Abstract
Background
The inner retinal complex is a well-defined layer in spectral-domain OCT scans of the retina. The central edge of this layer at the fovea provides anatomical landmarks that can be observed in serial OCT scans of developing full-thickness macular holes (FTMH). Measurement of the movement of these points may clarify the mechanism of FTMH formation.
Method
This is a retrospective study of primary FTMH that had a sequence of two OCT scans showing progression of the hole. Measurements were made of the dimensions of the hole, including measurements using the central edge of the inner retinal complex (CEIRC) as markers. The inner retinal separation (distance between the CEIRC across the centre of the fovea) and the Height-IRS (average height of CEIRC above the retinal pigment epithelium) were measured.
Results
Eighteen cases were identified in 17 patients. The average increase in the base diameter (368 microns) and the average increase in minimum linear dimension (187 microns) were much larger than the average increase in the inner retinal separation (73 microns). The average increase in Height-IRS was 103 microns.
Conclusion
The tangential separation of the outer retina to produce the macular hole is much larger than the tangential separation of the inner retinal layers. A model based on the histology of the Muller cells at the fovea is proposed to explain the findings of this study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Full-thickness macular holes (FTMH) have been extensively studied since Kelly and Wendel discovered surgical treatment in 1991 [1]. There is general agreement that FTMH is due to dehiscence and tangential separation of tissue rather than loss of tissue [2]. This would explain the characteristic pincushion distortion of vision produced by FTMH, and the recovery of vision that often occurs following macular hole surgery. However, the mechanism that produces tissue separation remains controversial. Hypotheses based on cortical vitreous contraction or glial cell proliferation have been proposed, but the prevailing view is that FTMH usually results from vitreomacular traction [3–5]. Vitreomacular traction is undoubtedly present in a large proportion of developing FTMH, and can be visualized in optical coherence tomography (OCT) scans of FTMH [3–5]. However, these forces appear to act mainly in the antero-posterior direction [6] and this would not of itself produce the tangential separation of the tissue required to produce a macular hole.
Modern OCT scanning of the retina produces images of sufficient quality to define the various layers of the retina and to give an accurate picture of the morphology of FTMH [7]. Serial OCT scanning of developing FTMH should be able to resolve how the retinal layers move relative to each other, and this should help clarify the mechanism of macular hole formation. Gentile used this technique to observe two cases of developing FTMH [8]. He found that there was initial vitreomacular traction but that the macular hole continued to enlarge following separation of the vitreomacular attachments. He also found that hole enlargement was associated with cyst formation. He concluded that hydration of the retina, as postulated by Tornambe [9], was the driving force in FTMH. Gentile’s observations were qualitative using an earlier generation of OCT scanner. He noted that modern spectral-domain OCT scanners, capable of differentiating the various layers of the retina, might further elucidate the process of FTMH pathogenesis.
Macular hole parameters currently in use are defined in terms of the shape of a macular hole rather than distances between anatomical points of the retina [10]. They are therefore not defined for measuring the movement of one anatomical point of the retina relative to another. The inner retinal complex, comprising the nerve fibre layer, the ganglion cell layer and the inner plexiform layer, is seen as a well-defined layer in modern OCT scans (Fig. 1a). This layer tapers to a sharp central edge, and this sharp edge is retained throughout the development of a FTMH. In cross-section, the inner retinal complex tapers to a point on each side of the foveola, and the distance between these anatomical points is easily measured on OCT scans. Measurement of the change in this distance in serial OCT scans of developing FTMH would provide a measure of the tangential movement of the central edge of the inner retina complex. Similarly, the change in height of the central edge of inner retinal complex above the retinal pigment epithelium would provide a measure of the antero-posterior movement of retinal tissue. We have examined serial OCT scans to provide quantitative data on the movement of the inner retinal complex, to clarify the mechanism of FTMH formation in cases of primary FTMH as defined by the International Vitreomacular Traction Study Group [5].
a The inner retinal complex (IRC) is seen as a well-defined layer on OCT scans. b Measurements made on the OCT scans: Inner retinal separation (IRS), minimum linear dimension (MLD), base diameter of hole (BD), and the height of a line joining the innermost points of the inner retinal complex (Height-IRS)
Material and methods
This is a retrospective study of primary FTMH that have had a sequence of OCT scans that showed progression of the FTMH. Consecutive cases were identified from theatre records of patients who have had surgery for FTMH. The OCT database of these patients was reviewed, and cases that had a sequence of OCT scans showing development or progression of an FTMH were identified. Cases were included if there was evidence of vitreomacular traction on the initial OCT scan. The study was approved by the Research and Development department of St. James’ Hospital, Leeds (R&D number: OP13/10828), and adhered to the tenets of the Declaration of Helsinki.
Horizontal raster OCT scans were taken using a Heidelberg OCT Spectralis spectral-domain OCT scanner. The OCT scans passing through the central fovea were used. Measurements were made with the horizontal and vertical scale set to the same value, using the Heidelberg software, by a single observer (WHW). The following parameters were measured (Fig. 1b):
-
1.
IRS, the inner retinal separation. The distance of a line joining the centre most points of the inner retinal complex either side of the fovea.
-
2.
MLD, the minimum linear dimension of the hole. Any flap attached to the edge of the hole is not included when taking this measurement.
-
3.
BD, the base diameter of the hole.
-
4.
Height-IRS. The average height, above the retinal pigment epithelial layer, of a line joining the central edges of the inner retinal complex.
For each sequence of two OCT scans, the changes in IRS, MLD, BD, and Height-IRS were calculated by subtracting the value in the first OCT scan from the value in the final OCT scan.
Statistical analysis was performed using GenStat 10th edition.
Results
Eighteen cases in 17 patients were identified with evidence of vitreomacular traction on an initial OCT and for which there was a further OCT showing progression or development of a FTMH. Eleven patients were female and six male. The average age of the patients at the time of the final OCT scan was 66.5 years (range 51–77). The median interval between the initial and final OCT scans was 84 days (interquartile range: 27–148 days).
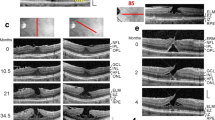
The average change in the parameters with standard error of mean are: (1) change in base diameter, 368 (±48) microns, (2) change in minimum linear dimension, 187 (±29) microns, (3) change in height, 103 (±20) microns, and (4) change in inner retinal separation, 73 (±20) microns. Boxplots for these data are shown in Fig. 2 left. Initial and final OCT scans of one of the cases are shown in Fig. 3.
Serial OCT scans from one of the cases. It can be seen that the initial and final OCT scans are taken from the same cross section. The horizontal positions of the central edge of the inner retinal complex have been arrowed. It can be seen that there is very little change in the inner retinal separation despite formation of a FTMH
The cases were divided into four groups according to the change that occurred between initial and final OCT scan. In group A, the change was from no full-thickness defect of the retina to a hole (full-thickness defect of the retina) but with persistent VMT at one or both edges of the hole. In group B, a hole with persistent VMT progressed whilst retaining VMT to one or both sides of the hole. In group C, a hole with VMT enlarged to become a hole with no VMT. In group D, the change was from no full-thickness defect to a hole with no persistent VMT.
The numbers in each group were as follows: group A, six; group B, four; group C, five; group D, three. One-way analysis of variance showed no significant difference for the mean change in base diameter, or mean change in minimum linear dimension, or mean change in height between the four groups (p-value of 0.79, 0.45, and 0.07 respectively). The p-value for the test of equal mean change in inner retinal separation in the four groups was 0.023. The box plot of the change in inner retinal separation for the four groups is shown in Fig. 2 right.
Discussion
This study of the development of a primary FTMH found that the average increase in the base diameter (368 microns) and the average increase in the minimum linear dimension (187 microns) are both considerably larger than the average increase in inner retinal separation (73 microns). The formation of a FTMH was also found to be associated with a change in the height of the inner retina which was larger than the change in inner retinal separation. This study is limited by being a retrospective study, and there is potential for bias in both the data collection and in the measurement of parameters. However, the quality of spectral-domain OCT scans is such that the end points for measurements are well-defined and allow reliable measurements. The errors in measurements are thus probably small compared to the changes in the parameters that have been found. Moreover, the findings are probably not unexpected, given the comparisons that can be made between FTMH and fellow eyes in everyday clinical practise. If there is no significant loss of tissue, the tangential separation of the outer retina must be at least equal to the minimum linear dimension. Thus, this study shows that during the formation of a FTMH, the tangential movement of the outer retina is much larger than the tangential movement of the inner retina, and that the formation of the hole is associated with a larger movement of the inner retina in an antero-posterior direction than in the plane of the retina.
Vitreomacular traction would explain the antero-posterior movement of the inner retinal complex noted in this study, but it would not of itself explain how tangential separation of the outer retina is produced by elevation of the inner retina. We propose a model that would explain how the tangential separation of the outer retina can be produced by elevation of the inner retina, with very little tangential movement of the inner retina. In addition, the model would explain other well-recognized features such as the rolling-up of the outer retina and the cyst formation that is seen in OCT scans. For the purpose of this model, the retina is separated into three layers (Fig. 4):
-
i.
A layer of inner retinal complex (IRC), using the three-layer definition of the IRC to include the nerve fibre layer in addition to the ganglion cell layer and the inner plexiform layer.
-
ii.
An intermediate layer consisting of the inner nuclear layer and the outer plexiform layer.
-
iii.
An outer retinal complex layer (ORC) consisting of the outer nuclear layer and the photoreceptor inner and outer segments
a OCT scan of a normal fovea and a FTMH. The images have the same horizontal and vertical scale for length b Drawing of OCT scans to show the three layers used in the proposed model. IRC: inner retinal complex layer; IML: intermediate layer; ORC: outer retinal complex layer. c The inner retinal complex in the region of the foveal depression moves like a drawbridge during the development of a FTMH
Our proposed model combines the concepts of drawbridge elevation of the inner retina [9], vector forces transmitted through the Muller cells [11, 12], and the special arrangement of Muller cells in the central area of the macula [13–15]:
-
1.
Drawbridge elevation of the inner retina complex:
In a normal eye, the inner retina follows the contour of the foveal depression. In cross-section, the inner retina on each side of the foveal depression follows a slope that is reversed in a developed FTMH with an elevated retinal rim. Thus, during the development of a FTMH, the inner retina on each side of the fovea has moved like a drawbridge pivoting about the edge of the normal foveal depression (Fig. 4c). Tornambe coined the term drawbridge elevation to describe this movement of the inner retina, and he postulated hydration of the retina as the cause for this elevation of the inner retina and FTMH formation [9]. In our model, the cyst formation in Henle’s layer in a FTMH is secondary to the drawbridge elevation.
-
2.
Vector forces:
Spaide and others have proposed that vector forces transmitted along the line of a Muller cell produces the tangential separation of the outer retina to form an FTMH [11, 12]. They noted that the Muller cells are long cells that pass obliquely through the entire thickness of the retina to connect the inner and outer retinal layers. The Muller cells are the main glial cell component of the retina, and it is probable that separation of the inner and outer retina would create tension within the Muller cells. An antero-posterior movement of the inner retina is thus transmitted to the outer retina along the line of these Muller cells, and this would produce an antero-posterior and tangential (centrifugal) movement of the outer retinal complex (Fig. 5a).
a Schematic of model showing oblique path of Muller cells. Inset: a vertical movement of the inner retina produces a vertical and tangential force on the outer retina. b Elevation of the inner retinal complex. The movement from the area of elevated inner retinal complex is directed to a smaller area of the outer retina. The length of the corresponding Muller cells in diagrams a and b are the same. It can be seen that the distances separating the Muller cells is increased in b compared to a, and this gives rise to the intra-retinal cysts. c The Muller cells have a z-shape. The length of the Miller cells increase with distance from the centre of the fovea. d Postulated path of Muller cells in a FTMH; the cells have been straightened from their z-shape and separated in Henle’s layer to form the retinal cysts
-
3.
Special arrangement of Muller cells in the central area of the macula:
Within a central area of the macula, the Muller cells have the following properties:
-
a)
Z-shape configuration. The Muller cells follow the path linking a photoreceptor with its corresponding ganglion cell, and have a characteristic z-shape (Fig. 5c). This path takes a centrifugal course as it follows the long cone axons in Henle’s layer [13].
-
b)
Increasing length of Muller cells with distance from the centre of the fovea. Within the central macula, the lateral displacement between photoreceptor and their corresponding ganglion cell increases with distance from the centre of the fovea up to a maximum for photoreceptors that are about 0.65 mm from the centre of the fovea [14]. This means that, in the foveal area, the length of the Muller cells must increase with distance from the centre of the fovea (Fig. 5). Muller cells linked to photoreceptors at a distance of about 0.65 mm from the centre of the fovea have a length of about 0.3 mm in Henle’s layer.
-
c)
The paths of the Muller cells are axially symmetric about the axis of the fovea [15].
With this arrangement of Muller cells at the central macula, a drawbridge elevation of the inner retina complex would produce forces acting on a smaller area of the outer retinal complex (Fig. 5a). In addition, within this affected area of outer retina, the effect of movement of the drawbridge falls off rapidly with distance from the centre of the fovea. This is because a more central point on the drawbridge moves through a larger arc, and the Muller cells more centrally have a shorter length (Fig. 5b). The effect is to cause the outer retinal complex to become rolled up as seen in two-dimensional OCT scans and to form the crown shape in three-dimensional OCT imaging as described by Hangai et al. [16].
The model also explains the intra-retinal cysts in FTMH as a consequence of FTMH formation rather than as a cause of FTMH pathogenesis. The Muller cells have a long path, and are packed tightly in parallel in Henle’s layer. In this region of the macula, adjacent Muller cells in Henle’s layer form two sides of a parallelogram that has been squashed by shearing forces. Separation of the inner and outer retina causes the parallelogram to open up and produce the cysts in Henle’s layer that are seen in a developed FTMH (Fig. 5). This parallelogram action is seen in a railway barrier that opens out as it falls into the closed position, or can be seen when a flattened wine rack is opened out for use.
This model explains the observations found in this study, but it does not explain why the inner retina should become elevated in a drawbridge fashion. Tornambe explains the drawbridge elevation by hydration of the retina. However, hydration pressure would act in all directions, and this would cause the outer retina to be pressed against the retinal pigment epithelium, while in fact the outer retina becomes elevated with FTMH formation. Swelling of tissue would also be expected to close a hole if there is no tissue loss. Vitreomacular traction would provide the antero-posterior force to elevate the drawbridge, but the point-like attachment of the vitreous to the fovea in FTMH [6] would be expected to produce a sharper curvature of the inner retina than the drawbridge elevation seen in FTMH. It is also difficult to explain the symmetry of FTMH, when vitreomacular traction is often asymmetric, unless there is some intrinsic force within the retina to maintain symmetry. Epiretinal membrane formation could also produce elevation of the inner retina, with shortening of the retina and loss of the foveal depression. However, further elevation of the inner retinal beyond a flattening of the foveal depression would be difficult to explain. The inner retinal complex that lines the foveal depression of a healthy eye has the shape of a shallow disc cone, and in three dimensions the drawbridge elevation of the inner retinal complex can be seen as the inversion of the shape of the inner retinal complex at the fovea. We have proposed a bistable hypothesis of FTMH formation in which the disc cone of inner retinal complex can be triggered to flip inside out by vitreomacular traction—in the same way that an umbrella can be flipped inside out by pressing on one edge [17]. The bistable hypothesis would thus explain the movement of the inner retinal complex that has been found, although there is of course no direct evidence that the disc cone of inner retinal complex is bistable in this study.
Dividing the cases into the groups chosen showed that there is some evidence that most of the separation in the inner retinal complex occurs in group C, when a FTMH with VMT progresses to become a FTMH without VMT. This finding may be worth further consideration, even though the data is from a small cohort of cases. The increase in inner retinal separation following separation of VMT could be explained if the presence of vitreomacular attachments were to act to prevent tangential separation of the inner retinal complex. The conventional view is that vitreous attachments to the macula produce a force with a tangential centrifugal component [18]. However, this is based on a model of the vitreous behaving like a string with one end attached to the macula. In this model, the vitreous face at the macula is behaving like a radial array of elastic bands. This may be an oversimplification, and the vitreous face may behave like an elastic sheet with a central defect at the fovea. In this case, there would be tension circumferentially in the vitreous face at the site of vitreomacular attachment, and this could act to limit tangential separation. Further work is required to differentiate these models of the posterior vitreous face.
This study has quantified the anatomical changes that occur during the development of a FTMH. We propose a mechanical model to link the movement of the inner retina to the outer retina to explain the observed changes. The model relies on a drawbridge elevation of the inner retinal complex rather than a tangential separation of the inner retina for FTMH formation. These changes must be explained by any complete hypothesis of FTMH formation.
References
Kelly NE, Wendel RT (1991) Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol 109:654–659
Gass JDM (1995) Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol 119:752–759
Stalmans P, Duker JS, Kaiser PK, Heier JS, Dugel PU, Gandorfer A, Sebag J, Haller JA (2013) OCT-based interpretation of the vitreomacular interface and indications for pharmacologic vitreolysis. Retina 33:2003–2011
Steel DHW, Lotery AJ (2013) Idiopathic vitreomacular traction and macular hole: a comprehensive review of pathophysiology, diagnosis, and treatment. Eye 27:S1–S21
Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, Sadda SR, Sebag J, Spaide RF, Stalmans P (2013) The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology 120:2611–2619
Takezawa M, Toyoda F, Kambara C, Yamagami H, Kakehashi A (2011) Clarifying the mechanism of idiopathic macular hole development in fellow eyes using spectral-domain optical coherence tomography. Clin Ophthalmol 5:101–108
Ko TH, Fujimoto JG, Duker JS, Paunescu LA, Drexler W, Baumal CR, Puliafito CA, Reichel E, Rogers AH, Schuman JS (2004) Comparison of ultrahigh and standard resolution optical coherence tomography for imaging macular hole pathology and repair. Ophthalmology 111(11):2033–2043
Gentile RC, Landa G, Pons ME, Eliott D, Rosen RB (2010) Macular hole formation, progression, and surgical repair: case series of serial optical coherence tomography and time lapse morphing video study. BMC Ophthalmol 10:24
Tornambe P (2003) Macular hole genesis: the hydration theory. Retina 23:421–424
Wakely L, Rahman R, Stephenson J (2012) A comparison of several methods of macular hole measurement using optical coherence tomography, and their value in predicting anatomical and visual outcomes. Br J Ophthalmol 96(7):1003–1007
Spaide RF (2000) Closure of an outer lamellar macular hole by vitrectomy: hypothesis for one mechanism of macular hole formation. Retina 20(6):587–590
Takahashi A, Nagaoka T, Ishiko S, Kameyama D, Yoshida A (2010) Foveal anatomic changes in a progressing stage 1 macular hole documented by spectral-domain optical coherence tomography. Ophthalmology 117:806–810
Reichenbach A, Bringmann A (2010) Muller cells in the healthy retina. In: Muller cells in the healthy and diseased retina. Springer, Heidelberg, pp 35–216
Sjostrand J, Popovic Z, Conradi N, Marshall J (1999) Morphometric study of the displacement of retinal ganglion cells subserving cones within the human fovea. Graefes Arch Clin Exp Ophthalmol 237:1014–1023
Elsner AE, Weber A, Cheney MC, VanNasdale DA (2007) Spatial distribution of macular birefringence associated with Henle fibres. Vis Res 48:2578–2585
Hangai M, Ojima Y, Gotoh N, Inoue R, Yasuno Y, Makita S et al (2007) Three-dimensional imaging of macular holes with high-speed optical coherence tomography. Ophthalmology 114:763–773
Woon WH, Greig D, Savage MD, Wilson MC, Grant CA, Bishop F, Mokete B (2014) Asymmetric vitreomacular traction and symmetrical full thickness macular hole formation. Graefes Arch Clin Ex Ophthalmol. doi:10.1007/s00417-014-2884-z
Theodossiadis G, Petrou P, Eleftheriadou M, Moustakas AL, Datseris I, Theodossiadis P (2014) Focal vitreomacular traction: a prospective study of the evolution to macular hole: the mathematical approach. Eye 28(12):1452–1460
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woon, W.H., Greig, D., Savage, M.D. et al. Movement of the inner retina complex during the development of primary full-thickness macular holes: implications for hypotheses of pathogenesis. Graefes Arch Clin Exp Ophthalmol 253, 2103–2109 (2015). https://doi.org/10.1007/s00417-015-2951-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-2951-0