Abstract
Purpose
Non-steroidal anti-inflammatory drugs (NSAIDs) are a class of anti-inflammatory drugs that are used in ophthalmologic surgery. These drugs do not have a steroid structure, but can inhibit surgery-induced miosis, anterior chamber inflammation, and cystoid macular edema (CME). However, the application of NSAIDs remains controversial. Therefore, we performed a meta-analysis to assess the efficacy and safety of NSAIDs for the treatment of anterior chamber inflammation after cataract surgery.
Methods
Relevant articles were identified from the PubMed, Embase, and Cochrane databases up to October 2016. The therapeutic effect of NSAIDs on anterior chamber inflammation was evaluated. The important outcomes of overall anterior chamber inflammation, freedom from ocular pain, and treatment-related/serious ocular adverse events were analyzed by using a random-effects network meta-analysis. The quality of evidence was assessed via the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) approach.
Results
A total of 19 trials assessing 7,234 patients were included in our meta-analysis. Diclofenac was the most likely to improve anterior chamber inflammation after cataract surgery, followed by nepafenac, ketorolac, bromfenac, and flurbiprofen. Nepafenac was most likely to improve postoperative ocular pain relief, followed by bromfenac and ketorolac. Our analysis of treatment-related/serious ocular adverse events revealed that piroxicam was most likely to have the fewest related adverse events, but the robustness of this finding was low. Diclofenac was another near-ideal drug, followed by nepafenac, bromfenac, and ketorolac.
Conclusions
NSAIDs are effective drugs compared to placebos for the relief of anterior chamber inflammation. Furthermore, diclofenac, nepafenac, ketorolac, and bromfenac demonstrated relatively greater significant effects than those of other NSAIDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A cataract is the clouding of the lens that may occur because of protein denaturation in the lens [1]. Nearly half of patients with blindness were found to have cataracts (approximately 20 million), and cataracts have also been found to be the leading cause of serious vision loss worldwide [2–4]. Cataract-related reductions in visual acuity cannot be rectified by wearing glasses; thus, cataract surgery is the treatment for cataract patients with advanced disease.
Cataract removal surgery can be performed at any disease stage, and 90% of patients can achieve a corrected vision of 20/40 or better [5, 6]. Phacoemulsification is the most widely used cataract surgery in the developed world and employs ultrasonic energy to emulsify the cataract lens [7]. Varying degrees of inflammation will occur after surgery due to mechanical damage and the reaction of the residual lens epithelium with the foreign intraocular lens [8]. These factors can cause membrane disorders of local ocular cells, the production of active phospholipase A2, and the release of arachidonic acid. Arachidonic acid may be transformed into prostaglandin (PG) by epoxidase catalysis [9]. The aggregation of PG in the eyes can lead to corestenoma during surgery and the release of inflammatory factors into aqueous fluid.
Non-steroidal anti-inflammatory drugs (NSAIDs) are a class of anti-inflammatory drugs without a steroid structure that can prevent the transformation of arachidonic acid into PG [10]. During cataract surgery, NSAIDs may inhibit surgery-induced miosis, anterior chamber inflammation, and cystoid macular edema (CME), as well as relieve perioperative ocular itching and pain. NSAIDs were first approved by the FDA to prevent surgically induced miosis [11]. Newer NSAIDs are being investigated for their ability to reduce the incidence of CME after cataract surgery. CME is a major complication after cataract surgery and remains the primary cause of surgical visual disorders. The pathogenesis of CME is unclear; however, most researchers believe that inflammation after cataract surgery is the primary cause of CME [12]. Whether NSAIDs can effectively prevent the development of CME remains controversial. Recent comprehensive analyses investigated the ability of NSAIDs to reduce the incidence of CME after cataract surgery; however, a positive effect was not observed [13–16].
Although ambiguity surrounds NSAIDs regarding CME prevention, NSAIDs play an important role in cataract surgery. In this study, we explored the value of topical NSAID application for inhibiting anterior chamber inflammation. NSAID ophthalmic preparations have very similar anti-inflammatory mechanisms, yet their therapeutic efficacies differ. Therefore, this study attempted to analyze the effects of various NSAIDs using a network meta-analysis.
Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines [17].
Data search strategy and selection criteria
A literature search was independently performed by two investigators using electronic databases, including PubMed, Embase, and the Cochrane Library, to identify articles published prior to October 2016 using the following search keywords: “cataract surgery,” “random*,” and “topical*.” The bibliographies of the obtained publications and the references of the relevant reviews were checked to ensure that no relevant studies were unintentionally omitted. The studies included in this meta-analysis met the following criteria: (1) the study had a blinded, randomized controlled trial (RCT) design, where one group was treated with NSAIDs and another group was treated with a blank, placebo, or alternate NSAID; (2) the study included patients after cataract surgery; (3) the patients received anti-inflammatory treatment after surgery; and (4) one of the following outcomes was included in the study: anterior chamber inflammation, ocular pain relief, or treatment-related/serious ocular adverse events. The exclusion criteria included the following: (1) non-cataract surgery studies; (2) steroid drug-related studies and experimental/control groups combined with steroid drug therapy; (3) anesthesia-related studies; (4) surgical method-related studies; and (5) undesired outcome studies. Reviews, case reports, conference reports, basic research, and editorial comments were also excluded.
Data extraction and quality assessment
Two investigators independently extracted the following information from each eligible study: the name of the first author, publication year, location, sample size, average age (total or experimental group), ratio of males to females, experimental intervention, control intervention, and follow-up time. We assessed the methodological quality of the included trials using the Cochrane Collaboration tool [18]. Studies were graded as having a “low risk,” “high risk,” or “unclear risk” of bias across the seven specified domains.
We were primarily interested in the treatment effect of NSAIDs on the relief of anterior chamber inflammation after cataract surgery. Therefore, we selected and analyzed three important outcomes for clinical decision making according to GRADE guidelines. Our analysis included overall anterior chamber inflammation, subjects with 0–5 anterior chamber cells and the complete absence of anterior chamber flare, the number of ocular pain-free patients during the early postoperative period following surgery, and the incidence of treatment-related/serious ocular adverse events. We also used the GRADE approach to assess the network meta-analysis quality, with four levels graded from high (best) to very low (worst) [19]. This method considered the quality of direct and indirect evidence, as well as the quality of network evidence according to the inconsistency between direct and indirect evidence and the intransitivity among all related pieces of evidence. We performed “node splitting” to separate the indirect evidence from the direct evidence to inform these evaluations [20].
Statistical analysis
We performed a meta-analysis using a random-effects model. For dichotomous outcomes, odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to determine the sizes of the effects. We also used a random-effects network meta-analysis for mixed multiple treatment comparisons because this approach fully preserves the within-trial randomized treatment comparisons in each trial [21]. To rank the treatments for each outcome, we used surface under the cumulative ranking (SUCRA) probabilities [22]. Comparison-adjusted funnel plots were used to determine whether small-study effects were present in our analysis [23].
Results
Literature search
Figure 1 shows the flow of study inclusion in the meta-analysis. We identified 1,127 articles after duplicates were removed. Of these, 1,042 were excluded after the titles and abstracts were screened. The full text of the remaining 85 articles was assessed, and 66 articles were excluded for the following reasons: undesired outcomes (36); experimental/control groups combined with steroid drug therapy studies (18); studies without a blinded design (7); duplicate publications (2); non-cataract surgery studies (2); and letters to the editor (1). Ultimately, 19 trials assessing 7,234 patients were included in our systematic review [24–42] (Fig. 1, Table 1).
The included studies were published between 1987 and 2015. The average subject age ranged from 65 to 75 years, and there were more women than men. All included patients received cataract surgery (phacoemulsification or extracapsular cataract extraction) with posterior chamber intraocular lens implantations, except one early article [42]. Three articles included patients with moderate-to-severe ocular inflammation after cataract surgery [34, 36, 41], which is an indirect degradation factor according to GRADE.
In our study, the researched topical NSAIDs were bromfenac, diclofenac, flurbiprofen, indomethacin, ketorolac, nepafenac, and piroxicam. One article included two RCTs [25], and one article had a three-arm design [39]. Three studies researched comparisons among NSAIDs [37–39], and another study researched comparisons between NSAIDs and placebos. The follow-up duration of the included studies ranged from one day to six weeks. In this meta-analysis, we included RCT studies with a blind design. Most studies were well designed; thus, the overall quality of the included studies was satisfactory (Fig. 2).
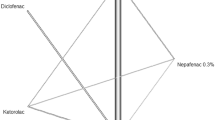
A total of 13 studies included findings of anterior chamber inflammation. The included NSAIDs were bromfenac, diclofenac, flurbiprofen, ketorolac, and nepafenac. All included drugs were directly compared with a placebo; there was also a direct comparison between diclofenac and flurbiprofen (Fig. 3a); in this figure, the nodes are weighted according to the number of studies that evaluated each treatment, and the edges are weighted according to the precision of the direct estimate for each pairwise comparison. In pairwise comparisons, bromfenac was significantly inferior to nepafenac regarding anterior chamber cells and flare reduction in indirect and network comparisons (logOR: −0.58; 95% CI: −1.11 to −0.05). Bromfenac was significantly superior to the placebo in direct and network comparisons (logOR: 1.04; 95% CI: 0.62 to 1.46). Diclofenac was superior to flurbiprofen in indirect (logOR: 1.38; 95% CI: 0.21 to 2.55) and network comparisons (logOR: 0.98; 95% CI: 0.06 to 1.90). Diclofenac was also superior to the placebo in direct (logOR: 2.19; 95% CI: 1.26 to 3.13) and network comparisons (logOR: 1.94; 95% CI: 1.13 to 2.75). Flurbiprofen was significantly better than the placebo for controlling ocular inflammation in all comparisons (network: logOR 0.96; 95% CI: 0.29 to 1.62). Ketorolac showed a significant advantage over the placebo in the network comparison (logOR: 1.31; 95% CI: 0.80 to 1.82), as did nepafenac (logOR: 1.63; 95% CI: 1.30 to 1.95) (Table 2). In terms of SUCRA rank, diclofenac was the most likely to improve anterior chamber inflammation after cataract surgery, followed by nepafenac, ketorolac, bromfenac, and flurbiprofen (Fig. 4a). Although the quality of the network data for diclofenac was low, the results remained robust due to the high quality of the studies overall. Additionally, the comparison-adjusted funnel plot used to assess publication bias and determine the presence of small-study effects did not indicate a publication bias (Fig. 5a).
Eight articles reported an outcome of ocular pain relief after surgery. Bromfenac, ketorolac, and nepafenac were included, all of which were directly compared to placebos but not to other drugs (Fig. 3b). In indirect and network comparisons, ketorolac was significantly inferior to nepafenac in relieving ocular pain relief after cataract surgery (logOR: −1.45; 95% CI: −2.30 to −0.59). In direct and network comparisons, a higher proportion of patients reported ocular pain relief with the application of an NSAID than the placebo; these NSAIDs included bromfenac (logOR: 1.80; 95% CI: 1.12), ketorolac (logOR: 0.94; 95% CI: 0.28 to 1.60), and nepafenac (logOR: 2.38; 95% CI: 1.84 to 2.92) (Table 2). Nepafenac was most likely to improve postoperative ocular pain relief, followed by bromfenac and ketorolac (Fig. 4b). Notably, only three NSAIDs were included in these comparisons. A global inconsistency was found in our test (p < 0.001); thus, further studies are needed to confirm these results. The comparison-adjusted funnel plot revealed no clear publication bias (Fig. 5b).
Fifteen articles examined treatment-related/seriously adverse events. They analyzed seven NSAIDs: bromfenac, diclofenac, flurbiprofen, indomethacin, ketorolac, nepafenac, and piroxicam. The drugs that were directly compared to a placebo included bromfenac, flurbiprofen, ketorolac, and nepafenac. The drugs that were directly compared to diclofenac included flurbiprofen, indomethacin, ketorolac, and piroxicam. There was also a direct comparison between flurbiprofen and indomethacin (Fig. 3c). For pairwise comparisons, there were no significant differences between NSAIDs and placebos regarding related adverse events. In direct and network comparisons, only bromfenac (logOR: 0.65; 95% CI: 0.14 to 1.16) and piroxicam (logOR: 3.26; 95% CI: 0.59 to 5.94) resulted in significantly fewer related adverse events than did the placebos. Additionally, flurbiprofen (logOR: −3.36; 95% CI: −6.13 to −0.59), indomethacin (logOR: −3.11; 95% CI: −6.08 to −0.13) and ketorolac (logOR: −2.78; 95% CI: −5.42 to −0.13) were significantly inferior to piroxicam in both indirect and network comparisons. However, the quality of evidence for these indirect comparisons was very low (Table 2). The SUCRA results showed that although there were fewer differences among the NSAIDs, these drugs were overall slightly better than the placebos regarding related adverse events. This finding indicated that piroxicam is most likely to have the fewest related adverse events. However, due to the very low evidence quality, further studies are needed for robust results. In addition to piroxicam, diclofenac is another nearly ideal drug, followed by nepafenac, bromfenac, and ketorolac (Fig. 4c). The comparison-adjusted funnel plot did not reveal any obvious publication bias (Fig. 5c).
Discussion
In this study, we performed a network meta-analysis to assess the efficacy and safety of NSAIDs for anterior chamber inflammation treatment after cataract surgery. The results included overall anterior chamber inflammation, ocular pain-free events, and treatment-related/serious ocular adverse events. Diclofenac was most likely to improve anterior chamber inflammation after cataract surgery followed by nepafenac, ketorolac, bromfenac, and flurbiprofen. Nepafenac was the most likely to reduce postoperative ocular pain, followed by bromfenac and ketorolac. Finally, piroxicam was the most likely to show the fewest related adverse events, but the evidence exhibited low robustness. Moreover, diclofenac was another nearly ideal drug, followed by nepafenac, bromfenac, and ketorolac. In a comprehensive analysis, compared with placebos, NSAIDs were shown to be effective drugs for reducing anterior chamber inflammation and ocular pain relief; NSAIDs also had fewer treatment-related/serious ocular adverse events. Furthermore, diclofenac, nepafenac, ketorolac, and bromfenac demonstrated relatively greater significant effects.
Anterior chamber cells and the presence of anterior chamber flare were the primary measurements for anterior chamber inflammation reduction; however, the evaluation criteria differed slightly among the included studies. Our analysis used 0–5 anterior chamber cells and the absence of flare as inflammation relief criteria. However, some studies used non-anterior chamber cells and the absence of flare as assessment criteria [25]. We included only three types of NSAIDs in our ocular pain relief analysis, and criteria were based on the subjective judgments of the patients. However, because only well-designed RCTs were included in our analysis, the influence of subjective assessment on the outcome was reduced. Adverse event-related outcomes were somewhat subjective due to varying assessment criteria among the assessors and studies. However, we analyzed only treatment-related or serious adverse events that emerged when these events had a negative impact on the administration of NSAIDs. Additionally, we did not analyze the pupil size results because the degree of miosis is also affected by individual differences, ocular stress reactions, and mydriatic drugs.
This study included the topical NSAIDs most commonly used in ophthalmology. Among them, diclofenac belongs to the phenyl acetic acid category, nepafenac belongs to the phenylacetamide category, and ketoprofen and bromfenac belong to the acetic acid category. Notably, diclofenac has unique characteristics. In addition to its ability to inhibit prostaglandin synthesis by suppressing cyclooxygenase, diclofenac shows bacteriostatic activity by inhibiting bacterial DNA synthesis and the lipoxygenase pathway, as well as reducing the formation of leukotrienes [43, 44]. These reactions may further suppress inflammation after cataract surgery with fewer serious adverse events.
The incidence of CME has been significantly reduced because cataract surgery has become more minimally invasive. Notably, cataract extraction with intraocular lens implantation is more minimally invasive and produces less inflammation than does intracapsular or extracapsular cataract extraction. However, constant technological optimization reducing the need for physical stimulation, ultrasonic influence, intraoperative perfusion fluid, viscoelastic agents, and other adjuvant drugs may also reduce inflammatory reactions after surgery. In aged cataract patients, phacoemulsification provided more advantages in uncorrected visual acuity and surgically induced astigmatism than did manual incision cataract surgery [45]. Theoretically, the perioperative application of NSAIDs may further prevent inflammatory reactions and reduce the incidence of CME. However, current systematic reviews could not make definitive conclusions because of a lack of high-quality evidence [13–16]. One study considered NSAIDs to be effective in chronic CME after cataract surgery [13]; however, another study suggested that while NSAIDs may accelerate visual recovery a few weeks after surgery, the long-term effects remain unclear [16]. NSAIDs were also found to have advantages in the treatment of CME compared to steroidal drugs [46]. Therefore, the effects of NSAID treatment on acute and chronic CME remain controversial.
Although this study excluded all steroid-related and combined treatment studies, corticosteroid drugs combined with antibiotics may reduce ocular bacterial flora and inflammation after cataract surgery [47], and a combination with NSAIDs may reduce the incidences of CME and macular thickening [48]. Treatment with steroid drugs can produce severe adverse reactions, including hypoadrenocorticism, ulcer disease, increases in intraocular pressure, and a high risk of secondary ocular infections. NSAIDs are superior to steroid drugs for inhibiting PG synthesis and reducing the incidence of CME. Moreover, with fewer adverse effects, NSAIDs have been reported to decrease visual acuity and sticky sensations. They also have a low probability of inducing corneal melting and perforation, which require monitoring in clinical applications.
In contrast with other reviews, we analyzed important inflammation-related outcomes according to GRADE recommendations and classified the quality of evidence into four levels using both direct and indirect comparisons. Although this approach required subjective assessments, the transparency of these choices should be enhanced.
There are several limitations to our study. First, our analysis was performed at the study level and not at an individual level. Second, our study included only inflammation-related outcomes; the effects on CME remain controversial. Third, our analysis had unexplained heterogeneity and global inconsistency, which may have resulted from differences in the administered doses, operation processes, concomitant treatments, or follow-up durations.
Conclusion
In conclusion, NSAIDs represent a class of drugs that are more effective for reducing anterior chamber inflammation and relieving ocular pain than placebos. NSAIDs also show fewer treatment-related/serious ocular adverse events. Diclofenac, nepafenac, ketorolac, and bromfenac have relatively greater significant effects than other topical NSAIDs.
References
Tsui PH, Huang CC, Zhou Q, Shung KK (2011) Cataract measurement by estimating the ultrasonic statistical parameter using an ultrasound needle transducer: an in vitro study. Physiol Meas 32:513–522. doi:10.1088/0967-3334/32/5/002
Isaacs R, Ram J, Apple D (2004) Cataract blindness in the developing world: is there a solution? J Agromedicine 9:207–220
Lam DS, Li EY, Chang DF, Zhang MZ, Zhan HK, Pang CP (2009) Project vision: a new and sustainable model for eliminating cataract blindness in China. Clin Exp Ophthalmol 37:427–430. doi:10.1111/j.1442-9071.2009.02084.x
Shen W, Yang Y, Yu M, Li J, Wei T, Li X, Li J, Su X, Zhong H, Yuan Y (2013) Prevalence and outcomes of cataract surgery in adult rural Chinese populations of the Bai nationality in Dali: the Yunnan minority eye study. PLoS One 8:e60236. doi:10.1371/journal.pone.0060236
Rofagha S, Bhisitkul RB (2011) Management of retained lens fragments in complicated cataract surgery. Curr Opin Ophthalmol 22:137–140. doi:10.1097/ICU.0b013e3283436fc5
Jammal HM, Khader Y, Shawer R, Al Bdour M (2012) Posterior segment causes of reduced visual acuity after phacoemulsification in eyes with cataract and obscured fundus view. Clin Ophthalmol 6:1843–1848. doi:10.2147/OPTH.S38303
Melancia D, Abegão Pinto L, Marques-Neves C (2015) Cataract surgery and intraocular pressure. Ophthal Res 53:141–148. doi:10.1159/000377635
Higashide T, Sugiyama K (2008) Use of viscoelastic substance in ophthalmic surgery - focus on sodium hyaluronate. Clin Ophthalmol 2:21–30
Waterbury LD, Galindo D, Villanueva L, Nguyen C, Patel M, Borbridge L, Attar M, Schiffman RM, Hollander DA (2011) Ocular penetration and anti-inflammatory activity of ketorolac 0.45% and bromfenac 0.09% against lipopolysaccharide-induced inflammation. J Ocul Pharmacol Ther 27:173–178. doi:10.1089/jop.2010.0135
Ruan KH, Cervantes V, So SP (2009) Engineering of a novel hybrid enzyme: an anti-inflammatory drug target with triple catalytic activities directly converting arachidonic acid into the inflammatory prostaglandin E2. Protein Eng Des Sel 22:733–740. doi:10.1093/protein/gzp058
Nichols J, Snyder RW (1998) Topical nonsteroidal anti-inflammatory agents in ophthalmology. Curr Opin Ophthalmol 9:40–44. doi:10.1097/00055735-199808000-00007
Rothova A (2007) Inflammatory cystoid macular edema. Curr Opin Ophthalmol 18:487–492. doi:10.1097/ICU.0b013e3282f03d2e
Sivaprasad S, Bunce C, Wormald R (2005) Non-steroidal anti-inflammatory agents for cystoid macular oedema following cataract surgery: a systematic review. Br J Ophthalmol 89:1420–1422. doi:10.1136/bjo.2005.073817
Sivaprasad S, Bunce C, Crosby-Nwaobi R (2012) Non-steroidal anti-inflammatory agents for treating cystoid macular oedema following cataract surgery. Cochrane Database Syst Rev CD004239. doi: 10.1002/14651858.CD004239.pub3
Yilmaz T, Cordero-Coma M, Gallagher MJ (2012) Ketorolac therapy for the prevention of acute pseudophakic cystoid macular edema: a systematic review. Eye (Lond) 26:252–258. doi:10.1038/eye.2011.296
Kim SJ, Schoenberger SD, Thorne JE, Ehlers JP, Yeh S, Bakri SJ (2015) Topical nonsteroidal anti-inflammatory drugs and cataract surgery: a report by the American Academy of Ophthalmology. Ophthalmology 122:2159–2168. doi:10.1016/j.ophtha.2015.05.014
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. doi:10.1371/journal.pmed.1000097
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, Cochrane Statistical Methods Group (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi:10.1136/bmj.d5928
Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, Nasser M, Meerpohl J, Post PN, Kunz R, Brozek J, Vist G, Rind D, Akl EA, Schünemann HJ (2013) GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol 66:719–725. doi:10.1016/j.jclinepi.2012.03.013
Hazlewood GS, Barnabe C, Tomlinson G, Marshall D, Devoe D, Bombardier C (2016) Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ 353:i1777. doi:10.1136/bmj.i1777
White IR, Barrett JK, Jackson D, Higgins JP (2012) Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods 3:111–125. doi:10.1002/jrsm.1045
Li D, Wang T, Shen S, Cheng S, Yu J, Zhang Y, Zhang C, Tang H (2015) Effects of fluroquinolones in newly diagnosed, sputum-positive tuberculosis therapy: a systematic review and network meta-analysis. PLoS One 10:e0145066. doi:10.1371/journal.pone.0145066
Trinquart L, Chatellier G, Ravaud P (2012) Adjustment for reporting bias in network meta-analysis of antidepressant trials. BMC Med Res Methodol 12:150. doi:10.1186/1471-2288-12-150
Hovanesian JA, Sheppard JD, Trattler WB, Gayton JL, Malhotra RP, Schaaf DT, Ng E, Dunn SH (2015) Intracameral phenylephrine and ketorolac during cataract surgery to maintain intraoperative mydriasis and reduce postoperative ocular pain: integrated results from 2 pivotal phase 3 studies. J Cataract Refract Surg 41:2060–2068. doi:10.1016/j.jcrs.2015.10.053
Modi SS, Lehmann RP, Walters TR, Fong R, Christie WC, Roel L, Nethery D, Sager D, Tsorbatzoglou A, Philipson B, Traverso CE, Reiser H (2014) Once-daily nepafenac ophthalmic suspension 0.3% to prevent and treat ocular inflammation and pain after cataract surgery: phase 3 study. J Cataract Refract Surg 40:203–211. doi:10.1016/j.jcrs.2013.07.042
Walters TR, Goldberg DF, Peace JH, Gow JA, Bromfenac Ophthalmic Solution 0.07% Once Daily Study Group (2014) Bromfenac ophthalmic solution 0.07% dosed once daily for cataract surgery: results of 2 randomized controlled trials. Ophthalmology 121:25–33. doi:10.1016/j.ophtha.2013.07.006
Numaga J (2011) Phase II placebo-controlled study of nepafenac ophthalmic suspension 0.1% for postoperative inflammation and ocular pain associated with cataract surgery in Japanese patients. J Ophthal Inflamm Infect 1:147–155. doi:10.1007/s12348-011-0036-8
Henderson BA, Gayton JL, Chandler SP, Gow JA, Klier SM, McNamara TR, Bromfenac Ophthalmic Solution (Bromday) Once Daily Study Group (2011) Safety and efficacy of bromfenac ophthalmic solution (Bromday) dosed once daily for postoperative ocular inflammation and pain. Ophthalmology 118:2120–2127. doi:10.1016/j.ophtha.2011.04.035
Donnenfeld ED, Nichamin LD, Hardten DR, Raizman MB, Trattler W, Rajpal RK, Alpern LM, Felix C, Bradford RR, Villanueva L, Hollander DA, Schiffman RM (2011) Twice-daily, preservative-free ketorolac 0.45% for treatment of inflammation and pain after cataract surgery. Am J Ophthalmol 151:420.e1–426.e1. doi:10.1016/j.ajo.2010.09.003
Maxwell WA, Reiser HJ, Stewart RH, Cavanagh HD, Walters TR, Sager DP, Meuse PA (2008) Nepafenac dosing frequency for ocular pain and inflammation associated with cataract surgery. J Ocul Pharmacol Ther 24:593–599. doi:10.1089/jop.2008.0023
Stewart RH, Grillone LR, Shiffman ML, Donnenfeld ED, Gow JA, Bromfenac Ophthalmic Solution 0.09% Study Group (2007) The systemic safety of bromfenac ophthalmic solution 0.09%. J Ocul Pharmacol Ther 23:601–612. doi:10.1089/jop.2007.0040
Lane SS, Modi SS, Lehmann RP, Holland EJ (2007) Nepafenac ophthalmic suspension 0.1% for the prevention and treatment of ocular inflammation associated with cataract surgery. J Cataract Refract Surg 33:53–58. doi:10.1016/j.jcrs.2006.08.043
Scuderi B, Driussi GB, Chizzolini M, Salvetat ML, Beltrame G (2003) Effectiveness and tolerance of piroxicam 0.5% and diclofenac sodium 0.1% in controlling inflammation after cataract surgery. Eur J Ophthalmol 13:536–540
Solomon KD, Cheetham JK, DeGryse R, Brint SF, Rosenthal A (2001) Topical ketorolac tromethamine 0.5% ophthalmic solution in ocular inflammation after cataract surgery. Ophthalmology 108:331–337. doi:10.1016/S0161-6420(00)00543-1
Stewart R, Grosserode R, Cheetham JK, Rosenthal A (1999) Efficacy and safety profile of ketorolac 0.5% ophthalmic solution in the prevention of surgically induced miosis during cataract surgery. Clin Ther 21:723–732. doi:10.1016/S0149-2918(00)88323-X
Heier J, Cheetham JK, Degryse R, Dirks MS, Caldwell DR, Silverstone DE, Rosenthal A (1999) Ketorolac tromethamine 0.5% ophthalmic solution in the treatment of moderate to severe ocular inflammation after cataract surgery: a randomized, vehicle-controlled clinical trial. Am J Ophthalmol 127:253–259. doi:10.1016/S0002-9394(98)00413-9
Flach AJ, Dolan BJ, Donahue ME, Faktorovich EG, Gonzalez GA (1998) Comparative effects of ketorolac 0.5% or diclofenac 0.1% ophthalmic solutions on inflammation after cataract surgery. Ophthalmology 105:1775–1779. doi:10.1016/S0161-6420(98)99053-4
Koçak I, Yalvaç IS, Koçak A, Nurözler A, Unlü N, Kasim R, Duman S (1998) Comparison of the anti-inflammatory effects of diclofenac and flurbiprofen eye drops after cataract extraction. Acta Ophthalmol Scand 76:343–345. doi:10.1034/j.1600-0420.1998.760318.x
Diestelhorst M, Schmidl B, Konen W, Mester U, Raj PS (1996) Efficacy and tolerance of diclofenac sodium 0.1%, flurbiprofen 0.03%, and indomethacin 1.0% in controlling postoperative inflammation. J Cataract Refract Surg 22(Suppl 1):788–793. doi:10.1016/S0886-3350(96)80163-5
Blaydes JE Jr, Kelley EP, Walt JG, DeGryse RE, Harper DG, Novack GD (1993) Flurbiprofen 0.03% for the control of inflammation following cataract extraction by phacoemulsification. J Cataract Refract Surg 19:481–487. doi:10.1016/S0886-3350(13)80611-6
Kraff MC, Martin RG, Neumann AC, Weinstein AJ (1994) Efficacy of diclofenac sodium ophthalmic solution versus placebo in reducing inflammation following cataract extraction and posterior chamber lens implantation. J Cataract Refract Surg 20:138–144. doi:10.1016/S0886-3350(13)80153-8
Sabiston D, Tessler H, Sumers K, Osterle C, Cheetham JK, Duzman E, DeGryse R (1987) Reduction of inflammation following cataract surgery by the nonsteroidal anti-inflammatory drug, flurbiprofen. Ophthal Surg 18:873–877
Dastidar SG, Ganguly K, Chaudhuri K, Chakrabarty AN (2000) The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis. Int J Antimicrob Agents 14:249–251. doi:10.1016/S0924-8579(99)00159-4
Serhan CN, Chiang N (2002) Lipid-derived mediators in endogenous anti-inflammation and resolution: lipoxins and aspirin-triggered 15-epi-lipoxins. ScientificWorldJournal 2:169–204. doi:10.1100/tsw.2002.81
Zhang JY, Feng YF, Cai JQ (2013) Phacoemulsification versus manual small-incision cataract surgery for age-related cataract: meta-analysis of randomized controlled trials. Clin Exp Ophthalmol 41:379–386. doi:10.1111/j.1442-9071.2012.02868.x
Wielders LH, Lambermont VA, Schouten JS, van den Biggelaar FJ, Worthy G, Simons RW, Winkens B, Nuijts RM (2015) Prevention of cystoid macular edema after cataract surgery in nondiabetic and diabetic patients: a systematic review and meta-analysis. Am J Ophthalmol 160:968.e33–981.e33. doi:10.1016/j.ajo.2015.07.032
van Endt JJ, Veraart HG, Kramer R, Janssen AG, Sunder Raj P (1997) A comparison of two ophthalmic steroid-antibiotic combinations after cataract surgery. Eur J Ophthalmol 7:144–148
Wittpenn JR, Silverstein S, Heier J, Kenyon KR, Hunkeler JD, Earl M, Acular LS for Cystoid Macular Edema (ACME) Study Group (2008) A randomized, masked comparison of topical ketorolac 0.4% plus steroid vs steroid alone in low-risk cataract surgery patients. Am J Ophthalmol 146:554–560. doi:10.1016/j.ajo.2008.04.036
Authors’ contributions
PD conceived of the study; PD and YL searched the literature and collected the data; YL performed the statistical analyses; PD drafted the manuscript; JwL reviewed the manuscript. All authors have read and approved the final paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81400418).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Rights and permissions
About this article
Cite this article
Duan, P., Liu, Y. & Li, J. The comparative efficacy and safety of topical non-steroidal anti-inflammatory drugs for the treatment of anterior chamber inflammation after cataract surgery: a systematic review and network meta-analysis. Graefes Arch Clin Exp Ophthalmol 255, 639–649 (2017). https://doi.org/10.1007/s00417-017-3599-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3599-8