Abstract
Background
To study morphological features of optic disc venous collaterals (OVCs) and neovascularization of optic disc (NVD) on optical coherence tomography angiography (OCTA).
Methods
Patients with OVCs and NVDs secondary to ischemic retinal diseases were prospectively enrolled. Multimodal imaging was performed using color fundus photography, fluorescein angiography (FA), and OCTA. Morphological evaluation of en-face structural OCT, cross-sectional and en-face OCTA was performed.
Results
Twenty eyes (20 patients; OVCs: n = 10 and NVD: n = 10) were included. OVCs appeared as small, loopy vessels distinct from surrounding peripapillary capillaries on OCTA in the radial peripapillary capillary frame. NVDs appeared as a mesh of fine caliber, raised vessels best seen in the vitreous slab of OCTA. Flow signals in these vascular alterations correlated well with hyperfluorescence on FA.
Conclusions
OCTA provides improved visualization of NVDs and OVCs in ischemic retinal diseases such as diabetic retinopathy and retinal vein occlusions compared to conventional FA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients with ischemic retinal diseases may develop a number of pathological vascular alterations at the optic disc such as new vessels, and venous collaterals (also termed as optociliary shunt vessels), among others. Optic nerve head collaterals may originate from the retinal capillary bed, and develop within the framework of the existing retinal vascular network. Blood flow through these channels may not adequately compensate for severe retinal ischemia [1]. On the other hand, retinal neovascularization is characterized by a rete of new vessels arising from venous circulation by penetrating the internal limiting membrane [2]. Differentiation between venous collaterals and neovascularization is clinically relevant because presence of collaterals may be protective from poorer visual outcomes, whereas neovascularization requires prompt laser photocoagulation to prevent sight-threatening complications [3–6]. Based on differences in the structural morphology and characteristics of vascular flow, these entities may be distinguished from each other by indirect ophthalmoscopy and/or fluorescein angiography (FA).
Optical coherence tomography angiography (OCTA) is a recently introduced non-invasive imaging modality that enables detailed analysis of retinochoroidal and optic nerve head vasculature without the need for dye injection [7]. The use of this technology has provided valuable insights into the disease pathophysiology and natural history for several vitreoretinal conditions such as age-related macular degeneration [8] and diabetic retinopathy [9], among others. The index study was performed with the aim of characterizing the morphology of optic nerve head venous collaterals and neovascularization using OCTA.
Materials and methods
The index study was performed prospectively at the Retina Services of the Post Graduate Institute of Medical Education and Research (PGIMER) between April 2015 and September 2015. The study was performed in adherence with the tenets of the Declaration of Helsinki, and was approved by the PGIMER Institute Ethics Committee (IEC). Written informed consent was obtained from all the study subjects.
Consecutive patients diagnosed with retinal vascular diseases such as diabetic retinopathy, central and branch retinal vein occlusion were evaluated for presence of optic disc venous collaterals (OVC) and optic disc neovascularization (NVD). OVC were identified clinically (on indirect ophthalmoscopy with 20D lens) as a patch of small, loopy, and thick vessels in an area of venous occlusion at the level of the retina, as defined in the Standard of Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study [10]. On FA, OVCs were defined as abnormal meandering vessels that do not demonstrate significant leakage [5]. Unlike OVCs, NVDs appear as a fine lacy network of vessels that are anterior to the retinal surface, and extend beyond the optic nerve head. On FA, NVDs show significant dye leakage and late hyperfluorescence. Two independent observers (AS and SK; ophthalmologists with subspecialty training in vitreoretina) evaluated the fundus photographs and FA images to identify OVCs and NVD. When both graders agreed with each other in identifying OVCs and NVD, the patient was selected for further imaging with OCTA.
In addition, all the patients included in the study underwent comprehensive ophthalmological examination, including measurement of the best-corrected visual acuity (BCVA) by Snellen’s chart, slit-lamp biomicroscopy, color fundus photography and FA (Carl Zeiss FF450, Zeiss Meditec, Germany), and OCTA (Optovue RTVue XR Avanti, Optovue Inc., Fremont, CA, USA). Patients with poor media clarity due to significant cataract or vitreous hemorrhage were excluded from study. In addition, patients with previous history of pan-retinal photocoagulation, intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections, surgery (pars plana vitrectomy), and vitreoretinal abnormalities such as tractional retinal detachment, epiretinal membranes, or taut hyaloid membrane were excluded from the study. Patients with OVCs due to tumors such as optic nerve head meningioma were excluded from the study.
The Optovue RTVue XR Avanti device utilizes the split-spectrum amplitude decorrelation angiography (SSADA) algorithm. OCTA imaging of the optic nerve head was performed using 3 × 3 mm and 4.5 × 4.5 mm scan protocols. Automated layer segmentation was performed by the inbuilt software in the machine consisting of various layers such as vitreous (to obtain images of superficial vascular layers) and radial peripapillary capillaries (RPC). Manual segmentation was performed to avoid misinterpretation of projection artefacts and to ensure accurate assessment of the capillary plexuses.
Two independent observers (AA and VG) analyzed the en-face and cross-sectional OCTA, and compared the findings with fundus photographs and FA. Discrepancies between the observers were resolved by open adjudication. Various features analyzed on OCTA imaging included the morphology, size, shape, and site of abnormal vessels. Automatic segmentation was manually altered to analyze all the images. Flow signal in the vascular networks was assessed to evaluate the blood flow pattern in these abnormal channels. The imaging features on OCTA were also compared to the findings on FA.
GraphPad Prism® (GraphPad Software Inc., La Jolla, CA, USA) version 6.0 was used for statistical analysis. Non-parametric tests were used for analyzing quantitative data. Qualitative descriptions of the imaging characteristics were performed. Descriptive analysis was used for characterizing OCTA features of OVCs and NVD.
Results
Ten eyes (ten subjects, mean age: 56.0 ± 6.60 years) with OVCs and ten eyes (ten subjects, mean age: 60.6 ± 5.89 years) (p = 0.12) with NVD were included in the study. The baseline demographic and clinical details of the patients is listed in Table 1.
Optical coherence tomography angiography features of OVCs
The en-face OCTA and cross-sectional OCTA images were analyzed to study the appearance of OVCs. On en-face OCTA, OVCs were visible as thin, loopy vessels in the RPC layer in all the eyes (10/10 eyes; 100%) (Fig. 1). The RPC system was well-preserved in eight out of ten eyes, while there was attenuation in the remaining two eyes. The OVCs appeared larger than RPCs but smaller than the retinal veins. These vessels could be easily differentiated from normal RPCs because the RPCs demonstrate a characteristic radial course whereas the OVCs appeared to run in loops. The OVCs were not visible anterior to the surface of the optic disc in the vitreous slab of OCTA by automatic segmentation (Fig. 2).
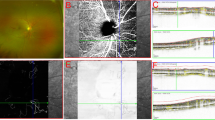
Top panel (a–d): patient #1. a Optic disc photograph of the right eye of a patient with central retinal vein occlusion shows disc collaterals (arrowheads). b Fundus fluorescein angiogram (FA) right eye during early frames shows collaterals as meandering vessels c Late-phase FFA shows no leakage from the collaterals. d Optical coherence tomography angiography (OCTA) (radial peripapillary capillary frame) of shows collaterals (arrowheads). Blue arrowheads demonstrate additional information provided by OCTA in comparison with FA. Bottom panel (e–h): patient #2. e Fundus photograph optic disc right eye shows optic disc collaterals (arrowheads). f Fluorescein angiogram (FA) right eye in the early phase shows presence of optic disc collaterals that do not leak in the late phase (g). Blue arrowheads demonstrate additional areas of vascular pathology seen on FA in comparison with fundus photography. h Optical coherence tomography angiography (OCTA) (radial peripapillary capillary frame) shows collaterals separately visible from peripapillary vessels (arrowheads). Blue arrowheads show additional details compared to FA
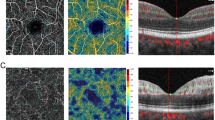
a and b En-face view at vitreo-retinal interface on Angioflow® and structural optical coherence tomography (OCT) respectively, showing hyper-reflectivity corresponding to the main trunk of vessel shown with arrow. c Cross-sectional optical coherence tomography angiography (OCTA) showing plane of acquisition as the vitreo-retinal interface (arrow) d Radial peripapillary frame of OCTA shows meandering vessel shown by blue arrows not visible on the en-face view
In comparison with fundus photography, anatomical delineation of the collaterals was better in all eyes on OCTA. While FA was able to identify the OVCs in all eyes, the exact extent and area of the OVCs was better appreciated on OCTA in six eyes. The findings in the remaining four eyes were comparable between FA and OCTA. Cross-sectional OCTA did not demonstrate any abnormal flow signal through the OVCs.
Optical coherence tomography angiography features of NVD
Ten eyes with NVD were evaluated using fundus photography, FA and OCTA (Fig. 3). On en-face OCTA, NVDs appeared as a mesh of fine caliber vessels over and around the disc with a tendency to rise above the retinal surface. These vessels were best visualized in the ‘vitreous’ frame of the OCTA in contrast to OVCs, which were best seen in the RPC frames (Fig. 4). The vitreous frames of OCTA showed minimal peripapillary capillaries (unlike the RPC frame) and NVDs were prominently seen rising above the retinal surface. Using the 4.5 × 4.5 mm en-face OCTA, neovascular tissue arising from the optic nerve head was also clearly delineated. This fibrovascular tissue appeared hyper-reflective on corresponding OCT B-scan, and showed abnormal flow signals on cross-sectional OCTA, in contrast to OVCs.
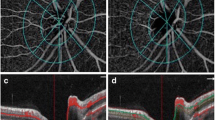
Top panel (a–d): patient #1. a Optic disc photograph of the right eye of a patient with diabetic retinopathy shows neovascularization of optic disc (arrowheads). b Fluorescein angiogram (FA) of the right eye during early phase shows neovascularization of the optic disc as rete of vessels with early hyperfluorescence. c Late phase of the FA shows diffuse leakage and hyperfluorescence from the new leaky vessels. d Optical coherence tomography angiography (OCTA) (vitreous frames) of shows neovascularization of the optic disc (arrowheads). Blue arrowheads show additional details seen on OCTA in comparison with FA. Bottom panel (e–h): patient #2. e Optic disc photographs of the right eye shows neovascularization of optic disc (arrowheads). f Fluorescein angiogram (FA) of the right eye in the early phase after injection shows early hyperfluorescence due to dye leakage. g The late-phase FA shows intense leakage from these vessels. h Optical coherence tomography angiography (OCTA) (vitreous frames) shows detailed architecture of the neovascularization of the optic disc (arrowheads). Blue arrowheads show additional details in comparison with FA
The findings on OCTA were compared with fundus photography and FA. The anatomical delineation of NVD on OCTA was superior in eight out of ten eyes and equivocal in one, while in one case FA showed better delineation of the new vessels. Hyperfluorescence due to dye leakage from fragile new vessels limited the view of detailed neovascular architecture on FA.
Discussion
The index study was performed with the objective of characterizing the morphology of OVCs and NVDs using OCTA. Assessment of morphological features using the non-invasive technique of OCTA has provided valuable insights into the disease pathophysiology, natural history, and response to therapy in various vitreoretinal conditions such as choroidal neovascularization [8, 11], glaucoma [12], retinal angiomatous proliferation [13], branch retinal vein occlusion [14], and ischemic central retinal vein occlusion [15]. Both NVDs and OVCs arise due to local retinal ischemia but represent different pathophysiological processes occurring in the retina. It has been suggested that OVCs develop as a protective mechanism for the perfusion of ischemic retina, whereas NVDs arise as a result of pathological imbalance, resulting in higher intraocular vascular endothelial growth factor (VEGF) levels [5, 10]. Thus, it is relevant to identify the differences between the two, since the management of NVDs includes prompt laser photocoagulation of the ischemic retina or intravitreal injections of anti-VEGF agents. The results of our study demonstrate important differences in the structural anatomy of these aberrant vessels using OCTA.
The incidence of collateral vessels may be up to 50% among patients with retinal vein occlusions [16]. OVCs are also found, though less commonly, in other pathologies such as high myopia [4], diabetic retinopathy [17, 18], long-standing glaucomatous optic neuropathy [4, 19], and occasionally in sickle-cell retinopathy [20]. Moreover, neovascular proliferation including NVDs may occur across a similar disease spectrum, highlighting the importance of differentiating the two entities. OVCs are characterized by small, sinusoidal vessels that may assume a straight course over a period of time. Histopathological studies hypothesize that tissue compensatory mechanisms may result in development of OVCs of similar caliber and cellular characteristics of the vessel whose function it replaces [1]. OVCs may also disappear if the obstructed vessel shows reperfusion. On the other hand, NVDs are characterized by a mesh of new vessels that arise from venous circulation, and tend to penetrate the internal limiting membrane [21]. These new vessels, driven by hypoxic stimulus, expand around a central peduncle and develop into ‘fronds’ of mature vessels resembling the shape of a cartwheel or an umbrella [22].
Retrospective analyses of patients enrolled in the SCORE trial assessed the development of venous collaterals among patients with central, branch, and hemiretinal vein occlusion [10]. In this study, the results showed that OVCs may not influence the visual outcome, and treatment with corticosteroids has no role in the development or regression of OVCs. Thus, development of OVCs, unlike NVDs, may be primarily driven by mechanical differences in the flow gradient which may be enhanced with larger areas of retinal non-perfusion [10]. If the development of vascular pathologies such as OVCs and NVDs are identified early, it may be possible to predict the biological behavior, course, and prognosis of the retinal disease. OCTA may permit non-invasive imaging of the optic nerve head improving our sensitivity in detecting development of OVCs compared to conventional dye-based angiography. In addition, careful examination of the optic nerve vessels may aid in detection of early venous changes suggestive of NVDs.
In the index study, features of OVCs and NVDs were clearly identified on OCTA. OVCs were identified as meandering, small vessels at the RPC plane of the OCTA. NVDs were identified as abnormal large vessels with a tendency to rise above the plane of retina, and were best observed in vitreous plane of OCTA. The vitreous frames have an offset set to ‘zero’, and are used to visualize vascular structures above the retinal surface. NVDs were found to have arborizing pattern and appeared as interwoven thin vascular complex above and around the disc. Unlike FA, the vascular growth pattern of NVDs and OVCs could be easily studied using OCTA. Such an ability may help in development of hypotheses such as the fractal analysis of the retinal vasculature [23]. Similarly, detailed evaluation of the flow signals on OCTA may predict the growth of neovascular fronds in comparison to OVCs. Such findings are not only relevant in studying the disease process, but also aid in other fields of medicine such as embryology or cardiology [24].
Similar to choroidal neovascularization, the diagnosis of NVDs and OVCs is performed using clinical examination aided with fluorescein angiography in cases of disagreement. However, the technology of OCTA provides additional relevant information that helps in the evaluation of these abnormal vascular networks and their implications on functional outcomes. The results of the index study may be useful in the future to assess the longitudinal course of these abnormal vessels and provides further opportunities in assessing the physical properties of blood flow through abnormal venous channels.
Potential weaknesses of the study include a small sample size. No formal sample size calculations were performed for this study. In addition, there was a difference in the etiologies of OVCs and NVDs, since the former are more common among patients with retinal vein occlusion. Thus, the differences in the groups may also be due to differences in the underlying disease. However, it may be argued that pathological changes occurring in the retinal vasculature may be independent of the etiology leading to retinal ischemia.
In conclusion, the index pilot study shows that OCTA can clearly demarcate microvascular architecture of OVCs and NVDs. Compared to FA, OCTA provides additional information and high quality in-vivo non-invasive imaging of the optic nerve head. OCTA may provide clinically meaningful information in the evaluation of abnormal retinal vascular pathologies that may help to predict the course and prognosis of patients with retinal ischemic diseases.
References
Henkind P, Wise GN (1974) Retinal neovascularization, collaterals, and vascular shunts. Br J Ophthalmol 58(4):413–422
Davis MD (1968) The natural course of diabetic retinopathy. Transactions — American Academy of Ophthalmology and Otolaryngology. Am Acad Ophthalmol Otolaryngol 72(2):237–240
Blinder KJ, Khan JA, Giangiacomo J, Ide CH (1989) Optociliary veins and visual prognosis after central retinal vein occlusion. Ann Ophthalmol 21(5):192–194, 197
Masuyama Y, Kodama Y, Matsuura Y, Sawada A, Harada K, Tsuchiya T (1990) Clinical studies on the occurrence and the pathogenesis of optociliary veins. J Clin Neuroophthalmol 10(1):1–8
Fuller JJ, Mason JO 3rd, White MF Jr, McGwin G Jr, Emond TL, Feist RM (2003) Retinochoroidal collateral veins protect against anterior segment neovascularization after central retinal vein occlusion. Arch Ophthalmol 121(3):332–336
Priluck IA, Robertson DM, Hollenhorst RW (1980) Long-term follow-up of occlusion of the central retinal vein in young adults. Am J Ophthalmol 90(2):190–202
Agrawal R, Xin W, Keane PA, Chhablani J, Agarwal A (2016) Optical coherence tomography angiography: a non-invasive tool to image end-arterial system. Expert Rev Med Devices 13(6):519–521
Jia Y, Bailey ST, Wilson DJ et al (2014) Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 121(7):1435–1444
Bandello F, Corbelli E, Carnevali A, Pierro L, Querques G (2016) Optical coherence tomography angiography of diabetic retinopathy. Dev Ophthalmol 56:107–112
Weinberg DV, Wahle AE, Ip MS, Scott IU, VanVeldhuisen PC, Blodi BA (2013) Score Study Report 12: development of venous collaterals in the Score Study. Retina 33(2):287–295
Kuehlewein L, Bansal M, Lenis TL et al (2015) Optical coherence tomography angiography of Type 1 neovascularization in age-related macular degeneration. Am J Ophthalmol 160(4):739–748, e732
Jia Y, Wei E, Wang X et al (2014) Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 121(7):1322–1332
Dansingani KK, Naysan J, Freund KB (2015) En face OCT angiography demonstrates flow in early type 3 neovascularization (retinal angiomatous proliferation). Eye 29(5):703–706
Kuehlewein L, An L, Durbin MK, Sadda SR (2015) Imaging areas of retinal nonperfusion in ischemic branch retinal vein occlusion with swept-source OCT microangiography. Ophthalmic Surg Lasers Imaging Retin 46(2):249–252
Casalino G, Williams M, McAvoy C, Bandello F, Chakravarthy U (2016) Optical coherence tomography angiography in paracentral acute middle maculopathy secondary to central retinal vein occlusion. Eye 30(6):888–893
Quinlan PM, Elman MJ, Bhatt AK, Mardesich P, Enger C (1990) The natural course of central retinal vein occlusion. Am J Ophthalmol 110(2):118–123
Karagiannis DA, Sampat V, Gregor Z (2006) Vascular shunt of the optic disc resembling neovascularization in a diabetic patient with optic disc drusen. Eur J Ophthalmol 16(5):764–766
Lee JJ, Yap EY (2004) Optociliary shunt vessels in diabetes mellitus. Singap Med J 45(4):166–169
West RH, Cebon L, Grant G, Gillies WE (1984) Solitary silent venous papillary loops and ocular hypertension. Aust J Ophthalmol 12(4):351–357
Dowhan TP, Bodnar ME, Daniels MB (1990) Optociliary shunts and sickle retinopathy in a woman with sickle cell trait. Ann Ophthalmol 22(2):66–69
Muqit MM, Stanga PE (2014) Fourier-domain optical coherence tomography evaluation of retinal and optic nerve head neovascularisation in proliferative diabetic retinopathy. Br J Ophthalmol 98(1):65–72
Dobree JH (1964) Proliferative diabetic retinopathy: evolution of the retinal lesions. Br J Ophthalmol 48:637–649
Mainster MA (1990) The fractal properties of retinal vessels: embryological and clinical implications. Eye 4(Pt 1):235–241
Liew G, Wang JJ, Mitchell P, Wong TY (2008) Retinal vascular imaging: a new tool in microvascular disease research. Circ Cardiovasc Imaging 1(2):156–161
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Ankur Singh and Aniruddha Agarwal share the joint authorship.
Ankur Singh and Aniruddha Agarwal contributed equally to this work.
Rights and permissions
About this article
Cite this article
Singh, A., Agarwal, A., Mahajan, S. et al. Morphological differences between optic disc collaterals and neovascularization on optical coherence tomography angiography. Graefes Arch Clin Exp Ophthalmol 255, 753–759 (2017). https://doi.org/10.1007/s00417-016-3565-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3565-x