Abstract
Background
With the advent of enhanced depth imaging optical coherence tomography (EDI-OCT), detailed visualisation of the choroid in vivo is now possible. Measurements of choroidal thickness (CT) have also enabled new directions in research to study normal and pathological processes within the choroid. The aim of the present study is to review the current literature on choroidal imaging using EDI-OCT.
Methods
Studies were identified by a systematic search using Medline (http://www.ncbi.nlm.nih.gov/pubmed). Papers were also identified based on the reference lists of relevant publications. Papers were included in the review if the focus of the study involved imaging of the choroid using EDI-OCT.
Results
Recent studies have demonstrated successful imaging of the choroid and high reproducibility of measurements of CT using EDI-OCT. There are much data confirming that abnormalities in choroidal structure and function contribute to major ocular diseases and patterns of CT variation may be observed in certain disease states and may be influenced by treatment. However, it is not clear whether these variations are a contributing factor or a consequence of the disease.
Conclusion
While more invasive methods such as indocyanine green (ICG) angiography remain the gold standard for detecting abnormalities of the choroidal vasculature in normal eyes and disease states, EDI-OCT has become an important adjunctive clinical tool in providing three-dimensional anatomical information of the choroid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Multifunctional role of the choroid
With the advent of enhanced depth imaging (EDI) optical coherence tomography (OCT), more detailed visualisation of the choroid in vivo is now possible. However, the exact role and mechanisms of the choroid in normal eyes and in pathological conditions are not completely understood. The principle role of the choroid is to supply oxygen to the outer retina up to the level of the inner nuclear membrane and, therefore, the neurosensory retina in the foveal avascular zone derives blood from the choroid [1]. However there is evidence to suggest that the choroid has multiple roles including thermoregulation via heat dissipation, positional adjustment of the retina by changes in choroidal thickness (CT), modulation of intraocular pressure via vasomotor control of blood flow, secretion of growth factors and drainage of aqueous humour via the uveoscleral pathway [2–7].
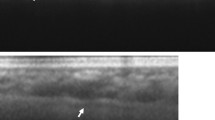
Most anatomical information regarding the choroid has been gained through histological post-mortem studies. Histologically the choroid can be subdivided into five layers: Bruch’s membrane, choriocapillaris, two vascular layers (Haller’s and Sattler’s), and the suprachoroidea [1]. Prior to the advent of OCT, clinical evaluation of the choroid has involved fundus fluorescein angiography (FFA), indocyanine green (ICG) angiography, and ultrasound [8]. While these methods are useful in detecting vasculature abnormalities, they do not provide cross-sectional anatomical information and poorly identify the depth of vascular pathology [8]. OCT involves a concept known as interferometry and utilises light waves to create high-resolution cross-sectional images of the retinal layers [9]. Recent developments in OCT have employed a longer wavelength than conventional OCT which has enabled penetration of the deeper ocular tissues such as the photoreceptor and retinal pigment epithelium layers, and visualisation of the choroid. The image of the choroid can be further enhanced by a technique first described by Spaide et al. known as EDI-OCT. By placing the OCT device closer to the eye and inverting the retinal image, the choroid is placed closer to the zero-delay line which results in higher depth of field and higher resolution of choroidal detail [9]. Measurements of CT in vivo have enabled new directions in research regarding the normal and disease states of the choroid. EDI-OCT is non-invasive and easy to use in the assessment of CT; however, image resolution does not enable accurate discrimination of the choriocapillaris. A detailed review of choroidal imaging using OCT was published by Mrejen and Spaide in 2013 [10]. The present article focuses on the clinical role of EDI-OCT imaging in relatively common conditions. Figure 1 presents an EDI-OCT image of a normal choroid.
Methods of literature search
Studies were identified by a systematic search using Medline (http://www.ncbi.nlm.nih.gov/pubmed). Terms searched were as follows: “Choroidal thickness,” “Enhanced depth imaging optical coherence tomography,” “Age related macular degeneration,” “Glaucoma,” “Retinal detachment,” Central serous chorioretinopathy,” “Retinitis pigmentosa,” “Diabetic retinopathy,” “Panretinal photocoagulation,” “Refractive error,” “Choroidal nevi,” “Choroidal tumours,” “Choroidal blood flow.” Papers were also identified based on the reference lists of relevant publications. Papers were included in the review if the focus of the study involved imaging of the choroid using EDI-OCT.
Reproducibility
In light of this new technique and due to the limitations inherent in taking manual and subjective measurements, various studies have recently assessed the repeatability of CT measurement. Studies have demonstrated that inter-observer and intra-observer correlation has been high for manual measurements as well as being highly reproducible [11–13]. A large study by Shao et al. assessed inter-observer reproducibility in 3,233 subjects and found an insignificant mean inter-observer difference of 3.14 ± 13.1 μm. The authors also found an excellent intra-observer reproducibility in 21 subjects with an intra-class correlation coefficient of 1.00 (P < 0.001) and a mean coefficient of variation of 0.85 ± 1.48 % [14].
OCT system comparison
Studies have also demonstrated a high level of agreement among the different instruments when using the optimal imaging techniques specific to each instrument [15, 16]. Variation in optimal image acquisition and enhanced imaging techniques may vary between instruments due to the drop in sensitivity from the zero-delay line. Lin et al. reported that the most favourable modes to visualise the choroidal-sclera junction and outer-choroidal vessels were to invert the Heidelberg Spectralis and Bioptigen SD-OCT images and to use the upright images produced by the Zeiss Cirrus OCT instrument [15].
Branchini compared reproducibility of CT measurements in 28 normal subjects on three SD-OCT instruments including the Zeiss Cirrus HD-OCT (Carl Zeiss Meditec Inc., Dublin, CA, USA), Heidelberg Spectralis (Heidelberg Engineering, Germany) and Optovue RTVue (Optovue Inc. Fremont, CA, USA) [16]. The choroid was measured subfoveally, 750 μm temporally and 750 μm nasally to the fovea. The authors reported good reproducibility for CT measurements acquired with Cirrus, Spectralis, and RTVue [16]. Another study by Yamashita (N = 43 healthy eyes) compared the Heidelberg Spectralis-OCT, Cirrus HD-OCT, and Topcon 3D OCT-1000 Mark-II and found a high inter-machine correlation coefficient and no trend on the Altman-Bland graphs indicating that all 3 instruments could be used interchangeably [17].
Trends in non-pathological choroidal imaging and healthy individuals
The choroid is a highly vascular structure and similar to any microvasculature in the body, it is likely to undergo structural and functional alterations with increasing age. This generally involves a reduction in perfusion to the outer retina, which may have an impact on visual function.
According to histological studies, the human subfoveal CT is approximately 194 μm at birth and decreases to approximately 80 μm by the age of 90 years [18]. In vivo studies have demonstrated that CT varies topographically within the posterior pole and is inversely correlated with age, which decreases by approximately 16 μm for each decade of life [19]. The study reviewed 54 eyes and demonstrated that CT was thinnest nasally, thickest subfoveally, and thinner temporally [19].
The Beijing Eye Study reviewed subfoveal CT in 3,233 subjects and found a number of associations. Hence, this study also highlights some of the potential confounders that should be considered when carrying out CT studies. Multivariate analysis found that subfoveal CT increased significantly with younger age, shorter axial length, deeper anterior chamber depth, thicker lens, and flatter corneas [20]. However, CT was not significantly associated with blood pressure, ocular perfusion pressure, intraocular pressure, cigarette smoking, alcohol consumption, serum concentrations of lipids and glucose, diabetes mellitus, and arterial hypertension [20]. There have also been gender differences reported, with CT being 18 % thicker in men than women [21]. It has been proposed that this may contribute to the prevalence differences in certain ocular conditions amongst males and females such as myopia, central serous chorioretinopathy, and age-related macular degeneration (AMD) [21].
Studies that have reviewed diurnal variation have demonstrated contrasting results although study numbers have been low. Tan et al. reported on 12 healthy individuals and found that CT was greatest in the morning compared to the evening [22]. Lee et al. reviewed 100 healthy volunteers and demonstrated that men, as well as those with a shorter axial lengths and thick baseline CT, had a greater pattern of diurnal variation with significantly greater amplitude [23].
Variations in choroidal imaging in pathology
Abnormalities in choroidal structure and function are well known contributors to major ocular diseases including central serous retinopathy and AMD, amongst others. However, what is unclear is whether choroidal thinning is a risk factor or a consequence of disease. While Yin et al. in 1997 demonstrated histologically that generalised choroidal thinning is associated with vessel loss, there is limited research evaluating the relationship between CT, choriocapillaris density and choroidal blood flow in vivo [24, 25]. It is biologically intuitive to consider that CT reduces with reduced blood flow; however, only one small cross-sectional retrospective study (N = 25 eyes) has been carried out and found no correlation between subfoveal CT and total and subfoveal choroidal blood flow in healthy young subjects [25].
Refractive error
Histological studies have demonstrated choroidal thinning, reduction in vessel density, and thinning of the choriocapillaris in high myopia [26, 27]. Various animal studies of myopia have reported a decrease in choriocapillaris density and diameter and demonstrated a decrease in choroidal circulation using various techniques including angiography, colour Doppler ultrasonography, and ocular pulse amplitude [28–31].
More recent studies with the aid of EDI-OCT have confirmed the correlation between refractive error and CT [20, 32, 33]. The Beijing study demonstrated a significant increase in subfoveal CT with shorter axial lengths and better best corrected visual acuities. Mean subfoveal CT was 253.8 μm (+/−107.4 μm) in the whole population; mean age was 64.3 years (+/− 9.6 years) and mean refractive error was −0.18 D (+/−1.98 D). The authors found that in the myopic refractive error range of greater than 1.00 diopter, subfoveal CT decreased by 15 μm for every diopter of myopic shift, or by 32 μm for every mm increase in axial length [20].
However, Fujiwara et al. found a mean subfoveal CT of 93.2 μm (+/− 62.5 μm) in 55 eyes of 31 patients with a mean refractive error of −11.9 D (+/− 3.7D) and a mean age of 59.7 years (+/− 17.6 years). In this series, regression analysis indicated a reduction in subfoveal CT by 8.7 μm for every diopter of myopic shift and 1.27 μm for each year of age [32]. Flores-Moreno et al. used the Topcon 3D-2000 SD OCT in eyes with myopia greater than 6 D without image inversion technique, but used the reference position “choroid” and image averaging 50, which moves the zero delay line behind the RPE to better image the choroid. The authors found a mean subfoveal CT of 131.3 μm (+/− 98.4 μm) in 120 eyes of 110 patients with a mean refractive error of −14.3 D (+/− 5.4D) and a mean age of 54.4 years (+/− 18.2 years). In this series, regression analysis indicated a regression of CT by 1.9 μm for each year of age [33].
In pathological myopia, elongation of the globe can result in posterior staphyloma due to scleral thinning and progressive scleral ectasia. Further complications include breaks in Bruch’s membrane, choroidal atrophy and lacquer lines. These dehiscences are associated with an increased risk for choroidal neovascularization (CNV). EDI-OCT studies have reported that posterior staphyloma formation was a key feature of choroidal thinning in highly myopic eyes [34]. A study by Yamagishi suggested a possible association with the development of serous retinal detachment; however, study numbers were low (N = 5) and there is a lack of literature regarding the relationship between retinal detachment and CT [34].
Age related macular degeneration (AMD)
The pathogenesis of AMD is thought to involve abnormalities of the choroidal circulation which, combined with poor choroidal perfusion, may lead to retinal pigment epithelium (RPE) hypoxia and ischaemia with the subsequent production of vascular endothelial growth factor (VEGF), angiogenesis, and formation of CNV [35–37].
The early stages of AMD can be defined by the presence of drusen, focal hyperpigmentation, and focal hypopigmentation, whereas late AMD is defined by the development of geographic atrophy (GA) or CNV [38]. In eyes with GA, loss of the RPE results in increased illumination of the choroid and subsequently a higher reflectivity signal to the choroid [39]. Image segmentation is the process of dividing an image into multiple parts in order to identify relevant parts in a digital image, which enables the identification of areas of RPE loss or atrophy [40]. Choroidal imaging using EDI-OCT has, therefore, been suggested as a possible method for monitoring GA [39]. In contrast, exudative AMD is characterised by the presence of CNV, the growth of new blood vessels from the choroid into the region underlying the RPE, or extending into the subretinal space.
Spaide et al. coined the term age-related choroidal atrophy, a condition which affects older individuals in whom there is a pronounced thinning of the choroid beyond the normal range of thinning with age. A tessellated fundus appearance with reduced CT are present and although a separate entity to AMD, it is associated with typical features of AMD [41, 42].
Histological studies have suggested that there are multiple morphological changes in the choroid and the choroidal vasculature in AMD. There is some evidence to suggest that there is a reduction in the density larger choroidal vessels, thinning of the choriocapillaris, and narrowing of the choriocapillaris lumen [43].
A number of EDI-OCT studies have documented a negative correlation between CT and AMD [44–46]. A study by Sigler et al. investigated the effects of early non-exudative AMD without GA and/or soft drusen on subfoveal CT in 150 eyes using EDI-OCT [44]. They found a decrease in mean macular CT compared to patients with no chorioretinal pathology.
Lee et al. assessed 176 eyes with non-exudative AMD changes and found a negative correlation between subfoveal CT, best corrected visual acuity, the severity of AMD in addition to the rate of GA progression [45]. The authors failed to find a significant decrease in CT in the early stages of non-exudative AMD and suggested that there may be a possible time lag from when the choroidal blood flow decreases to the subsequent thinning of the choroid in the later stages of the disease [45]. Switzer et al. studied 90 eyes with early AMD; although the study lacked a control group, they noted a correlation between CT and a number of the anatomical characteristics which have been associated with the progression of AMD [42]. Subfoveal CT was found to be significantly thinner in eyes with fundus tessellation, subretinal drusenoid deposit, lack of conventional drusen, or an absent band on OCT corresponding to overlap between outer segment and RPE apical processes [42]. In contrast, Jonas et al. compared 50 eyes with exudative AMD with 154 eyes with non-exudative eyes after excluding eyes with GA, and 228 healthy eyes. After adjusting for age and refractive error, AMD, in neither its non-exudative form nor exudative form, was significantly associated with a marked variation in CT in the foveal and parafoveal region [46]. While it is not entirely clear why these studies have had conflicting results, it is noteworthy that there is histological evidence of choriocapillaris atrophy in addition to reduced choroidal blood flow both in non-exudative AMD and exudative AMD the extent of which increases with progression of disease [35, 43, 47]. There may also be distinctions in the timing of choriocapillaris loss when considering non-exudative and exudative AMD relative to RPE loss [48]. However, as discussed previously there are a number of variables to consider when researching CT and indeed interpreting CT studies including age, gender, axial length, refractive error, anterior chamber depth, thicker lens, corneal diameter, smoking status, and diurnal variations [20–23, 49, 50].
EDI-OCT has also been a useful tool in differentiating between AMD and polypoidal choroidal vasculopathy (PCV) based on a thicker CT in eyes with PCV, which has been a consistent finding throughout studies [51–54]. PCV is characterized by a branching vascular network of subretinal polypoidal dilations arising from the choroidal vasculature. A study by Jirarattanasopa et al. reviewed 58 patients with AMD and 63 patients with PCV. They also found that subfoveal CT was greater in eyes with choroidal vascular hyperpermeability in eyes with either AMD or PCV where choroidal permeability was assessed using ICG angiography [51]. Koizumi et al. confirmed these findings when they compared 21 eyes with typical AMD to 23 eyes with PCV. They suggested that the choroidal vascular lesion seen in PCV may not be just the CNV accompanied by saccular capillary dilations, but may have a significant structural difference compared to typical AMD [52]. Chung et al. studied 25 eyes with PCV and 14 fellow eyes, 30 eyes with exudative AMD, 17 eyes with early AMD, and 20 eyes of age-matched normal subjects, and similarly propose that different pathogenic mechanisms in PCV are involved when compared to exudative AMD [53]. In addition, Yang et al. found that the choroid was thicker in eyes with PCV when they compared 18 eyes with PCV and 19 controls eyes. They further described the EDI-OCT characteristics of PCV which included dilated choroidal vessels, a double-layer sign at the level of the RPE–Bruch’s membrane–choriocapillaris complex, hyperreflectivity between the RPE and Bruch’s membrane and the association of choroidal macular swelling with vascular dilatation [54].
In a similar vein, whilst differentiating between central serous chorioretinopathy (CSCR) and AMD should be reasonably easy based on angiographic features, the diagnosis of CSCR in patients over the age of 50 years should be made carefully and OCT may be particularly helpful if eyes with chronic CSCR and diffuse leakage. Furthermore, CSCR may be complicated by secondary CNV. Thus, EDI-OCT can be a helpful additional tool to differentiate between AMD and CSCR based on a thicker CT in CSCR [55, 56].
Coscas et al. also compared AMD with adult onset foveomacular vitelliform dystrophy (AOFVD) [57]. The study was divided into five groups of 38 eyes and included AOFVD eyes with fluid accumulation; AOFVD fellow eyes without fluid (early stage); advanced exudative (wet) AMD; advanced dry AMD; and healthy normal eyes. The study found a significantly thicker choroid in the AOFVD group compared to the advanced AMD group which would suggest different pathogenic mechanisms in the two conditions [57]. It is, therefore, possible that CT measurements could also aid to differentiate AOFVD from AMD clinically.
The possible role of OCT imaging in monitoring the response to treatment to anti-VEGF therapy has also been studied; however. study numbers are small and studies are non-randomised [58–60]. Whilst three studies showed a reduction in CT using EDI-OCT after treatment, this effect was not observed in another study [58–60]. Rahman et al. compared patients with neovascular AMD in one eye which had received at least three anti-VEGF injections (n = 15) with patients with newly diagnosed neovascular AMD in one eye which had not received any treatment (N = 15) and demonstrated no significant reduction in CT following treatment [58]. However, Yamazaki et al. compared 40 eyes with neovascular AMD which were treated with ranibizumab and 40 untreated fellow eyes and found a significant reduction in CT in the treated group [59]. Kang et al. also noted a significant reduction in subfoveal CT in eyes with a favourable response (N = 22) to treatment with intravitreal Ranibizumab for neovascular AMD, but no significant change in non-responders (N = 18) where a favourable response to treatment was defined as a 100 μm reduction in subretinal fluid. The authors suggested that CT may be predictive of visual outcome and treatment response in exudative AMD [60]. Conversely, Ellabban et al. studied 20 with AMD, 20 with PCV and 20 with myopic CNV and found that Ranibizumab had a minimal effect on CT. The later study used EDI for imaging eyes with CNV and PCV only, on the basis that the sclera-choroidal interface is easily to identify in highly myopic eyes [61].
Glaucoma
Many earlier histological and angiographic studies have supported the vascular theory in glaucoma in that the choroidal supply to the papillary area may be compromised [62–67]. The anterior region of the optic nerve can be divided into four portions: The superficial nerve fibre layer and prelaminar, laminar, and retrolaminar portions. The prelaminar and laminar regions receive blood via the short posterior ciliary arteries which course through the choroid, but are not directly derived from the choroid [68]. However, Hayrey et al. in 2001 suggested that once the posterior ciliary arteries and the branches of the circle of Haller and Zinn penetrate the sclera, they then become part of the choroid and are no longer considered to be branches of their original arteries [69]. In support of the vascular theory, the Early Manifest Glaucoma Trial demonstrated that lower systolic perfusion pressure, lower systolic pressure, and cardiovascular disease emerged as predictors of disease progression [70].
Two recent studies by Mwanza et al. found no difference in macular CT between eyes with unilateral advanced primary open-angle glaucoma (POAG) (36 subjects) when compared to the fellow eye and found no difference in CT between eyes with POAG, normal tension glaucoma (NTG), and control eyes [71, 72]. The later study included 20 subjects with NGT, 56 subjects with POAG, and 38 control subjects [72]. Similar negative associations between glaucoma severity and macular and peri-papillary CT were found in another study which reviewed 74 glaucoma patients and glaucoma suspects [73]. In contrast, a study by Hirooka et al. compared 45 eyes with NGT with 62 control eyes and found a significant decrease in CT 3 mm nasal to the fovea when compared to the control group, as well as a significant correlation between CT 3 mm nasal to the fovea and the mean deviation slope based on Humphrey 30/2 visual field tests, and suggested a possible association between CT and visual field loss progression [74]. A further study by Hirooka, in 52 NTG subjects and 50 controls, found a significantly thinner inferior peripapillary CT in the NTG eyes, but no correlation between peri-papillary CT and retinal nerve fibre layer (RNFL) thickness by peri-papillary location [75].
Studies carried out to assess CT in patients with angle closure subtypes have found an increase in CT. Huang et al. compared 87 normal control eyes with 210 angle closure subtypes: 73 eyes with primary angle closure suspects, 46 eyes with acute primary angle closure, 35 eyes with primary angle closure, and 56 eyes with primary angle closure glaucoma [76]. After controlling for age and axial length, they found an increased subfoveal CT in all angle closure subtypes compared to control eyes and suggested that choroidal expansion may be a contributing factor to the development of angle closure. Similarly, Zhou found an increase in CT in the fellow eye of those that had experienced acute primary angle closure (44 eyes) compared to 43 control eyes [77].
Whilst EDI-OCT measurement of CT would be an attractive addition to the armamentarium of diagnostic and monitoring tools in glaucoma, the potential role of CT as a risk factor requires further investigation. The relatively higher proportion of patients with glaucoma in a series with age-related choroidal atrophy would support the role of impaired blood supply to the prelaminar optic nerve head in glaucoma, but CT is unlikely to prove to be the major variable in the context of a glaucoma diagnosis [41]. Furthermore, several confounders may bias measurements including blood pressure, refraction, diurnal variation, and operating software [73, 78, 79].
Diabetes
Diabetic retinopathy is in part characterised by capillary non-perfusion and ischaemic retinopathy. Hypoxia stimulates the production of cytokines including VEGF, resulting in retinal neovascularisation and intraretinal oedema [80, 81]. The retina, however, has a dual blood supply, the outer third being supplied by the choroidal capillaries and the inner two thirds being supplied by branches of the central retinal artery [82].
A number of studies have reported on the retinal and choroidal circulation in diabetic retinopathy using various methods including pulsatile ocular blood flow (POBF) and laser Doppler flowmetry. POBF determines the summed volume of blood entering the retina, choroid, and remaining uveal tract by measuring increases in intraocular pressure with each heart pulse. The choroidal circulation is responsible for 85 % of the POBF [83, 84]. Savage demonstrated that POBF was unaffected early in diabetic retinopathy, but increases significantly in eyes with moderate to severe non-proliferative diabetic retinopathy (NPDR), and Mackinnon reported that POBF increased as the severity of diabetic retinopathy progresses [84, 85]. The authors suggested this may be due to VEGF, released locally by the ischemic retina, resulting in vasodilation and increased blood flow. Geyer also demonstrated an initial decrease in POBF, which subsequently increased as the DR worsened [86]. However, Langham reported decreasing POBF with severity of diabetic retinopathy due to increased vascular resistance and increased ocular perfusion pressure [87]. POBF generally reflects the entire choroidal circulation, whereas laser Doppler flowmetry allows evaluation of the foveal region which may be more relevant to the current review of DR as CT measurements are generally taken subfoveally or parafoveally. Using this technique, a number of studies have demonstrated a significant decrease in choroidal blood flow and choroidal volume in the foveal region both in patients with NPDR and proliferative diabetic retinopathy (PDR) [88, 89].
There has been similar controversy in recent studies over the effects of diabetes and DR on CT. Some studies using conventional OCT have reported that in diabetic eyes there is an overall thinning of the choroid possibly due to choriocapillaris atrophy [90, 91]. Esmaeelpour, using longwave OCT (1,060 nm), also demonstrated a reduction in CT in diabetic eyes [92].
Two recent EDI-OCT studies have also demonstrated reduced subfoveal CT in eyes with DR compared to control eyes, but no pronounced thinning was found in the PDR group or, indeed, any significant difference between DR groups [93, 94]. This would be in keeping with the finding of decreased choroidal blood flow and choroidal volume in the foveal region in patients with DR. However, Kim et al. studied 195 treatment-naive eyes, which were divided into no DR, mild/moderate NPDR, severe NPDR, and PDR in addition to 40 eyes with a history of panretinal photocoagulation. In contrast they found that subfoveal CT was slightly greater in eyes with PDR compared to eyes with no DR or with mild-severe NPDR [95]. Although the exact mechanism is unknown, the authors suggested that the increased CT could be a reflection of increased POBF due to the production of VEGF or other cytokines mediating choroidal vasodilation. Furthermore, Kim et al. found that the subfoveal choroid was thicker in eyes with macular oedema than in those without and was thickest in eyes with a foveal serous retinal detachment and postulated a possible association with choroidal ischaemia, RPE dysfunction and increased vascular permeability [95].
Other studies have reviewed patients with diabetes mellitus, but without DR and results have been equally contrasting. Xu et al. in the Beijing Study reported on 246 subjects with diabetes mellitus, 23 of whom had DR [96]. They demonstrated a thicker subfoveal choroid in those with diabetes, whereas the presence and stage of DR were not associated with a variation in subfoveal CT after adjusting for various confounders. Yuluk et al. classified type-2 diabetic patients without DR into three groups according to duration of diabetes Group I (5–9 years, N = 63); Group II (10–14 years, N = 37); Group III (15–40 years, N = 34). While foveal retinal thickness was significantly decreased in patients with longer duration of diabetes, the duration of diabetes did not seem to be related to subfoveal CT [97].
It has been suggested that panretinal photocoagulation (PRP) treatment for diabetic retinopathy results in improved oxygen penetration to the inner retina from the choroidal vessels as a result of the direct destruction of the highly metabolically active photoreceptors in the outer retina [98, 99]. Similarly, studies have demonstrated variations in choroidal blood flow after PRP. Using pneumotonometry, POBF has been shown to decrease significantly 1, 3, and 9 months after PRP [100]. It is possible that the general choroidal blood flow could decrease due to damage to the peripheral choroid from photocoagulation while leaving the foveal region intact [84, 100]. According to a number of histological studies, the choriocapillaris is also compromised beneath the photoreceptor layers [101, 102]. Conversely, using laser Doppler flowmetry, subfoveal choroidal blood flow and volume have been shown to increase 1 month following PRP [103]. Although the exact underlying mechanisms are unclear, it is plausible that redistribution of choroidal blood flow is induced by PRP, both in the peripheral retina and the untreated macular area.
There is limited research assessing CT before and after PRP treatment. As previously mentioned, Kim et al. also assessed 40 eyes with a history of laser-PRP treatment at least 1 year previously and found a significant decrease in subfoveal CT when compared to the untreated PDR group [95]. However, Cho et al. noted an increase in CT in those with severe DR who had undergone three sessions of PRP (28 eyes) with the final session 1 week prior to EDI-OCT assessment [104]. It is, therefore, plausible that the timing of laser-PRP may influence CT measurements.
Further studies that directly investigate the correlation between CT, overall choroidal and subfoveal choroidal blood flow, choriocapillaris density, and vascular diameter would be particularly useful in the setting of diabetes. If there is an association, and in particular, if CT can be established as a proxy measure of choroidal blood flow, then EDI-OCT would play a significant clinical role in the management of patients with DR.
Vogt-Koyanagi-Harada disease
Vogt–Koyanagi–Harada (VKH) disease is a granulomatous inflammatory disorder that affects pigmented structures in genetically susceptible individuals and the choroid is the principle site of associated inflammation [105]. Studies have reported on CT in three or the four phases of VKH; the acute uveitic phase which is characterised by diffuse choroiditis, including focal areas of subretinal fluid and/or serous retinal detachment, the convalescent phase characterised by ocular fundus depigmentation, peripheral nummular chorioretinal depigmented scars, and pigmentary changes in the RPE, and in the chronic recurrent phase which mainly involves anterior uveitis.
Fong et al. first described the typical EDI-OCT findings of the acute phase and convalescent phase of VKH [106]. The authors used EDI-OCT to compare six eyes with VKH with six age- and gender-matched controls and demonstrated an increased CT in the acute phase and loss of focal hyper-reflectivity in the inner choroid leading to the suggestion that this may represent a permanent structural change to the choroid. Although study numbers have been low, studies have also found a reduction in CT in the convalescent phase, which correlated with the degree of depigmentation, and in longstanding recurrent disease was found to be associated with disease duration [107, 108]. The former studies assessed 19 eyes with VKH and 30 eyes with VKH compared with 32 control eyes, respectively. Furthermore, the acute uveitic phase of VKH with associated thickening of the choroid has been attributed not only to inflammatory infiltration, but also to increased exudation.
Thickening of the choroid was subsequently shown to reduce following treatment with corticosteroids in two separate studies which analysed 16 eyes each with VKH [109, 110]. EDI-OCT, therefore, appears to be of value in the assessment of the degree of choroidal involvement in the acute stages of VKH and may have a role in the assessment of response to therapy.
Behçet disease
The cause and the pathogenesis of Behçet disease remain unknown. Both genetic and environmental factors have been implicated, resulting in different manifestations of this multisystem inflammatory disorder. The ocular manifestations of Behçet disease are characterised by severe recurrent attacks of bilateral non-granulomatous panuveitis and retinal vasculitis [111]. While fundus fluorescein angiography remains the gold standard in monitoring inflammatory activity, the hyperfluorescent spots and hypofluorescent plaques, leakage from choroidal vessels, and irregular filling of choriocapillaris detected with ICG angiography suggest choroidal involvement [111, 112].
Kim et al. reported retrospectively on 30 eyes of 30 patients with Behçet’s posterior uveitis [113]. Subfoveal CT was measured using EDI-OCT in the active and quiescent phases of Behçet’s uveitis and compared with an age-, sex-, and spherical equivalent-matched healthy population. This study found choroidal thickening during the active phase of Behcet’s posterior uveitis. Subfoveal CT during the quiescent phase was also significantly greater than in normal eyes. The degree of reduction in choroidal thickening was significantly correlated with improvement in retinal vascular leakage as revealed by fluorescein angiography [113]. This, however, is in contrast to Coskun et al. who studied 35 patients with posterior uveitis (PU) associated with Behçet disease, 35 patients with Behçet disease without ocular involvement, and 30 healthy controls. They found that the choroid was thinner in those with Behçet disease-associated posterior uveitis and no significant difference between those with active disease (N = 26) and those in remission (N = 9) [114]. The above differences in CT could not be explained on the basis of disease duration as one would expect a thinner choroid in eyes with longer disease duration. As study numbers are low and the evidence is conflicting, the potential benefit of EDI-OCT in assessing choroidal morphology in Behçet disease remains unclear.
Choroidal naevi and choroidal tumours
EDI-OCT of the choroid enables evaluation of the thickness and reflective quality of both choroidal naevi and tumours. Shah et al. reviewed images of 51 eyes with choroidal naevi. They demonstrated that EDI-OCT measurements of tumour thickness were comparatively reduced relative to ultrasonography measurements [115]. Subretinal fluid was also identified more reliably with EDI-OCT (16 %) compared to ophthlamoscopic (8 %) and ultrasound evaluation (0 %). Choroidal naevi were found to have overlying choriocapillaris thinning in 94 % of cases. Other common features detected using EDI-OCT included partial (59 %) or complete (35 %) choroidal shadowing deep to the naevus, RPE atrophy (43 %), RPE loss (14 %), RPE nodularity (8 %), photoreceptor loss (43 %), and irregularities in the inner segment-outer segment junction, external limiting membrane, outer nuclear, outer plexiform, and inner nuclear layers [115].
Similarly EDI-OCT has demonstrated an over-estimation in small choroidal melanoma tumour thickness when using ultrasound. Characteristic features when compared with choroidal naevi include an increase in tumour thickness, subretinal fluid, subretinal lipofuscin deposition, and retinal irregularities [116]. A study by Torres et al. reported a series of EDI SD-OCT of 23 choroidal tumours, including small choroidal metastases that were visualized on examination, but undetectable by ultrasound. EDI SD-OCT was able to distinguish between the features of amelanotic and melanotic nevi, choroidal melanomas, hemangiomas, and metastases. Amelanotic naevi demonstrated a homogenous and medium reflective band with visible choroidal vessels; melanotic naevi and choroidal melanomas featured a highly reflective band in the anterior choroid with shadowing, but non-visualization of choroidal vessels and inner sclera; choroidal hemangiomas featured a medium/low reflective band without shadowing and choroidal metastasis showed a low reflective band in the deep choroid with enlargement of the suprachoroidal space [117].
Idiopathic macular hole (IMH)
Although the pathogenesis of IMH principally involves vitreomacular traction, a number of other factors are now considered to play a role such as degenerative macular thinning, macular cyst, intrinsic RPE disease, and systemic vascular disease. Studies have also suggested a contributing role of choroidal perfusion with an associated decrease in foveolar choroidal blood flow [118]. To date, however, EDI-OCT studies have demonstrated variable results. One small study of 12 eyes with IMH found no choroidal thinning and no change in CT following vitrectomy surgery [119]. Two other studies including Reibaldi et al. (22 eyes with IMH and 22 control eyes) and Zeng et al. (50 eyes with IMH and 50 control eyes) found a significant reduction in subfoveal CT in eyes with IMH compared to the unaffected fellow eyes, suggesting that reduced choroidal macular perfusion may contribute to the pathogenesis of IMH [120, 121]. While study numbers were low, the unaffected fellow eye also had reduced subfoveal CT compared to control eyes. The observed differences did not reach statistical significance in one study, thus further study is necessary to evaluate if fellow eyes with a thinner choroid may be prone to IMH [120, 121].
Central serous chorioretinopathy (CSCR)
The pathogenesis of CSCR is thought to involve serous detachment of the neurosensory retina over an area of leakage from the choriocapillaris due to hyperpermeability and distruption of the RPE causing functional loss. EDI-OCT studies have noted an increase in CT in patients with CSCR [56, 122, 123]. Imamura et al. studied 28 eyes of 19 patients with CSCR and suggested that the CT findings provide further evidence that CSCR is caused by focally increased hydrostatic pressure in the choroid [56]. Furthermore, Jirarattanasopa et al. (N = 44) also noted that thickening of the choroid was associated with leakage from the RPE, vascular hyperpermeability, and punctate hyperfluorescent lesions on angiography, and Spaide et al. noted that bilateral involvement is more common in patients over the age of 50 years [122, 123]. Kim et al. compared 30 eyes with unilateral CSCR with 30 controls and demonstrated an increase in CT in CSCR eyes as well as in the unaffected fellow eyes supporting the view that CSCR is a bilateral disorder [124].
Various treatment strategies have been used in the management of CSCR including laser photocoagulation and photodynamic therapy aimed at the underlying zone of choroidal hyperpermeability [125, 126]. A study by Maruko et al. observed 13 patients 1 year after half-dose photodynamic therapy with verteporfin and reported a reduction in CT with associated reduced vascular hyperpermeability on angiography [125]. If these findings are confirmed, then it is conceivable that CT assessment using EDI-OCT would be useful for monitoring treatment response. A similar effect, however, was not observed with laser photocoagulation, which would suggest that the two treatment modalities have differing mechanisms as shown by the reduced choroidal hyperpermeability in PDT-treated eyes only [126].
Retinitis pigmentosa (RP)
RP refers to a group of hereditary retinal disorders characterized by the loss of photoreceptors. The exact pathogenesis is not fully understood, and currently there is no curative treatment. Ocular vascular perfusion has been shown to be disrupted in RP and atrophy of the choriocapillaris has been described. While the reduction in the choroidal perfusion may be secondary to loss of demand associated with retinal atrophy, the exact pathogenic role of the choroid is unclear [127–129]. However, based on ocular pulsatile blood flow and using a mathematical model described by Silver et al. and confocal laser Doppler flowmetry, choroidal blood flow and velocity have been shown to be markedly reduced in RP and to correlate with the severity of RP [129–131].
EDI-OCT studies have demonstrated a reduction in CT in RP [132, 133]. Dhoot et al. compared 21 patients with RP and 25 age-matched controls, and while they found a reduction in CT in the RP group, the correlation with visual acuity was inconsistent [132]. Conversely, Ayton et al. reported on 44 patients with RP compared with 22 controls and found that RP eyes with poorer visual acuity or longer duration of symptoms tended to have thinner choroid and proposed that knowledge of the CT profile in RP is important for the field of restorative vision research [133].
Conclusions
The advent of EDI-OCT imaging of the choroid has enabled new directions in research relating to the normal and pathological roles of the choroid. There are much data confirming that abnormalities in choroidal structure and function contribute to major ocular diseases and patterns of CT variation may be observed in certain disease states and may be influenced by treatment. However, it is not clear whether these variations are a contributing factor or a consequence of disease. There is much speculation regarding the relationship between CT and choroidal blood flow; however, studies that directly investigate the correlation between CT, choroidal blood flow, and choriocapillaris density and vascular diameter are lacking. While invasive methods such as ICG angiography remain the gold standard for detecting abnormalities of the choroidal vasculature, EDI-OCT has become an important adjunctive clinical tool in providing 3-dimensional anatomical information of the choroid.
References
Nickla D, Wallman J (2010) The multifunctional choroid. Prog Retin Eye Res 29(2):144–168
Parver L (1991) Temperature modulating action of choroidal blood flow. Eye 5:181–185
Parver L, Auker C, Carpenter D (1980) Choroidal blood flow as a heat dissipating mechanism in the macula. Am J Ophthalmol 89:641–646
Parver L, Auker C, Carpenter D (1982) The stabilizing effect of the choroidal circulation on the temperature environment of the macula. Retina 2:117–120
Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, Christensen AM (1995) Moving the retina: choroidal modulation of refractive state. Vis Res 35:37–50
Wildsoet C, Wallman J (1995) Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vis Res 35:1175–1194
Alm A, Nilsson F (2009) Uveoscleral outflow: a review. Exp Eye Res 88:760–768
Regatieri C, Branchini L, Fujimoto J, Duker J (2012) Choroidal imaging using spectral domain optical coherence tomography. Retina 32(5):865–876
Spaide R, Koizumi H, Pozzoni M (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146:496–500
Mrejen S, Spaide R (2013) Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol 58(5):387–429
Rahman W, Chen F, Yeoh J, Patel P, Tufail A, Da Cruz L (2011) Repeatability of manual subfoveal choroidal thickness measurements in healthy subjects using the technique of enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci 52(5):2267–2271
Chhablani J, Barteselli G, Wang H, El-Emam S, Kozak I, Doede AL, Bartsch DU, Cheng L, Freeman WR (2012) Repeatability and reproducibility of manual choroidal volume measurements using enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci 53:2274–2280
Karaca EE, Ozdek S, Yalçin NG, Ekici F (2013) Reproducibility of choroidal thickness measurements in healthy Turkish subjects. Eur J Ophthalmol. doi:10.5301/ejo.5000351
Shao L, Xu L, Chen C, Yang LH, Du KF, Wang S, Zhou JQ, Wang YX, You QS, Jonas JB, Wei WB (2013) Reproducibility of subfoveal choroidal thickness measurements with enhanced depth imaging by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 54(1):230–233
Lin P, Mettu P, Pomerleau D, Chiu SJ, Maldonado R, Stinnett S, Toth CA, Farsiu S, Mruthyunjaya P (2012) Image inversion spectral-domain optical coherence tomography optimizes choroidal thickness and detail through improved contrast. Invest Ophthalmol Vis Sci 53:1874–1882
Branchini L, Regatieri C, Flores-Moreno I, Baumann B, Fujimoto J, Duker J (2012) Reproducibility of choroidal thickness measurements across three spectral domain optical coherence tomography systems. Ophthalmology 119:119–123
Yamashita T, Yamashita T, Shirasawa M, Arimura N, Terasaki H, Sakamoto T (2012) Repeatability and reproducibility of subfoveal choroidal thickness in normal eyes of Japanese using different SD-OCT devices. Invest Ophthalmol Vis Sci 53(3):1102–1107
Ramrattan R, van der Schaft T, Mooy C, de Bruijn W, Mulder P, de Jong P (1994) Morphometric analysis of Bruch’s membrane, the choriocapillaris and the choroid in aging. Investig Ophthalmol Vis Sci 35:2857–2864
Margolis R, Spaide R (2012) A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 147:811–815
Wei W, Xu L, Jonas J, Shao L, Du KF, Wang S, Chen CX, Xu J, Wang YX, Zhou JQ, You QS (2012) Subfoveal choroidal thickness: the Beijing eye study. Ophthalmology 120(1):175–180
Li X, Larsen M, Munch I (2011) Subfoveal choroidal thickness in relation to sex and axial length in 93 Danish university students. Invest Ophthalmol Vis Sci 52(11):8438–8441
Tan C, Ouyang Y, Ruiz H, Sadda S (2012) Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence.tomography. Invest Ophthalmol Vis Sci 53(1):261–266
Lee SW, Yu SY, Seo KH, Kim ES, Kwak HW (2014) Diurnal variation in choroidal thickness in relation to sex, axial length and baseline choroidal thickness in healthy Korean subjects. Retina 34(2):385–393
Yin Z, Vaegan M, Beaumont P, Sarks S (1997) Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma 6:23–32
Sogawa K, Nagaoka T, Takahashi A, Tanano I, Tani T, Ishibazawa A, Yoshida A (2012) Relationship between choroidal thickness and choroidal circulation in healthy young subjects. Am J Ophthalmol 153:1129–1132
Grossniklaus HE, Green WR (1992) Pathologic findings in pathologic myopia. Retina 12:127–133
Okabe S, Matsuo N, Okamoto S, Kataoka H (1982) Electron microscopic studies on retinochoroidal atrophy in the human eye. Acta Med Okayama 36:11–21
Moriyama M, Ohno-Matsui K, Futagami S, Yoshida T, Hayashi K, Shimada N, Kojima A, Tokoro T, Mochizuki M (2007) Morphology and long-term changes of choroidal vascular structure in highly myopic eyes with and without posterior staphyloma. Ophthalmology 114:1755–1762
Akyol N, Kükner A, Ozdemir T, Esmerligil S (1996) Choroidal and retinal flow changes in degenerative myopia. Can J Ophthalmol 31:113–119
To’mey K, Faris B, Jalkh A, Nasr A (1981) Ocular pulse in high myopia: a study of 40 eyes. Ann Ophthalmol 13:569–571
Linsenmeier R, Padnick-Silver L (2000) Metabolic dependence of photoreceptors on the choroid in the normal and detached retina. Invest Ophthalmol Vis Sci 41:3117–3123
Fujiwara T, Imamura Y, Margolis R, Slakter J, Spaide R (2009) Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol 148(3):445–450
Flores-Moreno I, Lugo F, Duker J, Ruiz-Moreno J (2012) The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol 155(2):314–319
Yamagishi T, Koizumi H, Yamazaki T, Kinoshita S (2012) Choroidal thickness in inferior staphyloma associated with posterior serous retinal detachment. Retina 32(7):1237–1242
Grunwald J, Hariprasad S, DuPont J (1998) Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci 39(2):385–390
Grossniklaus H, Green W (2004) Choroidal neovascularization. Am J Ophthalmol 137(3):496–503
Friedman E (1997) A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol 124(5):677–682
Klein R, Davis M, Magli Y, Segal P, Klein BE, Hubbard L (1991) The Wisconsin age-related maculopathy grading system. Ophthalmology 98:1128–1134
Sadda S (2011) ‘Under the C-Scan’: Value of Choroidal Imaging in AMD Management. American Academy of Ophthalmology. Orlando. Video Presentations
Zhang L, Lee K, Niemeijer M, Mullins R, Sonka M, Abramoff M (2012) Automated segmentation of the choroid from clinical SD-OCT. Invest Ophthalmol Vis Sci 53:7510–7519
Spaide R (2009) Age-related choroidal atrophy. Am J Ophthalmol 147(5):801–810
Switzer D, Mendonça L, Saito M, Zweifel S, Spaide R (2012) Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina 32(7):1265–1271
Spraul CW, Lang GE, Grossniklaus HE (1996) Morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci 37(13):2724–2735
Sigler EJ, Randolph JC (2013) Comparison of macular choroidal thickness among patients older than age 65 with early atrophic age-related macular degeneration and normal. Invest Ophthalmol Vis Sci 54(9):6307–6313
Lee JY, Lee DH, Lee JY, Yoon YH (2013) Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci. doi:10.1167/iovs. 13-12284
Jonas JB, Forster TM, Steinmetz P, Schlichtenbrede FC, Harder BC (2013) Choroidal thickness in age-related macular degeneration. Retina. [Epub ahead of print]
McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA (2009) Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci 50(10):4982–4991
Bhutto I, Lutty G. (2012) Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Aspects Med.Mol Aspects Med 21;33(4):295–317
Sigler E, Randolph J, Calzada J, Charles S (2014) Smoking and choroidal thickness in patients over 65 with early-atrophic age-related macular degeneration and normal. Eye 28:838–846
Sizmaz S, Küçükerdönmez C, Pinarci EY, Karalezli A, Canan H, Yilmaz G (2013) The effect of smoking on choroidal thickness measured by optical coherence tomography. Br J Ophthalmol 97(5):601–604
Jirarattanasopa P, Ooto S, Nakata I, Tsujikawa A, Yamashiro K, Oishi A, Yoshimura N (2012) Choroidal thickness, vascular hyperpermeability, and complement factor H in age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 53(7):3663–3672
Koizumi H, Yamagishi T, Yamazaki T, Kawasaki R, Kinoshita S (2011) Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 249(8):1123–1128
Chung SE, Kang SW, Lee JH, Kim YT (2011) Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology 118(5):840–845
Yang L, Jonas J, Wei W (2013) Optical coherence tomography enhanced depth imaging of polypoidal choroidal vasculopathy. Retina 33:1584–1589
Kim JH, Kang SW, Kim JR, Kim SJ (2013) Variability of subfoveal choroidal thickness measurements in patients with age-related macular degeneration and central serous chorioretinopathy. Eye (London) 27(7):809–815
Imamura Y, Fujiwara T, Margolis R, Spaide R (2012) Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina 29(10):1469–1473
Coscas F, Puche N, Coscas G, Srour M, Francais C, Glacet-Bernard A, Querques G, Souied E (2014) Comparison of macular choroidal thickness in adult onset foveomacular vitelliform dystrophy and age-related macular degeneration. Invest Ophthalmol Vis Sci 55:64–69
Rahman W, Chen FK, Yeoh J, da Cruz L (2013) Enhanced depth imaging of the choroid in patients with neovascular age-related macular degeneration treated with anti-VEGF therapy versus untreated patients. Graefes Arch Clin Exp Ophthalmol 251(6):1483–1488
Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S (2012) Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology 119(8):1621–1627
Kang HM, Kwon HJ, Yi JH, Lee CS, Lee SC (2014) Subfoveal choroidal thickness as a potential predictor of visual outcome and treatment response after intravitreal ranibizumab injections for typical exudative age-related macular degeneration. Am J Ophthalmol 157(5):1013–1021
Ellabban A, Tsujikawa A, Ogino K, Ooto S, Yamashiro K, Oishi A, Yoshimura N (2012) Choroidal thickness after intravitreal ranibizumab injections for choroidal neovascularization. Clin Ophthalmol 6:837–844
Hamard P, Hamard H, Dufaux J, Quesnot S (1994) Optic nerve head blood flow using a laser doppler velocimeter and haemorheology in primary open angle glaucoma and normal pressure glaucoma. Br J Ophthalmol 78:449–453
Hayreh S (1969) Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol 53:721–748
Hayreh S (1970) Pathogenesis of visual field defects. Role of the ciliary circulation. Br J Ophthalmol 54:289–311
Drance S, Sweeney V, Morgan R, Feldman F (1973) Studies of factors involved in the production of low tension glaucoma. Arch Ophthalmol 89:457–465
Kubota T, Jonas J, Naumann O (1993) Decreased choroidal thickness in eyes with secondary angle closure glaucoma. An aetiological factor for deep retinal changes in glaucoma? Br J Ophthalmol 77:430–432
Haefliger I, Flammer J, Luscher T (1993) Heterogeneity of endothelium-dependent regulation in ophthalmic and ciliary arteries. Invest Ophthalmol Vis Sci 34:1722–1730
Mackenzie P (2008) Vascular anatomy of the optic nerve. Can J Ophthmol 43(3):308–312
Hayreh S (2001) The blood supply of the optic nerve head and the evaluation of it-myth and reality. Prog Retin Eye Res 20(5):563–593
Leske M, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z (2007) EMGT Group. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology 114:1965–1972
Mwanza J, Sayyad F, Budenz D (2012) Choroidal thickness in unilateral advanced glaucoma. Invest Ophthalmol Vis Sci 53(10):6695–6701
Mwanza J, Hochberg J, Banitt M, Feuer W, Budenz D (2011) Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth imaging optical coherence tomography. Invest Ophthalmol Vis Sci 52(6):3430–3435
Maul E, Friedman D, Chang D, Boland MV, Ramulu PY, Jampel HD, Quigley HA (2011) Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology 118:1571–1579
Hirooka K, Fujiwara A, Shiragami T, Baba T, Shiraga F (2012) Relationship between progression of visual field damage and choroidal thickness in eyes with normal-tension glaucoma. Clin Exp Ophthalmol 40(6):576–582
Hirooka K, Tenkumo K, Fujiwara A, Baba T, Sato S, Shiraga F (2012) Evaluation of peripapillary choroidal thickness in patients with normal-tension Glaucoma. BMC Ophthalmol. doi:10.1186/ 1471-2415-12-29
Huang W, Wang W, Gao X, Li X, Li Z, Zhou M, Chen S, Zhang X (2013) Choroidal thickness in the subtypes of angle closure: an EDI-OCT study. Invest Ophthalmol Vis Sci 54(13):7849–7853
Zhou M, Wang W, Ding X, Huang W, Chen S, Laties AM, Zhang X (2013) Choroidal thickness in fellow eyes of patients with acute primary angle-closure measured by enhanced depth imaging spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 54(3):1971–1978
Rhew J, Kim Y, Choi K (2012) Measurement of subfoveal choroidal thickness in normal-tension glaucoma in Korean patients. J Glaucoma 23(1):46–49
Fénolland J, Giraud J, Maÿ F, Mouinga A, Seck S, Renard J (2011) Enhanced depth imaging of the choroid in open-angle glaucoma: a preliminary study. J Fr Ophtalmol 34(5):313–317
Silvestre J, Lévy B (2006) Molecular basis of angiopathy in diabetes mellitus. Circ Res 98:4–6
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Harris A, Gingaman P, Ciulla A, Martin B (2001) Retinal and choroidal blood flow in health and disease. In: Ryan SJ (ed) The retina, 3rd edn. Mosby, St Louis, pp 68–88
Langham ME, Farrell RA, O’Brien V, Silver DM, Schilder P (1998) Blood flow in the human eye. Acta Ophthalmol 191:9–13
Savage H, Hendrix J, Peterson D, Young H, Wilkinson C (2004) Differences in pulsatile ocular blood flow among three classifications of diabetic retinopathy. Invest Ophthalmol Vis Sci 45(12):4504–4509
MacKinnon J, O’Brien C, Swa K, Aspinall P, Butt Z, Cameron D (1997) Pulsatile ocular blood flow in untreated diabetic retinopathy. Acta Ophthalmol Scand 75:661–664
Geyer O, Neudorfer M, Snir T, Goldstein M, Rock T, Silver DM, Bartov E (1999) Pulsatile ocular blood flow in diabetic retinopathy. Acta Ophthalmol Scand 77:522–525
Langham ME, Grebe R, Hopkins S, Marcus S, Sebag M (1991) Choroidal blood flow in diabetic retinopathy. Exp Eye Res 52(2):167–173
Nagaoka T, Kitaya N, Sugawara R, Yokota H, Mori F, Hikichi T, Fujio N, Yoshida A (2004) Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol 88(8):1060–1063
Schocket L, Brucker A, Niknam R, Grunwald J, DuPont J, Brucker A (2004) Foveolar choroidal hemodynamics in proliferative diabetic retinopathy. Int Ophthalmol 25(2):89–94
Regatieri C, Branchini L, Carmody J, Fujimoto J, Duker J (2012) Choroidal thickness in patients with diabetic retinopathy analysed by spectral-domain optical coherence tomography. Retina 32(3):563–568
Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E (2012) Macular and peripapillary choroidal thickness in diabetic patients. Retina 32(9):1781–1790
Esmaeelpour M, Považay B, Hermann B, Hofer B, Kajic V, Hale SL, North RV, Drexler W, Sheen NJ (2011) Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm Optical coherence tomography. Invest Ophthalmol Vis Sci 52:5311–5316
Querques G, Lattanzio R, Querques L, Del Turco C, Forte R, Pierro L, Souied EH, Bandello F (2012) Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci 53:6017–6024
Lee Kyung H, Won Lim J, Cheol Shin M (2013) Comparison of choroidal thickness in patients with diabetes by spectral-domain optical coherence tomography. Korean J Ophthalmol 27(6):433–439
Kim J, Lee D, Joe S, Kim J, Yoon Y (2013) Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci 54(5):3378–3384
Xu J, Xu L, Du KF, Shao L, Chen CX, Zhou JQ, Wang YX, You QS, Jonas JB, Wei WB (2013) Subfoveal choroidal thickness in diabetes and diabetic retinopathy. Ophthalmology 120(10):2023–2028
Yülek F, Uğurlu N, Onal ED, Kocamış SI, Cağıl N, Ersoy R, Cakır B (2013) Choroidal changes and duration of diabetes. Semin Ophthalmol 29(2):80–84
Stefansson E, Machemer R, de Juan E Jr, McCuen BW 2nd, Peterson J (1992) Retinal oxygenation and laser treatment in patients with diabetic retinopathy. Am J Ophthalmol 113:36–38
Stefansson E, Landers MB III, Wolbarsht ML (1981) Increased retinal oxygen supply following pan-retinal photocoagulation and vitrectomy and lensectomy. Trans Am Ophthalmol Soc 79:307–334
Sandhu R, Sivaprasad S, Chong V (2005) Pulsatile ocular blood flow changes after pan–retinal photocoagulation and macular laser for diabetic retinopathy – results at 9 months follow–up. Invest Ophthalmol Vis Sci 46: E-Abstract 385
Lee C, Smith J, Kang-Mieler J, Budzynski E, Linsenmeier R (2011) Decreased circulation in the feline choriocapillaris underlying retinal photocoagulation lesions. Invest Ophthalmol Vis Sci 52(6):3398–3403
Stitt A, Gardiner T, Archer D (1995) Retinal and choroidal responses to panretinal photocoagulation: an ultrastructural perspective. Graefes Arch Clin Exp Ophthalmol 233(11):699–705
Takahashi A, Nagaoka T, Sato E, Yoshida A (2008) Effect of panretinal photocoagulation on choroidal circulation in the foveal region in patients with severe diabetic retinopathy. Br J Ophthalmol 92:1369–1373
Cho G, Cho H, Kim Y (2013) Change in subfoveal choroidal thickness after argon laser panretinal photocoagulation. Int J Ophthalmol 6(4):505–509
Rao NA (2007) Pathology of Vogt–Koyanagi–Harada disease. Int Ophthalmol 27:81–85
Fong AH, Li KK, Wong D (2011) Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi-Harada disease. Retina 31(3):502–509
Takahashi H, Takase H, Ishizuka A, Miyanaga M, Kawaguchi T, Ohno-Matsui K, Mochizuki M (2013) Choroidal thickness in convalescent Vogt-Koyanagi-Harada Disease. Retina 34(4):775–780
da Silva FT, Sakata VM, Nakashima A, Hirata CE, Olivalves E, Takahashi WY, Costa RA, Yamamoto JH (2013) Enhanced depth imaging optical coherence tomography in long-standing Vogt-Koyanagi-Harada disease. Br J Ophthalmol 97(1):70–74
Nakayama M, Keino H, Okada AA, Watanabe T, Taki W, Inoue M, Hirakata A (2012) Enhanced depth imaging optical coherence tomography of the choroid in Vogt-Koyanagi-Harada disease. Retina 32(10):2061–2069
Maruko I, Iida T, Sugano Y, Oyamada H, Sekiryu T, Fujiwara T, Spaide RF (2011) Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina 31(3):510–517
Matsuo T, Sato Y, Shiraga F, Shiragami C, Tsuchida Y (1999) Choroidal abnormalities in Behçet disease observed by simultaneous indocyanine green and fluorescein angiography with scanning laser ophthalmoscopy. Ophthalmology 106(2):295–300
Atmaca LS, Sonmez PA (2003) Fluorescein and indocyanine green angiography findings in Behçet’s disease. Br J Ophthalmol 87(12):1466–1468
Kim M, Kim H, Kwon HJ, Kim SS, Koh HJ, Lee SC (2013) Choroidal thickness in Behcet’s uveitis: an enhanced depth imaging-optical coherence tomography and its association with angiographic changes. Invest Ophthalmol Vis Sci 54(9):6033–6039
Coskun E, Gurler B, Pehlivan Y, Kisacik B, Okumus S, Yayuspayı R, Ozcan E, Onat AM (2013) Enhanced depth imaging optical coherence tomography findings in behçet disease. Ocul Immunol Inflamm 21(6):440–445
Shah SU, Kaliki S, Shields CL, Ferenczy SR, Harmon SA, Shields JA (2012) Enhanced depth imaging optical coherence tomography of choroidal nevus in 104 cases. Ophthalmology 119(5):1066–1072
Shields C, Kaliki S, Rojanaporn D, Ferenczy S, Shields J (2012) Enhanced depth imaging optical coherence tomography of small choroidal melanoma comparison with choroidal nevus. Arch Ophthalmol 130(7):850–856
Torres VL, Brugnoni N, Kaiser PK, Singh AD (2011) Optical coherence tomography enhanced depth imaging of choroidal tumors. Am J Ophthalmol 151(4):586–593
Aras C, Ocakoglu O, Akova N (2004) Foveolar choroidal blood flow in idiopathic macular hole. Int Ophthalmol 25(4):225–231
Schaal K, Pollithy S, Dithmar S (2012) Is choroidal thickness of importance in idiopathic macular hole? Ophthalmologe 109(4):364–368
Reibaldi M, Boscia F, Avitabile T, Uva MG, Russo V, Zagari M, Bonfiglio V, Reibaldi A, Longo A (2011) Enhanced depth imaging optical coherence tomography of the choroid in idiopathic macular hole: a cross-sectional prospective study. Am J Ophthalmol 151(1):112–117
Zeng J, Li J, Liu R et al (2012) Choroidal thickness in both eyes of patients with unilateral idiopathic macular hole. Ophthalmology 119(11):2328–2333
Jirarattanasopa P, Ooto S, Tsujikawa A, Yamashiro K, Hangai M, Hirata M, Matsumoto A, Yoshimura N (2012) Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology 119(8):1666–1678
Spaide R, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR, Slakter JS, Sorenson JA, Orlock DA (1996) Central serous chorioretinopathy in younger and older adults. Ophthalmology 103(12):2070–2079
Kim Y, Kang S, Bai K (2011) Choroidal thickness in both eyes of patients with unilaterally active central serous chorioretinopathy. Eye 25(12):1635–1640
Maruko I, Lida T, Sugano Y, Furuta M, Sekiryu T (2011) One-year choroidal thickness results after photodynamic therapy for central serous chorioretinopathy. Retina 31(9):1921–1927
Maruko I, Lida T, Sugano Y, Ojima A, Ogasawara M, Spaide R (2010) Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology 117(9):1792–1799
Grunwald J, Maguire A, Dupont J (1996) Retinal hemodynamics in retinitis pigmentosa. Am J Ophthalmol 122:502–508
Schmidt K, Pillunat L, Kohler K, Flammer J (2001) Ocular pulse amplitude is reduced in patients with advanced retinitis pigmentosa. Br J Ophthalmol 85:678–682
Langham M, Kramer T (1990) Decreased choroidal blood flow associated with retinitis pigmentosa. Eye (London) 4:374–381
Falsini B, Anselmi GM, Marangoni D, D’Esposito F, Fadda A, Di Renzo A, Campos EC, Riva CE (2011) Subfoveal choroidal blood flow and central retinal function in retinitis pigmentosa. Invest Ophthalmol Vis Sci 52:1064–1069
Silver DA, Farrell RA, Langham ME, O’Brien V, Schilder P (1989) Estimation of pulsatile ocular blood flow from intraocular pressure. Acta Ophthalmol (Supplement) 191: 67: 25–29
Dhoot D, Huo S, Yuan A, Xu D, Srivistava S, Ehlers JP, Traboulsi E, Kaiser PK (2012) Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. Br J Ophthalmol 97(1):66–69
Ayton L, Guymer R (2012) Choroidal thickness profiles in retinitis pigmentosa clinical and experimental. Ophthalmology 41(4):396–403
Acknowledgments
Contributions to authors in each of these areas: Design and conduct of the study (HL, HZ); Collection, management, analysis, and interpretation of the data (HL, HZ); Preparation, review, and approval of manuscript (HL, HZ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laviers, H., Zambarakji, H. Enhanced depth imaging-OCT of the choroid: a review of the current literature. Graefes Arch Clin Exp Ophthalmol 252, 1871–1883 (2014). https://doi.org/10.1007/s00417-014-2840-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2840-y