Abstract
Purpose
To investigate the relationship between optic nerve head (ONH) blood flow and color tone.
Methods
Retrospective observational study conducted between February 2014 and August 2014. We examined 29 eyes of 17 young healthy subjects and 37 eyes of 26 cataract patients undergoing cataract surgery. Blood flow was measured using laser speckle flowgraphy, and color tone was quantified using the public domain ImageJ software. Blood flow and color tone of the ONH before and after cataract surgery were compared. The influence of age, axial length, and color tone on ONH blood flow were also investigated.
Results
Mean blur rate (MBR) in the ONH decreased with increasing age (R = –0.437, P < 0.001) and axial length (R = –0.306, P = 0.012). In young subjects, ONH redness had a moderate positive correlation with MBR (R = 0.376, P = 0.044); however, this correlation was not observed in the study population as a whole (R = 0.066, P = 0.601). MBR in the ONH was higher after cataract surgery (P < 0.001). Moreover, the ONH redness reduced postoperatively from that preoperatively (P < 0.001). An increase in MBR after cataract surgery correlated with improved visual acuity (R = –0.399, P = 0.014) and decreased redness the of ONH (R = –0.433, P < 0.01).
Conclusions
Ocular blood flow decreased in older people and in myopic eyes. The reddish appearance of the ONH was not an indicator of a circulatory condition, particularly in older people. Lens opacity appeared to underestimate hemodynamic quantification using laser speckle flowgraphy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Observing the optic nerve head (ONH) is an essential part of ophthalmology practice. If the ratio of optic cup vertical diameter to optic disc vertical diameter is more than 0.7 or a localized loss of neuroretinal rim is observed, the possibility of glaucomatous optic neuropathy is likely higher [1]. Ophthalmologists examine not only the morphology, but also the color of the ONH, and they often subjectively judge whether ONH status is normal by looking at the red color of the disc in a clinical setting. In clinical practice, ONH color is a sign of optic nerve atrophy, loss of axons due to various etiologies [2]. When the ONH has a strong red color and is swollen, this may be indicative of optic nerve inflammation [3]. ONH color is thought to be an important clinical sign and provides clues for understanding a patient’s ocular condition. Several previous trials reported methods of quantifying ocular fundus color [4–6], however, there is no established method for assessing ONH color tone in clinical practice.
Ocular circulation disturbances can negatively affect vision. Ophthalmologists often assess circulatory condition by observing the redness of the ONH, retinal vessels and other sighs of ocular ischemia. Abnormalities in blood flow have been suggested to play important roles in the pathogenesis of several eye diseases, including glaucoma, diabetic retinopathy, and age-related macular degeneration [7–12]. Although it is important to evaluate ocular blood flow, it is not easy to quantitatively examine ocular microcirculation. Fluorescein angiography (FA) and indocyanine green angiography (IA) are the most prevalent techniques and the gold standards for determining ocular microcirculation. However, it is difficult to evaluate blood flow quantitatively using FA and IA and, furthermore, they are invasive and qualitative methods with the potential side effects of angiographic agents. Therefore, other tools that do not rely on angiographic agents for measuring ocular blood flow quantitatively have been developed, including laser Doppler velocimetry [13], scanning laser Doppler flowmetry [14] and retinal functional imaging [15].
Recently, laser speckle flowgraphy (LSFG) has been used to investigate ocular blood flow [16–21]. The advantage of LSFG includes its noninvasive and quantitative estimation of blood flow of the ONH and retinal vessels in living eyes. LSFG was reported to have high reproducibility for measuring ONH microcirculation and it is an effective and objective instrument for monitoring ocular microcirculation [22].
The association between ONH color and microcirculation in healthy subjects is still not well understood; however, pallor of the optic atrophy was reported to result from axonal loss and gliosis rather than from decreased microcirculation [23]. The aim of this study was to evaluate whether ONH color tone can be a measure of ONH microcirculation. The influence of age, axial length, and the presence of cataracts on assessment of ONH hue and blood flow were also investigated.
Methods
The Ethics Committee at Kyoto University Graduate School of Medicine approved this study, which was conducted in accordance with the tenets of the Declaration of Helsinki.
Subjects
We retrospectively studied the medical records of 29 eyes of 17 young healthy subjects from our database of normal volunteers, and 37 eyes of 26 consecutive cataract patients who visited Kyoto University Hospital between February 2014 and August 2014 as older subjects. Inclusion criteria of enrolled patients included: (1) no ocular disease, e.g., glaucoma, retinal vein occlusion, intraocular inflammation, or diabetic retinopathy; and (2) no history of ocular laser therapy or surgery. Exclusion criteria included eyes with poor LSFG images due to media opacity or poor fixation. Patients underwent a comprehensive ocular examination, including autorefractometry (ARK1; Nidek, Gamagori, Japan), best-corrected visual acuity measurement with a Landolt C chart, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement, fundus photography (TRC-NW8F, Topcon Corp., Tokyo, Japan), and axial length measurement using ocular biometry (IOLMaster; Carl Zeiss Meditec, Jena, Germany). Healthy volunteer subjects underwent slit-lamp biomicroscopy, fundus photography, and axial length assessment using ocular biometry. All cataract patients were treated with small incision cataract surgery and intraocular lens implantation under topical anesthesia. We graded the degree of cataract progression by evaluating nuclear sclerosis using Emery–Little classification [24]. There were no intraoperative or postoperative complications. LSFG, fundus photography, and IOP measurements were performed before and 2 weeks after cataract surgery.
Evaluation of microcirculation at the optic nerve head
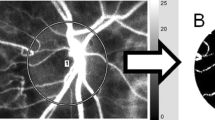
Microcirculation at the ONH was evaluated using LSFG-NAVI (Softcare, Ltd, Fukuoka, Japan). The detailed mechanism of LSFG has been described previously [16]. LSFG-NAVI consists of a fundus camera, a diode laser (wavelength, 830 nm), an image sensor, an infrared charged-coupled device (CCD) camera, and a high-resolution digital CCD camera [750 (width) × 360 (height) pixels] [25]. LSFG-NAVI assesses ocular circulation using the laser speckle phenomenon. The laser speckle phenomenon is an interference event observed when coherent light sources are scattered by a rough surface. The speckle pattern is obtained in the image plane when light is reflected by tissue. The speckle pattern varies depending on velocity when light reflects from moving objects, and produces blurring within the speckle pattern. The pupils of subjects were dilated with 0.5 % tropicamide and 0.5 % phenylephrine hydrochloride. The subject’s face was set on the head holder. Eye position was achieved by using an internal fixation to obtain the site of interest within the fundus image. The examiner performed the measurements three times. Each measurement was acquired in 4 seconds. LSFG-NAVI scans 30 flames per second. Mean blur rate (MBR) was quantified by calculating and averaging the blur rates of the speckle pattern, and a composite color map was produced by showing MBR as pseudo colors (Fig. 1a). We selected subjectively the best image of three composite color maps for assessment. MBR is an arbitrary unit calculated from variations in the speckle pattern blurring, and is used as a parameter reflecting the amount of tissue blood flow. Previous studies reported that MBR was highly correlated with blood flow obtained by the hydrogen gas clearance method in rabbit ONH (R = 0.73) and by the microsphere method in nonhuman primates (R2 = 0.87) [26, 27]. MBR was calculated at each pixel using LSFG analyzer software and displayed as a composite color map showing the two-dimensional variation in the MBR level over the site of interest. The margin of the ONH was demarcated manually in an oval field and MBR in the ONH was calculated (Fig. 1a).
Representative result of quantitative analysis of digital color and blood flow in the optic nerve head. Right eye of a 25-year-old man. (a) The composite color map measured by laser speckle flowgraphy. Mean blur rate = 24.9. (b) The optic nerve head was selected (yellow line) using ImageJ. (c–e) Each histogram shows the mean red, green, and blue intensity
Optic nerve head redness quantification
Quantitative analysis of digital color fundus photographs was evaluated using the public domain software ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) and its plugin software “Measure RGB” (Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/plugins/rgb-measure.html) [28]. Digital color fundus photographs consist of red, green, and blue components (the RGB images). We used ImageJ to demarcate the ONH and separately measured the intensity of red, green, and blue pixels of which the RGB images are composed within the selected area (Fig. 1b). To quantify ONH color, we used calculation algorithms described in detail in a previous report [6]. In the current study, we defined a “disc color index” (DCI) for quantifying ONH redness as follows:
where R, G, and B are the mean intensities of red, green, and blue, respectively (Fig. 1b, c, d, e).
Statistical analysis
All values are presented as means ± standard deviation. Visual acuity measured with a Landolt chart was converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analyses. Multiple regression analysis by forward stepwise selection was performed to determine factors associated with MBR and DCI. A paired t test was used to compare between preoperative and postoperative parameters. Pearson product-moment correlation test was used to evaluate the relationship between MBR change rate and DCI change rate, visual acuity change or IOP change rate. Statistical significance was defined as a P value < 0.05 for two-tailed testing using SPSS statistical software (version 17.0, SPSS, Inc., Chicago, Illinois, USA).
Results
Differences in MBR and color tone between healthy young subjects and older subjects with cataracts
In total, 66 eyes of 43 patients were successfully examined by LSFG. Of the 66 eyes, 29 were eyes of young healthy subjects and 37 were older eyes with cataracts. Table 1 shows the demographics of the study population. MBR in older subjects was significantly lower than in young patients (17.3 ± 3.7 vs. 25.9 ± 4.9, P < 0.001), and DCI was significantly higher in older subjects than in young patients (0.59 ± 0.03 vs. 0.52 ± 0.04, P < 0.001).
Association between color tone and MBR in the optic nerve head
In young subjects, correlation analysis showed that higher DCI (P = 0.044, R = 0.376) was significantly associated with higher MBR (Table 2), while significant correlations were not found with age or axial length. Stepwise multiple regression analysis demonstrated that younger age and higher DCI were associated with higher MBR (P = 0.015, 0.003, respectively), but axial length was not correlated with MBR (P = 0.579; Table 2). Meanwhile, in older subjects with cataracts, no significant correlations were found between MBR and age, axial length, or DCI (Table 2).
Effect of cataract surgery on color tone and MBR in the optic nerve head
Of the 37 eyes that underwent cataract surgery, 33 were classified as Emery–Little classification II and 4 were class III. Of the 37 implanted intraocular lenses, 30 were AvanseePreset PN6A (Kowa, Japan), 5 were AcrySof IQ SN60WF (Alcon, USA) and 2 were AcrySof ReSTOR SN6AD1 (Alcon, USA). Figure 2 shows representative fundus color photographs and LSFG composite color maps before and after cataract surgery. Table 3 shows comparisons between before and after cataract surgery. Postoperative MBR was significantly higher than preoperative MBR, and postoperative DCI and IOP were lower than preoperative values. Moreover, MBR change rate had a negative correlation with DCI change rate (P < 0.01, R = –0.433; Fig. 3a) and visual acuity change (P = 0.014, R = –0.399; Fig. 3b). However, there was no significant correlation between MBR change rate and IOP change rate (P = 0.071; Fig. 3c). Stepwise multiple regression analysis demonstrated that MBR change rate had a negative correlation with DCI change rate (P = 0.037), but visual acuity change and IOP change rate were not significantly correlated with MBR change rate (P = 0.262, 0.071, respectively). Moreover, postoperative MBR was still lower in older subjects than in young subjects, while significant differences were not found between postoperative DCI and DCI in young subjects (Table 3).
Representative results of patients who underwent cataract surgery. (a–d) Left eye of a 70-year-old woman. Visual acuity was 20/32 and 20/16 before and after cataract surgery, respectively. (e–h) Right eye of an 85-year-old woman. Visual acuity was 20/50 and 20/20 before and after cataract surgery, respectively. (i–l) Right eye of a 64-year-old woman. Visual acuity was 20/20 and 20/16 before and after cataract surgery, respectively (a, e, i). Preoperative color fundus photographs. (b, f, j) Preoperative composite color-coded maps measured by laser speckle flowgraphy. (c, g, k) Postoperative color fundus photographs. (d, h, l) Postoperative composite color-coded maps measured by laser speckle flowgraphy. Postoperative DCI was lower than preoperative DCI. Postoperative MBR was higher than preoperative value. DCI = disc color index; MBR = mean blur rate; VA = visual acuity shown as Snellen equivalent in feet
Scatter diagrams of MBR change and DCI change, visual acuity change and IOP change. (a) There was a significantly negative correlation between MBR change and DCI change (R = –0.433, P < 0.01). (b) There was a significantly negative correlation between MBR change and visual acuity change (R = –0.399, P = 0.014). (c) There was no significant correlation between MBR change and IOP change (P = 0.071). DCI = disc color index; IOP = intraocular pressure; MBR = mean blur rate
Factors associated with MBR and DCI after cataract surgery
We investigated factors associated with MBR and DCI after surgery in order to exclude the possible effects of cataract on these parameters. In older subjects, correlation analysis showed that MBR negatively correlated with axial length (P < 0.001, R = –0.592). However, contrary to expectations, no significant correlation was found between MBR and DCI (P = 0.518). Next, the analyses were conducted in all subjects, including young subjects and older patients after cataract surgery. These results revealed that older age (P < 0.001, R = –0.437) and longer axial length (P = 0.012, R = –0.306) were significantly associated with lower MBR, while no significant correlation was found between MBR and DCI (P = 0.601). Stepwise multiple regression analysis demonstrated that older age and longer axial length were associated with lower MBR (P < 0.001, < 0.001, respectively), but DCI was not associated with MBR (P = 0.152; Table 4). On the other hand, we evaluated the factors associated with DCI (Table 5). Stepwise multiple regression analysis showed that older age was only associated with higher DCI (P = 0.031, R = 0.265), but neither axial length nor MBR had a significant association with DCI (P = 0.396, 0.094, respectively).
Discussion
The aim of this study was to clarify the relationship between ONH blood flow and color tone, as well as to investigate associated factors. We quantitatively analyzed ONH color tone and blood flow and found a modest correlation between these factors in younger subjects, but not in older subjects or in the entire subject cohort after cataract surgery. Meanwhile, older age showed a significant correlation with lower MBR in all subjects, suggesting that ONH blood flow changes in older subjects might contain factors that cannot be elucidated from ONH color tone alone.
Our findings are in agreement with previous studies that reported that ocular blood flow decreases with aging [29–32]. The reduction of ocular blood flow was suggested to be a risk factor for some ocular diseases, e.g., neovascular age-related macular degeneration and open-angle glaucoma [33–35]. Also age-related macular degeneration and glaucoma are more prevalent among older people. Taken together, it is plausible that a decrease in ocular blood flow with aging is partially related to such ocular diseases.
In the present study, we found that axial length had a negative influence on MBR independently of the age. According to previous studies, retinal blood flow in high myopia decreases due to the narrowing of the retinal vessel diameter [36], and choroidal blood flow may decrease as axial length increases [37, 38]. Our result appeared not to contradict them. However, LSFG might underestimate MBR in the ONH because of myopia-induced disc changes, including a tilted disc [39] and macrocupping in the macrodisc [2]. Therefore, it is unclear that LSFG was valid for assessment of myopic disc.
On the other hand, we could not find a correlation between reddish appearance of the ONH and axial length. Recently, Neelum et al. quantified ONH color change in eyes with myopic chorioretinal degeneration to show that axial length had a positive correlation with the color change of myopic chorioretinal degeneration, using the formula: R/(R + G + B) [5]. Because hypoplasia of the retinal pigment epithelium following axial elongation reduces pigment epithelium and increases visibility of choroidal vessels [40], the retina in myopic eyes appears much redder. However, in this study, we quantified ONH color and, as the ONH does not contain pigment like the retinal pigment epithelium and choroid, axial length did not seem to affect ONH color.
We also examined the influence of cataract on red color tone and blood flow assessment in the ONH. Postoperative MBR was significantly higher and postoperative DCI was lower than preoperative values. Lens opacity is a possible reason for these differences. Light transmission through the lens decreases with aging, particularly at shorter wavelengths [41], and we calculated mean blue intensity (B) as the denominator of the DCI calculation formulas. Therefore, DCI might become relatively high in eyes with cataracts. In addition, LSFG might not be able to perform accurate measurements because measuring beams and reflected lights were interrupted by cataracts. Indeed, we demonstrated that MBR change rate correlated with red color change rate. Accordingly, the presence of a cataract is not negligible when assessing ocular blood flow in older people using LSFG. Thus, caution should be paid when interpreting LSFG data due to MBR underestimation caused by lens opacity.
This study has several limitations. First, the study is retrospective in nature and investigates a relatively small sample size. Second, we did not evaluate ocular perfusion pressure (OPP) between before and after cataract surgery, and OPP is important in ocular blood flow research. Earlier studies have reported that ONH blood flow is autoregulated [42, 43], and uncomplicated phacoemulsification surgery does not affect ocular hemodynamics [44]. In addition, because OPP is calculated as OPP = 2/3 (1/3[systolic blood pressure] + 2/3[diastolic blood pressure]) – IOP, an approximately 2-mmHg reduction in IOP after surgery was thought to have a small influence on OPP. Our result showed that there was no significant correlation between MBR change and IOP change before and after surgery. Therefore, the lack of evaluating OPP in the current study is not thought to have much of an influence on our results. Third, there is no established method for objective evaluation of fundus color. Although DCI was employed in this study, this objective technique may not always be consistent with subjective evaluation. Accordingly, further investigation should be performed to confirm valid measurements of ocular fundus color. Fourth, LSFG does not measure blood flow itself, but velocity. Finally, we examined the entire ONH area, which shows great variability in the number of vessels in the disc and cup/disc ratios among individuals. Thus, these methods may be inappropriate for quantitatively assessing ONH color and microcirculation. Further studies should be performed to explore new algorithms and techniques for quantifying fundus color and ocular microcirculation.
This study quantitatively evaluated ONH color and blood flow. We showed that older subjects had greater ONH redness and lower blood flow than the younger subjects. Moreover, it is difficult to assess ONH hemodynamics by evaluating redness, especially in older subjects. In addition, ocular blood measurements using laser speckle phenomenon are underestimated with lens opacity and long axial length.
References
Pederson JE, Anderson DR (1980) The mode of progressive disc cupping in ocular hypertension and glaucoma. Arch Ophthalmol 98:490–495
Jonas JB, Budde WM, Panda-Jonas S (1999) Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol 43:293–320
Moorthy RS, Inomata H, Rao NA (1995) Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol 39:265–292
Suzuki S (1999) Quantitative evaluation of "sunset glow" fundus in Vogt-Koyanagi-Harada disease. Jpn J Ophthalmol 43:327–333
Neelam K, Chew RY, Kwan MH, Yip CC, Au Eong KG (2012) Quantitative analysis of myopic chorioretinal degeneration using a novel computer software program. Int Ophthalmol 32:203–209
Yoshihara N, Yamashita T, Ohno-Matsui K, Sakamoto T (2014) Objective analyses of tessellated fundi and significant correlation between degree of tessellation and choroidal thickness in healthy eyes. PLoS One 9, e103586
Hayreh SS (1969) Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol 53:721–748
Grunwald JE, Riva CE, Stone RA, Keates EU, Petrig BL (1984) Retinal autoregulation in open-angle glaucoma. Ophthalmology 91:1690–1694
Flammer J, Orgul S, Costa VP et al (2002) The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 21:359–393
Grunwald JE, Brucker AJ, Grunwald SE, Riva CE (1993) Retinal hemodynamics in proliferative diabetic retinopathy. A laser Doppler velocimetry study. Invest Ophthalmol Vis Sci 34:66–71
Boltz A, Luksch A, Wimpissinger B et al (2010) Choroidal blood flow and progression of age-related macular degeneration in the fellow eye in patients with unilateral choroidal neovascularization. Invest Ophthalmol Vis Sci 51:4220–4225
Pemp B, Schmetterer L (2008) Ocular blood flow in diabetes and age-related macular degeneration. Can J Ophthalmol 43:295–301
Riva CE, Grunwald JE, Petrig BL (1986) Autoregulation of human retinal blood flow. An investigation with laser Doppler velocimetry. Invest Ophthalmol Vis Sci 27:1706–1712
Nicolela MT, Hnik P, Drance SM (1996) Scanning laser Doppler flowmeter study of retinal and optic disk blood flow in glaucomatous patients. Am J Ophthalmol 122:775–783
Izhaky D, Nelson DA, Burgansky-Eliash Z, Grinvald A (2009) Functional imaging using the retinal function imager: direct imaging of blood velocity, achieving fluorescein angiography-like images without any contrast agent, qualitative oximetry, and functional metabolic signals. Jpn J Ophthalmol 53:345–351
Sugiyama T, Araie M, Riva CE, Schmetterer L, Orgul S (2010) Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol 88:723–729
Watanabe G, Fujii H, Kishi S (2008) Imaging of choroidal hemodynamics in eyes with polypoidal choroidal vasculopathy using laser speckle phenomenon. Jpn J Ophthalmol 52:175–181
Nagahara M, Tamaki Y, Tomidokoro A, Araie M (2011) In vivo measurement of blood velocity in human major retinal vessels using the laser speckle method. Invest Ophthalmol Vis Sci 52:87–92
Shiga Y, Omodaka K, Kunikata H et al (2013) Waveform analysis of ocular blood flow and the early detection of normal tension glaucoma. Invest Ophthalmol Vis Sci 54:7699–7706
Murakami Y, Ikeda Y, Akiyama M, et al. (2015) Correlation between macular blood flow and central visual sensitivity in retinitis pigmentosa. Acta Ophthalmol doi: 10.1111/aos.12693 [Epub ahead of print].
Soma R, Moriyama M, Ohno-Matsui K (2015) Hemodynamics of focal choroidal excavations. Int Ophthalmol 35:261–268
Aizawa N, Yokoyama Y, Chiba N et al (2011) Reproducibility of retinal circulation measurements obtained using laser speckle flowgraphy-NAVI in patients with glaucoma. Clin Ophthalmol 5:1171–1176
Radius RL, Anderson DR (1979) The mechanism of disc pallor in experimental optic atrophy. A fluorescein angiographic study. Arch Ophthalmol 97:532–535
Emery JM, Little JH (1979) Phacoemulsification and aspiration of cataracts: Surgical techniques, complications, and results. CV Mosby, St Louis, pp 45–48
Senarathna J, Rege A, Li N, Thakor NV (2013) Laser speckle contrast imaging: theory, instrumentation and applications. IEEE Rev Biomed Eng 6:99–110
Takahashi H, Sugiyama T, Tokushige H et al (2013) Comparison of CCD-equipped laser speckle flowgraphy with hydrogen gas clearance method in the measurement of optic nerve head microcirculation in rabbits. Exp Eye Res 108:10–15
Wang L, Cull GA, Piper C, Burgoyne CF, Fortune B (2012) Anterior and posterior optic nerve head blood flow in nonhuman primate experimental glaucoma model measured by laser speckle imaging technique and microsphere method. Invest Ophthalmol Vis Sci 53:8303–8309
Touchon J, Warkentin K (2008) Fish and dragonfly nymph predators induce opposite shifts in color and morphology of tadpoles. Oikos 117:634–640
Ravalico G, Toffoli G, Pastori G, Croce M, Calderini S (1996) Age-related ocular blood flow changes. Invest Ophthalmol Vis Sci 37:2645–2650
Emeterio Nateras OS, Harrison JM, Muir ER et al (2014) Choroidal blood flow decreases with age: an MRI study. Curr Eye Res 39:1059–1067
Groh MJ, Michelson G, Langhans MJ, Harazny J (1996) Influence of age on retinal and optic nerve head blood circulation. Ophthalmology 103:529–534
Grunwald JE, Hariprasad SM, DuPont J (1998) Effect of aging on foveolar choroidal circulation. Arch Ophthalmol 116:150–154
Grunwald JE, Metelitsina TI, Dupont JC, Ying GS, Maguire MG (2005) Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci 46:1033–1038
Xu W, Grunwald JE, Metelitsina TI et al (2010) Association of risk factors for choroidal neovascularization in age-related macular degeneration with decreased foveolar choroidal circulation. Am J Ophthalmol 150:40-47–e42
Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B (2008) Risk factors for incident open-angle glaucoma: the Barbados Eye studies. Ophthalmology 115:85–93
Shimada N, Ohno-Matsui K, Harino S et al (2004) Reduction of retinal blood flow in high myopia. Graefes Arch Clin Exp Ophthalmol 242:284–288
Mori F, Konno S, Hikichi T, Yamaguchi Y, Ishiko S, Yoshida A (2001) Factors affecting pulsatile ocular blood flow in normal subjects. Br J Ophthalmol 85:529–530
Lam AK, Wong S, Lam CS, To CH (2002) The effect of myopic axial elongation and posture on the pulsatile ocular blood flow in young normal subjects. Optom Vis Sci 79:300–305
Kim TW, Kim M, Weinreb RN, Woo SJ, Park KH, Hwang JM (2012) Optic disc change with incipient myopia of childhood. Ophthalmology 119:21–26.e
Tokoro T (1998) Types of fundus changes in the posterior pole. Atlas of posterior fundus changes in pathologic myopia. Springer, Tokyo, pp 5–22
Mellerio J (1987) Yellowing of the human lens: nuclear and cortical contributions. Vision Res 27:1581–1587
Boltz A, Told R, Napora KJ et al (2013) Optic nerve head blood flow autoregulation during changes in arterial blood pressure in healthy young subjects. PLoS One 8, e82351
Wang L, Burgoyne CF, Cull G, Thompson S, Fortune B (2014) Static blood flow autoregulation in the optic nerve head in normal and experimental glaucoma. Invest Ophthalmol Vis Sci 55:873–880
Turk A, Mollamehmetoglu S, Imamoglu HI, Kola M, Erdol H, Akyol N (2013) Effects of phacoemulsification surgery on ocular hemodynamics. Int J Ophthalmol 6:537–541
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported, in part, by the Japan Society for the Promotion of Science (JSPS), Tokyo, Japan (Grant-in-Aid for Scientific Research, no. 21592256), the Japan National Society for the Prevention of Blindness, Tokyo, Japan and the Innovative Techno-Hub for Integrated Medical Bio-Imaging of the Project for Developing Innovation Systems, from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) in Japan.
Conflict of interest
Y. Kuroda and A. Uji have no conflicts of interest. N. Yoshimura reports grants and personal fees from Nidek, Canon, Novartis Japan, Bayer, Santen, Senju, Ohtsuka, Wakamoto and Alcon Japan, and grants from Topcon and the Japan Society for the Promotion of Science, outside the submitted work.
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Kuroda, Y., Uji, A. & Yoshimura, N. Factors associated with optic nerve head blood flow and color tone: a retrospective observational study. Graefes Arch Clin Exp Ophthalmol 254, 963–970 (2016). https://doi.org/10.1007/s00417-015-3247-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3247-0