Abstract
Purpose
To evaluate quantitatively the choroidal vascularity in polypoidal choroidal vasculopathy (PCV) and neovascular age-related macular degeneration (AMD) patients compared to healthy controls.
Methods
All eyes underwent swept source optical coherence tomography (OCT), and choroidal images were binarized into blood vessels lumen and stroma. The choroidal vascular index (CVI) was defined as the ratio of luminal area (LA) over total choroidal area of the subfoveal region with a width of 1500 μm.
Results
The study included 73 patients with neovascular AMD or PCV with mean ± standard deviation (SD) age of 71.8 ± 9.3 years, which was older than the mean age of 65.1 ± 10.8 years of 72 healthy eyes from control group (p < 0.01). The 44 PCV eyes had significantly higher mean SFCT of 214.23 ± 95.21 μm than neovascular AMD eyes (172.74 ± 96.48 μm, p = 0.03) and greater luminal area (0.23 ± 0.09 mm2 vs. 0.19 ± 0.08 mm2, p = 0.05). After adjusting for age, axial length, and gender in multivariate regression analysis, the SFCT of PCV and neovascular AMD eyes were not significantly different from healthy eyes (195.55 ± 93.11 μm), but the CVI of both PCV (64.94 ± 5.43%, p = 0.01) and neovascular AMD (62.54 ± 5.57%, p = <0.01) were significantly lower than control (68.53 ± 5.91%).
Conclusion
Despite physiological changes of choroidal vasculature due to aging, the choroidal morphology is different in PCV, neovascular AMD and healthy eyes, which has implication on disease pathogenesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is one of the leading causes of vision loss worldwide [1]. The prevalence of polypoidal choroidal vasculopathy (PCV), a subtype of exudative AMD, was reported to be between 22.3–61.6% among Asians and 8–13% among Caucasians [2]. Whether PCV is a distinct disease entity or lies within the spectrum of exudative AMD is still a subject of controversy [3]. While AMD and PCV patients share some common genetic and clinical features, they also have different histopathology, risk factors, natural history, and responses to treatment [4–7].
Choroidal thickness has been proposed to be a main surrogate marker for the assessment of structural changes of the choroid in AMD and PCV eyes [8–10]. In advanced AMD, the choroid has been demonstrated to become thinner than age-matched controls [11, 12]. However, the choroidal thickness in early or intermediate AMD may not deviate much from normal eyes [13]. In contrast, thick choroid has been found in a significant proportion of patients with PCV [10]. Nevertheless, the reported choroidal thickness measurements have not been consistent among studies [12–15]. It remains uncertain whether change in choroidal thickness is contributed by altered extravascular space or remodelling of choroidal vasculature. Indeed, various physiological factors may affect the thickness of both the stroma and vasculature of the choroid [16]. This reflects the limitation of using choroidal thickness alone as an imaging marker because both vascular and stromal elements contribute towards thickness.
In the present study, we performed quantitative evaluation of choroidal vascularity in the eyes of patients with neovascular AMD, symptomatic PCV, and their uninvolved fellow eyes and compared with healthy eyes. Using binarized images of cross-sectional swept source optical coherence tomography (SS OCT) scans, we calculated the choroidal vascularity index (CVI), which is the ratio of choroidal blood vessels luminal area to total choroidal area within a specified subfoveal area. The CVI has been reported in a population-based study, and in patients with exudative AMD, central serous chorioretinopathy (CSC), uveitis, and diabetic retinopathy as a surrogate marker for choroidal vascularity [17–19]. However, there has not been a comparison between neovascular AMD and PCV with healthy eyes from a control group.
Methods
This is a cross-sectional, observational study of consecutive patients with symptomatic exudative maculopathy recruited from the University Eye Centre at the Hong Kong Eye Hospital and the Prince of Wales Hospital from November 2015 to May 2016. Control data were obtained from healthy patients with immature cataracts. The study was approved by institutional review boards and adhered to the tenets of the 1964 Declaration of Helsinki. Informed consent was signed by all subjects before the posterior segment imaging procedures. Exclusion criteria included the presence of refractive errors more than 3.0 diopters, significant cataract, massive subfoveal hemorrhage or media opacities that obscured choroidal images, history of ocular inflammation, history of retinal detachment, previous vitrectomy, intraocular surgery (including cataract surgery) in the study eye within 1 year, history of CSC, ocular trauma, and glaucoma in the study eye. Eyes that had undergone photodynamic therapy (PDT), intravitreal corticosteroid injection, or anti-vascular endothelial growth factor (VEGF) less than 3 months ago were also excluded from this study.
All patients received comprehensive ophthalmic examinations, including best corrected visual acuity (BCVA), intraocular pressure (IOP) measurement with Goldmann tonometry, blood pressure measurement, axial length measurement with IOL master (Zeiss, Germany), slit lamp biomicroscopy, dilated fundal examination, fluorescein angiography (FA), and indocyanine green angiography (ICGA) with a confocal scanning laser ophthalmoscopy system (HRA Spectralis; Heidelberg Engineering, Dossenheim, Germany) and SS OCT (DRI Triton, Topcon, Tokyo, Japan).
Two masked retinal specialists, experienced in the assessment and management of AMD and PCV, independently reviewed the images from early to late phases of FA and ICGA. Diagnosis of PCV was based on identification of characteristic polypoidal lesions, often in association with a branching vascular network (BVN) in ICGA [20, 21]. The diagnosis of exudative AMD was determined based on FA finding of choroidal neovascularization (classic, occult, or both), without polypoidal lesions or BVN shown on ICGA. A third retinal specialist arbitrated when there was disagreement between the two observers.
All patients underwent SS OCT imaging, which incorporated a light source at 1050 nm wavelength and acquired 100,000 A-scans per second. It had an axial resolution of 8 μm and lateral resolution of 20 μm. The macular region was scanned using 12 raster radial lines (30° × 5°), 1 clock hour apart and centered on the fovea, with 16 frames averaged in each B-scan. Each scan was 12 mm in length. Bruch’s membrane and the chorioscleral interface were delineated with the machine’s built-in autosegmentation software and the subfoveal choroidal thickness (SFCT) was automatically measured by built-in calliper.
Choroidal vascular index (CVI)
The entire length of the 12 mm radial OCT B-scans were binarized using public domain software, Image J (Version 1.51; https://imagej.nih.gov/ij/) with the Niblack autolocal threshold tool [22]. Using the polygonal selection tool, the choroidal area of interest bounded by the Bruch’s membrane as the upper border and choroidal-scleral interface as the lower border was marked (Fig. 1). For each study eye, the total choroidal area (TCA) for the subfoveal region within a standard width of 1500 μm (750 μm either side of the fovea) was computed. In the binarized images, dark pixels represented the lumen of blood vessels and white pixels represented the stroma. To calculate the area of dark pixels defined as luminal area (LA), the image was converted into RGB (red, green, blue) colors to allow the color threshold tool to select the dark pixels [23]. The stromal area (SA) and was calculated by subtracting LA from TCA. The ratio of LA over TCA was termed CVI. Image grading was done by one of the authors who was masked to the patients’ information. Quality assurance checks were performed separate from the time of image acquisition and images that contained segmentation algorithm failures, motion artifacts, or poorly focused were excluded from the analysis. Failure of the automated chorioscleral interface segmentation line was defined when corroboration with visual inspection differed in more than 25% of the scan area.
Imaging processing for obtaining CVI. (Top) Original SSOCT image of a raster scan through the fovea. (Middle) After binarization, the choroidal vessels lumen are represented by dark pixels, and the stromal tissue by light pixels. (Bottom) CVI is calculated by dividing the luminal area (LA) by total choroidal area (TCA). TCA is obtained by computing the area bounded by the Bruch’s membrane (red line) at the top, the choroid-sclera interface (yellow line) at the bottom and within a standard width of 1.50 mm (0.75 mm on either side of fovea, bounded by the purple lines). The choroidal thickness of this healthy eye of a 69 year-old man was 267.62 μm and CVI was 69.71
Statistical analysis
Inter-observer agreement for diagnosis of PCV or neovascular AMD was evaluated by the intra-class correlation coefficient (ICC) value. Chi square test was used for comparing categorical data. Mann–Whitney U test was used compared the means between different subjects and Wilcoxon signed-rank test for comparison of the means of two eyes from the same subject. Univariate regression analyses were performed to determine whether demographics, ocular factors or systemic factors could influence subfoveal choroidal thickness and CVI. Multivariate linear regression analysis of SFCT and CVI in neovascular was performed to evaluate for the differences between neovascular AMD and PCV with healthy eyes after adjusting for age, gender, axial length, BCVA, and CI (when CVI was the dependent variable). The relationships between SFCT and CVI were examined using Pearson correlation test. A p value of <0.05 was considered to be statistically significant. All of the statistical analyses were performed with SPSS software version 18.0 (SPSS Inc, Chicago, IL, USA).
Results
Seventy-nine patients with exudative maculopathy and 72 controls were recruited. Six patients were excluded from the analysis due to massive subretinal haemorrhage, media opacities and poor eye fixation which obscured the quality of image acquisition. The study ultimately included 73 patients with mean ± standard deviation (SD) age of 71.8 ± 9.3 years (range 54 to 89 years). Table 1 summarizes the demographics and clinical data of patients and control subjects. The mean age of the control group was 65.1 ± 10.8 years (range 51 to 81 years), which was significantly younger than the disease group (p = <0.01). Eyes with exudative maculopathy also had significantly worse BCVA than the control group (LogMAR 0.70 ± 0.48 vs. 0.30 ± 0.22, p = <0.01). One eye from each control subject was randomly selected for analysis in this study. Univariate analysis of demographic, ocular factors and blood pressure showed that only age was significantly correlated with SFCT (Table 2). None of these factors were significantly associated with CVI.
All exudative maculopathy eyes were diagnosed with PCV (44 eyes) or neovascular AMD (29 eyes) by dye angiography. The ICC value for diagnosis of PCV and neovascular AMD for the inter-observer agreement between the two independent observers was 0.88, indicating good agreement. In Table 3, we compared control eyes with PCV and neovascular AMD eyes. PCV eyes had significantly higher mean SFCT than neovascular AMD (214.23 ± 95.21 vs. 172.74 ± 96.48, p = 0.03,) and also greater luminal area (0.23 ± 0.09 mm2 vs. 0.19 ± 0.08 mm2 , p = 0.05). There were no significant differences in SFCT and LA when compare between control vs. PCV eyes and control vs. neovascular AMD eyes. The CVI was 68.46 ± 5.92% for normal eyes, 64.94 ± 5.43% for PCV eyes, and 62.54 ± 5.57% for neovascular AMD eyes. The CVI for both PCV and neovascular AMD eyes were significantly lower than control eyes (p < 0.01 and p < 0.01, respectively), and the mean difference of CVI between PCV and neovascular AMD eyes did not reach statistical significance.
Eighteen patients had bilateral diseases and, therefore, we analyzed only the 55 patients for comparison of their uninvolved fellow eyes with disease eyes (Table 4). The CVI of uninvolved fellow eyes of neovascular AMD was significantly higher than neovascular AMD eyes (65.70 ± 14.37% vs. 62.54 ± 5.57%, p = 0.02). Although the uninvolved fellow eyes of PCV had higher mean CVI, its difference compared to PCV eyes did not reach statistical significance (64.94 ± 5.43% vs. 67.22 ± 4.16%, p = 0.09).
Multivariate linear regression analyses for the mean SFCT and CVI for neovascular AMD, PCV, and control eyes are shown in Table 5. Using the control eyes as reference, the mean CVI values of PCV and neovascular AMD were still significantly reduced when compared to controls, after adjusting for SFCT, age, gender, axial length, and BCVA. The adjusted mean difference of CVI in PCV eyes CVI was 4.29% lower than normal, 95% confidence intervals (95%CI 0.92–7.66, p = 0.01). The adjusted mean difference of CVI in AMD eyes was 5.90% lower than normal, (95%CI 1.74–10.06, p <0.01). There were no significant differences comparing the uninvolved fellow eyes of PCV and neovascular AMD with control eyes. The relationships between SFCT and CVI are shown in Fig. 2. The Pearson correlation coefficients were 0.666 for PCV, 0.675 for neovascular AMD, and 0.009 for control eyes. Figure 3 illustrates the binarized OCT scans for the analysis of CVI in representative PCV and neovascular AMD cases.
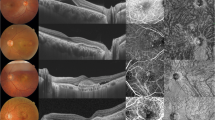
Representative fluorescence angiography (FA) (a & e), confocal scanning laser ophthalmoscopy indocyanine green angiography (ICGA) (b & f), swept source optical coherence tomography (SS OCT) (c & g), and binarized SS OCT images (d & h) of PCV eye (a–d) and AMD eye (e–h). a FA showed staining of retinal pigment epithelial defect and mild leakage from polyps. b ICGA showed a cluster of polyps that is hyperfluorescent. c SS OCT scan showed a pigment epithelial defect with subretinal fluid. Two parallel horizontal green lines segmented the choroid from Bruch’s membrane to chorioscleral interface. The vertical green line at the fovea for calculation of subfoveal choroidal thickness, which was 241.36 μm. d Based on the binarized image (red and yellow segmentation lines, blue line through the fovea), the choroidal vascularity index (CVI) was 65.62. e Early phase FA showed a well demarcated leakage and f ICGA reveal a choroidal neovascular network without any hyperfluorescent spot. This is a type 2 CNV. g A subretinal hyperreflective material was found in the SS OCT. h The subfoveal choroidal thickness was 155.43 μm and CVI was 53.68
Discussion
In our study, PCV eyes and their uninvolved fellow eyes had the highest SFCT measurements when compared to AMD eyes and controls; however, the differences were not statistically significant. A number of previous studies had demonstrated quantifiably greater choroidal thickness in PCV eyes [10, 14, 24]. Nevertheless, a thick choroid in PCV has not been consistently reported in other studies [12, 13, 15, 25]. A prospective study of 163 patients with AMD and PCV did not find significant difference in choroidal thickness [25]. The normative value for SFCT has not been determined in normal population because it was shown to be highly variable [26–28]. Population-based studies reported that SFCT could fluctuate due to physiological factors including age, gender, refraction, and axial length [29, 30]. Indeed, the thickness of the choroid can be influenced by vascular permeability, irrigation of fluid, and ionic molecules through RPE layers, the amount of proteoglycans and nonvascular smooth muscles [16]. In a population based study of 354 healthy eyes, physiological and ocular factors were shown to influence SFCT measurements, whereas CVI had less variability [23]. In our univariate analysis, we also found that age was strongly associated with SFCT measurement, but its association with CVI was not statistically significant.
Histological study of the choroid has also suggested that reduction in size and density of choroidal vessels occur with physiological aging [31]. In vivo imaging of the choroid vasculature in healthy eyes with high penetration OCT also had concordant findings [32]. After adjusting for age, as well as gender and axial length in multivariate linear regression analysis, the CVI of both PCV and neovascular AMD eyes were significantly reduced compared to control. Wei et al. also reported lower CVI measurements in 42 eyes with exudative AMD [33]. CVI is the proportion of blood vessel lumen within a designated area of choroid. A reduction in CVI represents reduction in choroidal vessels diameter, with or without reduction in density. In histology studies, neovascular AMD eyes consisted of smaller vascular channels and loss of choroidal vascular density suggesting the role of choroidal ischemia in its pathogenesis [34–36]. During normal aging, progressive morphologic changes occur in the choroid, Bruch’s membrane, RPE, and photoreceptors, but these processes become pathologic in macular degeneration [31]. Degeneration of the RPE has been hypothesized to limit the diffusion to choriocapillaris because of the accumulation of deposits at the Bruch’s membrane resulting in choriocapillary atrophy [34, 35]. On the other hand, Doppler flowmetry study suggested impaired choroidal perfusion may be responsible for dysfunction of the RPE in neovascular AMD [37].
PCV eyes had larger mean total luminal area of choroidal vessels than normal eyes and neovascular AMD eyes. The difference was significantly larger compared to neovascular AMD eyes, but not significantly larger than normal eyes. A number of qualitative studies have observed abnormal dilatations of outer choroidal vessels in PCV cases using structural en face OCT scans [32, 38–40]. Balaratnasingam et al. proposed using pachychoroid features in the classification of patients with PCV [41]. The term pachychoroid was used to describe features of thick choroid in a number of retinal diseases including CSC, PCV, and retinal pigment epitheliopathy [38, 42–45]. These features include reduced tessellation overlying the area of thick choroid in fundoscopy exam, choroidal vascular hyperpermeability (CVH) in ICGA, increased choroidal thickness in OCT B-scans, and focal or diffusely dilation of outer choroidal vessels sometimes with club-shaped posterior termination (referred as “pachyvessels”) observed using en face OCT scans. The underlying choroidal abnormality suggests venous congestion and stasis leading to choroidal hyperpermeability in the pathogenesis of PCV.
Although many eyes with pachychoroid will have quantifiably greater choroidal thickness measurements, it is possible for an eye to be defined as pachychoroid to have normal choroidal thickness [12–15]. The discordance occurs when the increased luminal volume secondary to choroidal vessels dilation is offset by the reduction in tissue volume from the stroma. Therefore, pachychoroid is not simply a thick choroid, rather it implicates the structural and functional alterations of the choroid leading to increased choroidal vascularity. This reflects the limitation of using choroidal thickness as a marker for choroidal vascularity as both vascular and stromal elements contribute towards thickness [16]. We did not find significant differences in CVI between PCV and neovascular AMD eyes, consistent with Wei et al.’s subgroup analysis [33]. Both mean LA and SA were higher in PCV eyes than AMD eyes, which may lead to similar CVI values. It appears that the choroidal vessels diameter increases with the expansion of extravascular space in PCV eyes.
Previous studies have suggested that changes in the choroid could be both a cause and a result of AMD or PCV development [46, 47]. Dansingani et al. has identified genetic risk alleles associated with neovascularization in the pachychoroid phenotype [46]. Hence, genetic predisposition may explain the changes in the choroid as a cause of AMD/PCV development. Furthermore, SFCT is not stagnant in AMD and PCV eyes. Therapeutic trial has shown that reduction in SFCT might occur during intravitreal anti-VEGF therapy and might increase during the disease recurrence [47]. Longitudinal and detailed choroidal imaging studies in AMD and PCV eyes will further elucidate whether CVI changes as a result of AMD/PCV development.
In our study, the CVI of neovascular AMD eyes was significantly lower than their uninvolved fellow eyes, which had less advanced stages of AMD with less compromised choroidal circulation. For PCV eyes in our study, the CVI was not significantly lower than their uninvolved fellow eyes. Patients with PCV could be predisposed to develop PCV lesions in both eyes as bilateral involvement had been reported with rates between 10 and 50% in retrospective studies [48]. Prospective studies are yet to confirm the incidence of bilateral symptomatic PCV. A thick choroid has been reported in uninvolved fellow eyes of PCV patients, and a number of observational studies in patients with pachychoroid configuration detected similar morphological changes in the choroid bilaterally [10, 32, 38, 42]. OCT enables non-invasive and regular longitudinal examinations of the outer choroid morphological changes in asymptomatic eyes to elucidate whether increased choroidal vascularity is a forme fruste change preceding sight-threatening serosanguinous maculopathy and the involvement of systemic factors in the evolution of PCV. Our study was limited by the lack of longitudinal analysis in the change of CVI associated with various treatments. We were not able to recruit a sufficient number of eyes which had various stages of non-exudative AMD for comparative analysis of choroidal morphological changes. The CVI is derived from two-dimensional scans and may not represent the topography of choroidal vascularity of the entire globe. Enface OCT had demonstrated both diffuse and focal distribution of pathologically dilated outer choroidal vessels [38]. Sampling error can be reduced by analysis of three-dimensional volume scans of the choroid. Validation of an automated algorithm for characterization of choroidal vascularity in the enface view will further elucidate the detail morphological changes in AMD and PCV eyes [49]. OCT scans of the choroid were not performed during 9 am to 5 pm, but not at the same time of the day; however, diurnal variations may not significantly influence our results [50].
In conclusion, we have demonstrated reduction of CVI in both AMD and PCV eyes compared with healthy eyes after adjusting for age, axial length and gender in multivariate analysis. There was marked reduction of choroidal vascularity in neovascular AMD eyes, while increased mean choroidal vessels lumen area was found in PCV eyes. Binarized images of the choroid allow quantitative analysis of the different compartments within the choroid. There are a myriad of overlapping clinical manifestations between neovascular AMD and PCV, particularly among Asians [2]. Hence, studying the in vivo choroidal morphology has important implications for the understanding of histopathology of AMD and PCV, which may lead to significant consequences to treatment outcomes.
References
Lim LS, Cheung CM, Wong TY (2013) Asian age-related macular degeneration: current concepts and gaps in knowledge. Asia Pac J Ophthalmol (Phila) 2(1):32–41. doi:10.1097/APO.0b013e31827ff5bc
Wong CW, Yanagi Y, Lee WK, Ogura Y, Yeo I, Wong TY, Cheung CM (2016) Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res 53:107–139. doi:10.1016/j.preteyeres.2016.04.002
Laude A, Cackett PD, Vithana EN, Yeo IY, Wong D, Koh AH, Wong TY, Aung T (2010) Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res 29(1):19–29. doi:10.1016/j.preteyeres.2009.10.001
Ozkaya A, Alagoz C, Garip R, Alkin Z, Perente I, Yazici AT, Taskapili M (2016) The role of indocyanine green angiography imaging in further differential diagnosis of patients with nAMD who are morphologically poor responders to ranibizumab in a real-life setting. Eye (London). doi:10.1038/eye.2016.71
Ma L, Li Z, Liu K, Rong SS, Brelen ME, Young AL, Kumaramanickavel G, Pang CP, Chen H, Chen LJ (2015) Association of genetic variants with polypoidal choroidal vasculopathy: a systematic review and updated meta-analysis. Ophthalmology 122(9):1854–1865. doi:10.1016/j.ophtha.2015.05.012
Cho M, Barbazetto IA, Freund KB (2009) Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol 148(1):70–78 e71. doi:10.1016/j.ajo.2009.02.012
Cheung CM, Yang E, Lee WK, Lee GK, Mathur R, Cheng J, Wong D, Wong TY, Lai TY (2015) The natural history of polypoidal choroidal vasculopathy: a multi-center series of untreated Asian patients. Graefes Arch Clin Exp Ophthalmol 253(12):2075–2085. doi:10.1007/s00417-015-2933-2
Koizumi H, Yamagishi T, Yamazaki T, Kawasaki R, Kinoshita S (2011) Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 249(8):1123–1128. doi:10.1007/s00417-011-1620-1
Yang LH, Jonas JB, Wei WB (2013) Optical coherence tomographic enhanced depth imaging of polypoidal choroidal vasculopathy. Retina 33(8):1584–1589. doi:10.1097/IAE.0b013e318285cbb3
Chung SE, Kang SW, Lee JH, Kim YT (2011) Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology 118(5):840–845. doi:10.1016/j.ophtha.2010.09.012
Coscas F, Puche N, Coscas G, Srour M, Francais C, Glacet-Bernard A, Querques G, Souied EH (2014) Comparison of macular choroidal thickness in adult onset foveomacular vitelliform dystrophy and age-related macular degeneration. Invest Ophthalmol Vis Sci 55(1):64–69. doi:10.1167/iovs.13-12931
Manjunath V, Goren J, Fujimoto JG, Duker JS (2011) Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmol 152(4):663–668. doi:10.1016/j.ajo.2011.03.008
Wood A, Binns A, Margrain T, Drexler W, Povazay B, Esmaeelpour M, Sheen N (2011) Retinal and choroidal thickness in early age-related macular degeneration. Am J Ophthalmol 152(6):1030–1038 e1032. doi:10.1016/j.ajo.2011.05.021
Kim SW, Oh J, Kwon SS, Yoo J, Huh K (2011) Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina 31(9):1904–1911. doi:10.1097/IAE.0b013e31821801c5
Jonas JB, Forster TM, Steinmetz P, Schlichtenbrede FC, Harder BC (2014) Choroidal thickness in age-related macular degeneration. Retina 34(6):1149–1155. doi:10.1097/IAE.0000000000000035
Nickla DL, Wallman J (2010) The multifunctional choroid. Prog Retin Eye Res 29(2):144–168. doi:10.1016/j.preteyeres.2009.12.002
Tan KA, Laude A, Yip V, Loo E, Wong EP, Agrawal R (2016) Choroidal vascularity index—a novel optical coherence tomography parameter for disease monitoring in diabetes mellitus? Acta Ophthalmol. doi:10.1111/aos.13044
Agrawal R, Salman M, Tan KA, Karampelas M, Sim DA, Keane PA, Pavesio C (2016) Choroidal vascularity index (CVI)—a novel optical coherence tomography parameter for monitoring patients with panuveitis? PLoS ONE 11(1), e0146344. doi:10.1371/journal.pone.0146344
Agrawal R, Chhablani J, Tan KA, Shah S, Sarvaiya C, Banker A (2016) Choroidal vascularity index in central serous chorioretinopathy. Retina. doi:10.1097/IAE.0000000000001040
Japanese Study Group of Polypoidal Choroidal Vasculopathy (2005) Criteria for diagnosis of polypoidal choroidal vasculopathy. Nippon Ganka Gakkai Zasshi 109(7):417–427
Koh AH, Expert PCVP, Chen LJ, Chen SJ, Chen Y, Giridhar A, Iida T, Kim H, Yuk Yau Lai T, Lee WK, Li X, Han Lim T, Ruamviboonsuk P, Sharma T, Tang S, Yuzawa M (2013) Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina 33(4):686–716. doi:10.1097/IAE.0b013e3182852446
Niblack W (1986) An introduction to digital image processing. Prentice-Hall, Englewood Cliffs
Agrawal R, Gupta P, Tan KA, Cheung CM, Wong TY, Cheng CY (2016) Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep 6:21090. doi:10.1038/srep21090
Rishi P, Rishi E, Mathur G, Raval V (2013) Ocular perfusion pressure and choroidal thickness in eyes with polypoidal choroidal vasculopathy, wet-age-related macular degeneration, and normals. Eye (London) 27(9):1038–1043. doi:10.1038/eye.2013.106
Ting DS, Ng WY, Ng SR, Tan SP, Yeo IY, Mathur R, Chan CM, Tan AC, Tan GS, Wong TY, Cheung CM (2016) Choroidal thickness changes in age-related macular degeneration and polypoidal choroidal vasculopathy: a 12-month prospective study. Am J Ophthalmol 164:128–136 e121. doi:10.1016/j.ajo.2015.12.024
Branchini LA, Adhi M, Regatieri CV, Nandakumar N, Liu JJ, Laver N, Fujimoto JG, Duker JS (2013) Analysis of choroidal morphologic features and vasculature in healthy eyes using spectral-domain optical coherence tomography. Ophthalmology 120(9):1901–1908. doi:10.1016/j.ophtha.2013.01.066
Wei WB, Xu L, Jonas JB, Shao L, Du KF, Wang S, Chen CX, Xu J, Wang YX, Zhou JQ, You QS (2013) Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology 120(1):175–180. doi:10.1016/j.ophtha.2012.07.048
Spaide RF (2009) Age-related choroidal atrophy. Am J Ophthalmol 147(5):801–810. doi:10.1016/j.ajo.2008.12.010
Ferrara D, Waheed NK, Duker JS (2016) Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res 52:130–155. doi:10.1016/j.preteyeres.2015.10.002
Barteselli G, Chhablani J, El-Emam S, Wang H, Chuang J, Kozak I, Cheng L, Bartsch DU, Freeman WR (2012) Choroidal volume variations with age, axial length, and sex in healthy subjects: a three-dimensional analysis. Ophthalmology 119(12):2572–2578. doi:10.1016/j.ophtha.2012.06.065
McLeod DS, Lutty GA (1994) High-resolution histologic analysis of the human choroidal vasculature. Invest Ophthalmol Vis Sci 35(11):3799–3811
Adhi M, Ferrara D, Mullins RF, Baumal CR, Mohler KJ, Kraus MF, Liu J, Badaro E, Alasil T, Hornegger J, Fujimoto JG, Duker JS, Waheed NK (2015) Characterization of choroidal layers in normal aging eyes using enface swept-source optical coherence tomography. PLoS ONE 10(7), e0133080. doi:10.1371/journal.pone.0133080
Wei X, Ting DS, Ng WY, Khandelwal N, Agrawal R, Cheung CM (2016) Choroidal vascularity index: a novel optical coherence tomography based parameter in patients with exudative age-related macular degeneration. Retina. doi:10.1097/IAE.0000000000001312
McLeod DS, Taomoto M, Otsuji T, Green WR, Sunness JS, Lutty GA (2002) Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci 43(6):1986–1993
Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J (2011) Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Invest Ophthalmol Vis Sci 52(3):1606–1612. doi:10.1167/iovs.10-6476
Seddon JM, McLeod DS, Bhutto IA, Villalonga MB, Silver RE, Wenick AS, Edwards MM, Lutty GA (2016) Histopathological insights into choroidal vascular loss in clinically documented cases of age-related macular degeneration. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.3519
Metelitsina TI, Grunwald JE, DuPont JC, Ying GS, Brucker AJ, Dunaief JL (2008) Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci 49(1):358–363. doi:10.1167/iovs.07-0526
Dansingani KK, Balaratnasingam C, Naysan J, Freund KB (2016) En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina 36(3):499–516. doi:10.1097/IAE.0000000000000742
Sayanagi K, Gomi F, Akiba M, Sawa M, Hara C, Nishida K (2015) En-face high-penetration optical coherence tomography imaging in polypoidal choroidal vasculopathy. Br J Ophthalmol 99(1):29–35. doi:10.1136/bjophthalmol-2013-304658
Semoun O, Coscas F, Coscas G, Lalloum F, Srour M, Souied EH (2015) En face enhanced depth imaging optical coherence tomography of polypoidal choroidal vasculopathy. Br J Ophthalmol. doi:10.1136/bjophthalmol-2015-307494
Balaratnasingam C, Lee WK, Koizumi H, Dansingani K, Inoue M, Freund KB (2016) Polypoidal choroidal vasculopathy: a distinct disease or manifestation of many? Retina 36(1):1–8. doi:10.1097/IAE.0000000000000774
Ferrara D, Mohler KJ, Waheed N, Adhi M, Liu JJ, Grulkowski I, Kraus MF, Baumal C, Hornegger J, Fujimoto JG, Duker JS (2014) En face enhanced-depth swept-source optical coherence tomography features of chronic central serous chorioretinopathy. Ophthalmology 121(3):719–726. doi:10.1016/j.ophtha.2013.10.014
Warrow DJ, Hoang QV, Freund KB (2013) Pachychoroid pigment epitheliopathy. Retina 33(8):1659–1672. doi:10.1097/IAE.0b013e3182953df4
Pang CE, Freund KB (2015) Pachychoroid neovasculopathy. Retina 35(1):1–9. doi:10.1097/IAE.0000000000000331
Gallego-Pinazo R, Dolz-Marco R, Gomez-Ulla F, Mrejen S, Freund KB (2014) Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol 3(4):111–115
Dansingani KK, Perlee LT, Hamon S, Lee M, Shah VP, Spaide RF, Sorenson J, Klancnik JM Jr, Yannuzzi LA, Barbazetto IA, Cooney MJ, Engelbert M, Chen C, Hewitt AW, Freund KB (2016) Risk alleles associated with neovascularization in a pachychoroid phenotype. Ophthalmology. doi:10.1016/j.ophtha.2016.06.060
Koizumi H, Kano M, Yamamoto A, Saito M, Maruko I, Sekiryu T, Okada AA, Iida T (2016) Subfoveal choroidal thickness during aflibercept therapy for neovascular age-related macular degeneration: twelve-month results. Ophthalmology 123(3):617–624. doi:10.1016/j.ophtha.2015.10.039
Kim YT, Kang SW, Chung SE, Kong MG, Kim JH (2012) Development of polypoidal choroidal vasculopathy in unaffected fellow eyes. Br J Ophthalmol 96(9):1217–1221. doi:10.1136/bjophthalmol-2012-301644
Duan L, Hong YJ, Yasuno Y (2013) Automated segmentation and characterization of choroidal vessels in high-penetration optical coherence tomography. Opt Express 21(13):15787–15808. doi:10.1364/OE.21.015787
Seidel G, Hausberger S, Herzog SA, Palkovits S, Poschl EM, Wackernagel W, Weger M (2015) Circadian macular volume changes in the healthy human choroid. Am J Ophthalmol 159(2):365–371 e362. doi:10.1016/j.ajo.2014.11.002
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Meeting presentation
None.
Rights and permissions
About this article
Cite this article
Bakthavatsalam, M., Ng, D.SC., Lai, F.HP. et al. Choroidal structures in polypoidal choroidal vasculopathy, neovascular age-related maculopathy, and healthy eyes determined by binarization of swept source optical coherence tomographic images. Graefes Arch Clin Exp Ophthalmol 255, 935–943 (2017). https://doi.org/10.1007/s00417-017-3591-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3591-3