibercept injection had significantly better vision outcomes at 24 weeks than those that did not (6.8 ± 7.1 letters vs. 1.0 ± 4.7 letters, p < 0.01). There was no significant difference in CMT between these groups of patients (−48 ± 75 μm vs. -29 ± 55 μm, p = 0.34).
Eyes with a baseline BCVA <69 ETDRS letters showed a greater reduction in CMT at 24 weeks (−58 ± 60 μm vs. -3 ± 54 μm, p < 0.01) compared with those with a better visual acuity. This difference was not explained by a higher baseline CMT in patients with baseline BCVA <69 letters compared to those with better acuity (437 ± 109 μm vs. 404 ± 77 μm, p < 0.27).
However, there was no significant difference in vision for eyes with a baseline BCVA < 69 ETDRS letters compared to those with a better acuity (4.6 ± 7.0 letters vs. 2.0 ± 5.6 letters, p = 0.19). Similarly, there was no difference in 24-week BCVA for eyes with a baseline CMT ≥400 μm compared to those with a CMT <400 μm (4.7 ± 7.5 letters vs. 1.9 ± 5.0 letters, p = 0.15). Inclusion of potential confounding variables in the multiple regression analysis did not significantly alter BCVA or CMT outcomes at 24 weeks. Other vision and anatomical outcomes are presented in Table 3.
Baseline characteristics and response to therapy
All patients had diffuse DME, with seven of these having subretinal fluid and two displaying a cystoid pattern. No difference was found between baseline DME morphology and change in mean CMT or BCVA at 24 weeks (p > 0.05 for all comparisons). Baseline ISe band, DRIL or ELM disruption did not predict the visual outcome at 24 weeks.
Non-vitrectomized eyes at baseline had a greater mean reduction in CMT at 24 weeks (−46 ± 59 μm vs. 30 ± 51 μm, p = 0.01) but no significant difference was noted in BCVA (3.1 ± 6.6 vs. 4.2 ± 4.5 ETDRS letters, p = 0.72). Eyes with VMA at baseline had a greater mean reduction in CMT at 24 weeks (−84 ± 83 μm vs. -27 ± 55 μm, p = 0.03) but no significant difference in BCVA (6.3 ± 4.9 vs. 2.6 ± 6.5 ETDRS letters, p = 0.17). Two of these seven patients had subsequent separation of the vitreous from the macula at 24-week of follow up.
Adverse events
Ocular and systemic adverse events are listed in Table 4. The one serious ocular adverse event was a rhegmatogenous retinal tear and detachment in the study eye occurring after the second injection. There were no cases of endophthalmitis. There were no occurrences of raised IOP or progression of cataract. One patient commenced on renal hemodialysis for diabetic nephropathy related chronic renal failure during the study.
Discussion
This clinical trial demonstrates a visual and anatomical benefit in switching therapy to aflibercept for patients with DME incompletely responsive to bevacizumab. The improvements observed in this study are likely due to the differing pharmacodynamics of these two drugs.
Aflibercept is a recombinant fusion protein consisting of the binding domains of VEGF receptor (VEGFR) -1 and VEGFR-2, binding all isoforms of VEGF-A, VEGF-B and PlGF [16]. This contrasts with bevacizumab, a monoclonal antibody that only binds to and inactivates VEGF-A. PlGF may play a role in the pathogenesis of DME and blockade of this protein may be beneficial in its management. Increasing intravitreal concentrations of PlGF have been associated with progressively advancing degrees of diabetic retinopathy [17]. Additionally, intravitreal injection of PlGF into rat eyes has been shown to disrupt the outer blood retinal barrier, leading to edema [18].
Pathological elevation of VEGF-A appears to be higher in DME than other exudative retinal conditions driven by VEGF-A, including retinal vein occlusion and neovascular age-related macular degeneration [19]. Increased binding affinity of aflibercept to VEGF-A compared to bevacizumab may be another reason why a switch in therapy is effective [16, 20]. Furthermore, the trough binding activity of aflibercept is 200-fold to 800-fold greater than that of bevacizumab, suggesting that the effect of aflibercept may be longer lasting [21].
Incomplete response to therapy reflects the multifactorial and complex pathophysiology of DME [7]. There is no consensus regarding how to define patients with persistent DME following treatment with one anti-VEGF agent. There are differing opinions about persisting with the same agent, as well as when a switch in therapy may be appropriate [22]. Alternate management options may include increasing the dose of drug, increasing dose frequency, intravitreal steroid therapy, macular laser, vitrectomy surgery or any combination of these [7].
Switching from bevacizumab or ranibizumab to aflibercept for persistent DME has been previously reported [9–12]. In the only other published prospective study with 1 month of follow up, there was a significant reduction in CMT in 14 eyes but no significant change in BCVA [11]. Three retrospective series have also showed a benefit in reduction of CMT, with the larger of these not demonstrating a significant improvement in visual function [9, 10, 12].
In our study, eyes with prior vitrectomy had poorer anatomical outcomes, as reported in a previous study of DME [23]. This may be due to a significantly shorter half-life of intravitreal drugs in vitrectomized eyes [24]. Conversely, patients with VMA had a significantly improved CMT at 24 weeks in our trial. This was not explained by subsequent PVD, which occurred in two of these seven patients by 24 weeks. Previous studies have shown that VMA is associated with DME [25]. Additionally, patients with VMA may respond better to anti-VEGF treatment [26]. An attached posterior hyaloid may trap VEGF-A in the macula making DME in these cases more responsive to anti-VEGF therapy [27].
The effect of additional macular focal/grid laser photocoagulation, utilised as an adjuvant in Protocol T, was eliminated in this study. The dose of bevacizumab used prior to switch (2.5 mg) was higher than that in Protocol T as well as other randomized clinical trials of bevacizumab in DME, which all utilize a 1.25 mg dose [4, 28, 29]. Higher doses of anti-VEGF drugs may be more effective in a treatment-resistant cohort in which DME may be increasingly driven by VEGF-A [30].
Additionally, there was no washout period between cessation of bevacizumab therapy and initiation of aflibercept therapy, that is, treatment with aflibercept was on average initiated within 42 days of prior intravitreal bevacizumab injection. This is another confounding factor in studies including VIVID/VISTA and Protocol T, which had washout periods of 3 and 12 months, respectively. These washout periods likely allowed DME to progress so that patients had poorer vision and CMT at baseline, exaggerating the benefits of treatment. This may also explain why in VISTA/VIVID, there was no apparent difference between participants who had and had not received prior anti-VEGF treatment [31].
In Protocol T, poorer baseline visual acuity (<69 ETDRS letters) and thicker CMT (>400 μm) were shown to be predictive factors for visual outcomes at 12 months. These factors were not found to be associated with outcomes at 24 weeks in this study cohort. The reasons for this may relate to the demographic of the study group presented, with participants having significant recent history of treatment with anti-VEGF drugs. Furthermore, the effect of baseline vision and CMT may not be apparent due to the smaller sample size of this study.
However, we did find that patients with a poorer baseline BCVA (<69 letters) had improved reduction in CMT at 24 weeks. Interestingly, this was not explained by a thicker CMT at baseline for these patients. Poorer visual acuity may be a consequence of increased macular ischemia in patients with diabetic retinopathy. [32] Consequently, DME in these patients may be increasingly driven by VEGF released in response to macular, and perhaps peripheral, ischemia and switching from bevacizumab to aflibercept may better treat these patients.
The significant ocular adverse event encountered in this study was a macula-on rhegmatogenous retinal detachment. This occurred approximately three weeks following the second intravitreal injection and was considered to be related to a posterior vitreous detachment [33]. The systemic adverse events encountered are more likely to be complications of diabetes, which is associated with significant comorbidity, rather than relating to intravitreal injection. The local and systemic safety of aflibercept has been validated in multiple clinical trials [34].
The strengths of this study are the prospective trial design, standardized examinations, inclusion and exclusion criteria as well as a significant prior history of therapy with bevacizumab. The limitations of this study include the lack of a control group, a relatively small sample size and short follow up. The small sample size limits the power of the subgroup statistical analyses performed, which are included not to guide treatment but for exploratory purposes. Further follow up is planned in this cohort to continue with an extended injection interval of 8 weeks through 48 weeks to assess maintenance of these changes in the longer-term.
Patients with persistent DME despite regular anti-VEGF therapy represent a management challenge. This prospective clinical trial shows that switching therapy to aflibercept may be an effective strategy for patients who have incomplete response to bevacizumab.
References
Liew G, Michaelides M, Bunce C (2014) A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 4, e004015. doi:10.1136/bmjopen-2013-004015
Early Treatment Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Arch Ophthalmol 103:1796–1806
Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK (2010) Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117(1064–1077), e1035. doi:10.1016/j.ophtha.2010.02.031
Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, Arnold-Bush B, Baker CW, Bressler NM, Browning DJ, Elman MJ, Ferris FL, Friedman SM, Melia M, Pieramici DJ, Sun JK, Beck RW (2015) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372:1193–1203. doi:10.1056/NEJMoa1414264
Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Zeitz O, Metzig C, Brown DM (2014) Intravitreal aflibercept for diabetic macular edema. Ophthalmology 121:2247–2254. doi:10.1016/j.ophtha.2014.05.006
Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR, Jhaveri C, Melia M, Beck RW, Diabetic Retinopathy Clinical Research Network (2016) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. doi:10.1016/j.ophtha.2016.02.022
Bahrami B, Zhu M, Hong T, Chang A (2016) Diabetic macular oedema: pathophysiology, management challenges and treatment resistance. Diabetologia. doi:10.1007/s00125-016-3974-8
Nguyen QD, Tatlipinar S, Shah SM, Haller JA, Quinlan E, Sung J, Zimmer-Galler I, Do DV, Campochiaro PA (2006) Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol 142:961–969. doi:10.1016/j.ajo.2006.06.068
Lim LS, Ng WY, Mathur R, Wong D, Wong EY, Yeo I, Cheung CM, Lee SY, Wong TY, Papakostas TD, Kim LA (2015) Conversion to aflibercept for diabetic macular edema unresponsive to ranibizumab or bevacizumab. Clin Ophthalmol 9:1715–1718. doi:10.2147/OPTH.S81523
Rahimy E, Shahlaee A, Khan MA, Ying GS, Maguire JI, Ho AC, Regillo CD, Hsu J (2016) Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol 164(118–127):e112. doi:10.1016/j.ajo.2015.12.030
Wood EH, Karth PA, Moshfeghi DM, Leng T (2015) Short-term outcomes of aflibercept therapy for diabetic macular edema in patients with incomplete response to ranibizumab and/or bevacizumab. Ophthalmic Surg Lasers Imaging Retina 46:950–954. doi:10.3928/23258160-20151008-08
Shah CP, Heier JS (2016) Aflibercept for diabetic macular edema in eyes previously treated with ranibizumab and/or bevacizumab may further improve macular thickness. Ophthalmic Surg Lasers Imaging Retina 47:836–839. doi:10.3928/23258160-20160901-06
Otani T, Kishi S, Maruyama Y (1999) Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol 127:688–693
Maheshwary AS, Oster SF, Yuson RM, Cheng L, Mojana F, Freeman WR (2010) The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol 150(63–67):e61. doi:10.1016/j.ajo.2010.01.039
Sun JK, Radwan SH, Soliman AZ, Lammer J, Lin MM, Prager SG, Silva PS, Aiello LB, Aiello LP (2015) Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes 64:2560–2570. doi:10.2337/db14-0782
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15:171–185. doi:10.1007/s10456-011-9249-6
Kovacs K, Marra KV, Yu G, Wagley S, Ma J, Teague GC, Nandakumar N, Lashkari K, Arroyo JG (2015) Angiogenic and inflammatory vitreous biomarkers associated with increasing levels of retinal ischemia. Invest Ophthalmol Vis Sci 56:6523–6530. doi:10.1167/iovs.15-16793
Miyamoto N, de Kozak Y, Jeanny JC, Glotin A, Mascarelli F, Massin P, BenEzra D, Behar-Cohen F (2007) Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia 50:461–470. doi:10.1007/s00125-006-0539-2
Pfister M, Koch FH, Cinatl J, Rothweiler F, Schubert R, Singh P, Ackermann H, Koss MJ (2013) Cytokine determination from vitreous samples in retinal vascular diseases. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft 110:746–754. doi:10.1007/s00347-012-2719-4
Yang JH, Wang XD, Fuh G, Yu LL, Wakshull E, Khosraviani M, Day ES, Demeule B, Liu J, Shire SJ, Ferrara N, Yadav S (2014) Comparison of binding characteristics and in vitro activities of three inhibitors of vascular endothelial growth factor A. Mol Pharm 11:3421–3430. doi:10.1021/mp500160v
Stewart MW, Rosenfeld PJ, Penha FM, Wang F, Yehoshua Z, Bueno-Lopez E, Lopez PF (2012) Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina 32:434–457. doi:10.1097/IAE.0B013E31822C290F
Moshfeghi DM, Kaiser PK, Michels S, Midena E, Kitchens JW, Prenner JL, Regillo CD, Reichel E (2016) The role of anti-VEGF therapy in the treatment of diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina 47:S4–S14. doi:10.3928/23258160-20160415-01
Mehta S, Blinder KJ, Shah GK, Kymes SM, Schlief SL, Grand MG (2010) Intravitreal bevacizumab for the treatment of refractory diabetic macular edema. Ophthalmic Surg Lasers Imaging 41:323–329. doi:10.3928/15428877-20100430-05
Kakinoki M, Sawada O, Sawada T, Saishin Y, Kawamura H, Ohji M (2012) Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Invest Ophthalmol Vis Sci 53:5877–5880. doi:10.1167/iovs.12-10164
Gaucher D, Tadayoni R, Erginay A, Haouchine B, Gaudric A, Massin P (2005) Optical coherence tomography assessment of the vitreoretinal relationship in diabetic macular edema. Am J Ophthalmol 139:807–813. doi:10.1016/j.ajo.2004.12.084
Sadiq MA, Soliman MK, Sarwar S, Agarwal A, Hanout M, Demirel S, Rentiya ZS, Khan W, Do DV, Nguyen QD, Sepah YJ, Group R-S (2016) Effect of Vitreomacular Adhesion on Treatment Outcomes in the Ranibizumab for Edema of the Macula in Diabetes (READ-3) Study. Ophthalmology 123:324–329. doi:10.1016/j.ophtha.2015.09.032
Robison CD, Krebs I, Binder S, Barbazetto IA, Kotsolis AI, Yannuzzi LA, Sadun AA, Sebag J (2009) Vitreomacular adhesion in active and end-stage age-related macular degeneration. Am J Ophthalmol 148(79–82):e72. doi:10.1016/j.ajo.2009.01.014
Gillies MC, Lim LL, Campain A, Quin GJ, Salem W, Li J, Goodwin S, Aroney C, McAllister IL, Fraser-Bell S (2014) A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 121:2473–2481. doi:10.1016/j.ophtha.2014.07.002
Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, Boos CJ, Xing W, Egan C, Peto T, Bunce C, Leslie RD, Hykin PG (2010) A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology 117(1078–1086):e1072. doi:10.1016/j.ophtha.2010.03.045
Dhoot DS, Pieramici DJ, Nasir M, Castellarin AA, Couvillion S, See RF, Steinle N, Bennett M, Rabena M, Avery RL (2015) Residual edema evaluation with ranibizumab 0.5 mg and 2.0 mg formulations for diabetic macular edema (REEF study). Eye 29:534–541. doi:10.1038/eye.2014.338
Do DV, Nguyen QD, Vitti R, Berliner AJ, Gibson A, Saroj N, Soo Y, Boyer DS (2016) Intravitreal aflibercept injection in diabetic macular edema patients with and without prior anti-vascular endothelial growth factor treatment: outcomes from the phase 3 program. Ophthalmology 123:850–857. doi:10.1016/j.ophtha.2015.11.008
Balaratnasingam C, Inoue M, Ahn S, McCann J, Dhrami-Gavazi E, Yannuzzi LA, Freund KB (2016) Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology 123:2352–2367. doi:10.1016/j.ophtha.2016.07.008
Meyer CH, Michels S, Rodrigues EB, Hager A, Mennel S, Schmidt JC, Helb HM, Farah ME (2011) Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol 89:70–75. doi:10.1111/j.1755-3768.2010.02064.x
Kitchens JW, Do DV, Boyer DS, Thompson D, Gibson A, Saroj N, Vitti R, Berliner AJ, Kaiser PK (2016) Comprehensive review of ocular and systemic safety events with intravitreal aflibercept injection in randomized controlled trials. Ophthalmology. doi:10.1016/j.ophtha.2016.02.046
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Funding
Bayer Corporation Global provided financial support in the form of an unrestricted grant. The sponsor had no role in the design, conduct or analysis of this research or in the preparation of the manuscript. None of the authors have any proprietary interest in any material or method presented.
Conflict of interest
Dr Andrew Chang has received research grant funding from Bayer. He has also acted as a consultant for Alcon, Bayer and Novartis. All other authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Rights and permissions
About this article
Cite this article
Bahrami, B., Hong, T., Zhu, M. et al. Switching therapy from bevacizumab to aflibercept for the management of persistent diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 255, 1133–1140 (2017). https://doi.org/10.1007/s00417-017-3624-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3624-y