Abstract
Purpose
The purpose is to study the effect of Enhanced Extracorporeal Counterpulsation (EECP) in patients with non-arteritic anterior ischaemic optic neuropathy (NAION).
Methods
EECP is a noninvasive, mechanical, and circulatory support therapy. Sixteen patients with unilateral NAION were treated with EECP (twelve 1-h daily treatment sessions for each patient). Color Doppler imaging (CDI) was applied to measure the mean flow velocity (MFV), peak-systolic velocity (PSV), end-diastolic velocity (EDV) of the ophthalmic artery (OA) and central retinal artery (CRA). Intraocular pressure (IOP) was measured by Goldmann applanation tonometry. Measurements were collected before and immediately after the first and the last sessions of EECP in both eyes, and they were compared with the baseline measurement before EECP. The measurements were also compared between the NAION eyes and the normal fellow eyes. Visual acuity (VA) and visual field (VF) were assessed before EECP and after the last EECP.
Results
EECP progressively increased blood flow velocities of the OA and CRA and progressively decreased IOP in both eyes (P < 0.05). After the first session of EECP, there was a 16 ± 5.3 % increase in EDV and a 13.9 ± 9.5 % increase in MFV of the OA, and a 17.1 ± 2.5 % increase in PSV, a 21.2 ± 9.3 % increase, in EDV and a 16.5 ± 3.3 % increase in MFV of the CRA in NAION eyes. After the last EECP treatment, there was a 16.8 ± 6.7 % increase in EDV and a 14.0 ± 5.1 % increase in MFV of the OA, and a 17.7 ± 12.3 % increase in PSV, a 23.1 ± 6.3 % increase in DSV, and a 21.1 ± 8.4 % increase in MFV of the CRA in NAION eyes (P < 0.05). The change of the PSV, EDV, and MFV in the CRA were more significant in NAION eyes than that of their fellow eyes (P < 0.05). VA was improved and VF mean deviation was decreased in NAION eyes after the last EECP treatment (P = 0.003 and 0.049, respectively), and VA improvement was correlated positively with the blood flow parameter.
Conclusions
EECP could be a clinically effective and safe treatment for NAION.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-arteritic anterior ischaemic optic neuropathy (NAION) is the most common type of acute optic neuropathy in individuals over 50 years of age [1, 2]. An acute incident of hypoperfusion is one of the primarily risk factors for NAION [1]. Color Doppler imaging (CDI) on NAION patients has shown markedly different retrobulbar haemodynamics with reduced circulation velocity in orbital vessels [3, 4]. Approximately 15 % of patients developed NAION in the fellow eye within five years [2]. Currently, there is no effective treatment for acute NAION and no preventive method for the involvement of fellow eyes.
The enhanced extracorporeal counterpulsation (EECP) device is a noninvasive mechanical circulatory support device consisting of a series of cuffs that are wrapped around the patient’s lower body (shank, thigh, and buttocks), with a specially made airbag that exerts sequential compression on the lower body during ventricular diastole. Sequentially, the aortic diastolic pressure and cardiac output are increased by the back flow of artery blood and the increased venous return to the heart. Thus, the blood perfusion of ischemic organs can be improved. Currently, EECP is used as an adjunctive therapy for patients with myocardial ischemia and/or ischemic stroke [5–7]. EECP significantly increases the blood flow velocity of the ophthalmic artery in elderly patients with atherosclerosis [8]. Furthermore, Werner et al., reported that the reperfusion of the affected retina in central retinal artery occlusion and branch retinal artery occlusion was accelerated after a short-term EECP treatment [9]. Since occlusive vasculopathy of the microcirculation may be one proposed mechanism for NAION, which may be related to the decreased blood flow velocity in the retrobulbar vessels [3, 4], EECP may have a favorable effect on NAION through the augmentation of retrobulbar blood flow.
However, little is known about the effect of EECP on NAION. In the present study, we measured the blood flow velocity of the ophthalmic artery (OA) and central retinal artery (CRA) in patients over 50 years of age who were diagnosed with acute unilateral NAION. The ocular blood flow velocity, visual function, and intraocular pressure (IOP) were measured before and after the EECP treatment to assess the effect of EECP in these patients. To the best of our knowledge, this is the first study to observe the effect of EECP on NAION.
Materials and methods
This prospective study was approved by the Ethics Committee of the Sun Yat-sen University. Written consents were obtained from all study participants according to the Declaration of Helsinki. Sixteen patients (from 50 to 78 years old, mean age 65.70 ± 4.30 years) with acute unilateral NAION were enrolled in the study from December 1, 2010 to March 31, 2014. They were patients who visited the First Affiliated Hospital of Sun Yat-sen University and Zhongshan ophthalmic center without being admitted to other medical facilities. All these patients presented acute painless loss of VA, relative afferent pupillary defect, optic disc oedema, visual field (VF) defect, and the early phase filling defect of the optic disc (by fundus fluorescein angiography, FFA) in the affected eyes without evidence of other neurological, systemic, or ocular diseases. The fellow eyes showed normal situations after the IOP, VA, VF, funduscopy, and FFA examination. The exclusion criteria included: 1) patients with an elevated erythrocyte sedimentation rate, history of giant cell arteritis or relevant clinical features, glaucoma or severe cataract; 2) patients with history of corneal surgery and/or intraocular surgery recently (one month), and those who had erythralgia of the eye; 3) patients who had aortic regurgitation, frequent premature ventricular complexes (>10/min), thrombophlebitis or infectious lesions, and severe hypertension (>170/100 mmHg), which could not be controlled by medications.
Patients were treated with 1-h EECP daily (the same protocol for all patients) for 12 consecutive weekdays [5, 6]. The study was conducted at the center of EECP, the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China). The EECP device (Shuangshan EECP-MCI, Guangzhou, China) was composed of an air compressor, a set of cuffs, a computer module, and a treatment table. The cuffs were connected by air hoses to the air compressor unit, and were wrapped around the calves and upper thighs (including the buttocks) of patients. The EECP device inflates cuffs with air and then deflates them in a sequence that is synchronized to the patient’s cardiac cycle. The cuffs were sequentially inflated with compressed air from the distal area of the body to the proximal area of the body in early ventricular diastole and rapidly deflated right before ventricular systole. The pressure applied to the cuffs was set at 0.035 to 0.040 mPa/cm2. Medications, which the patients used, were not changed during the study.
A thorough ophthalmological examination, including the assessment of Snellen VA, was performed on all patients. IOP was measured by Goldmann applanation tonometry (AIT-20, TOPCON, Japan) before CDI examination, as well as before and immediately after the first 1 h and last 1 h EECP. VF was measured by the Humphrey perimeter (Model 740i, Humphrey–Zeiss, San Leandro, California, USA). The first examination of VA and VF were performed before EECP. A second test of VA and VF was carried out after the last EECP treatment. All patients were fasting and refrained from exercise, smoking, and drinking coffee or alcohol for at least 2 h prior to the first CDI examination. Velocities of blood flow in the OA and CRA were measured using a color Doppler unit (PROSOUND SSD—5500SV, ALOKA, Japan) by an experienced radiologist who was blinded to the condition of each patient. Velocities of blood flow were measured again immediately after the first 1 h and last 1 h EECP (after the measurement of IOP). Patients received CDI in the supine position. A 7.5 MHZ linear-array transducer (4–13MHZ, UST-5410, Japan) was applied on bilateral closed eyelids using a sterile coupling gel with the examiner’s hand resting on the orbital margin to minimize the pressure on the eyes. We obtained peak-systolic velocity (PSV) and end-diastolic velocity (EDV) measurements of bilateral OA and CRA in all patients. The mean flow velocity (MFV) was calculated with the formula: MFV = 1/3 (PSV − EDV) + EDV [10].
Complaints after EECP were taken from all of the study participants. Funduscopy was performed after EECP to examine whether fundus bleeding had happened after the elevation of blood pressure.
Continuous variable data in our study were presented as mean ± SD. Snellen VA was converted to the logarithm of the minimum angle of resolution (logMAR) scale. The Mann–Whitney U test was used to compare VA and VF before and after EECP. A generalized estimating equation was used for analyzing the repeated measurement data. The baseline data (before EECP) were also compared to the data from sequential time points. Multiple linear regression models were performed to identify the correlations of the variability of blood flow parameter with IOP changes, with duration between symptom onset and beginning of treatment, and with VA and VF changes. Statistical analyses were performed with SPSS (Version 19.0, Chicago, USA), and a p-value less than 0.05 was considered statistically significant.
Results
The interval from onset to treatment ranged from six to 18 days and the mean interval from the onset to the first treatment was 12.23 ± 2.32 days (Table 1). EECP was well tolerated by the patients. Only one patient complained of pain in the lower legs from a skin abrasion induced by EECP. No retinal bleeding was observed in the study.
Blood flow velocities
From the first EECP to the last EECP treatment, the EDV and MFV of the OA were increased significantly and progressively in both eyes of the patients, as well as the PSV, EDV, and MFV of the CRA (Tables 2, 3 and 4, Figs. 1, 2 and 3). After the first hour of EECP, there was a 16 ± 5.3 % increase in EDV and a 13.9 ± 9.5 % increase in MFV of the OA, and a 17.1 ± 2.5 % increase in PSV, a 21.2 ± 9.3 % increase in EDV, and a 16.5 ± 3.3 % increase in MFV of the CRA in NAION eyes. After the last EECP treatment, there was a 16.8 ± 6.7 % increase in EDV and a 14.0 ± 5.1 % increase in MFV of the OA, a 17.7 ± 12.3 % increase in PSV, a 23.1 ± 6.3 % increase in DSV, and a 21.1 ± 8.4 % increase in MFV of the CRA in NAION eyes (P < 0.05). Compared to normal fellow eyes, the change of PSV, EDV, and MFV in the CRA of NAION eyes were significantly increased (P < 0.05), while the change of blood flow velocities in the OA showed no statistically significant differences. The PSV of the OA after EECP showed a tendency to be lower than that of baseline in both eyes, but no statistically significant differences were observed (P > 0.05, Table 2).
Changes of the mean systolic flow velocity, mean diastolic flow velocity, and mean flow velocity in the ophthalmic artery (OA) and central retinal artery (CRA) after the first hour of Enhanced Extracorporeal Counterpulsation (EECP) in patients with non-arteritic anterior ischaemic optic neuropathy (NAION) (%)
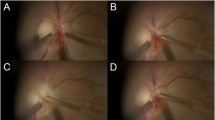
Color Doppler imaging (CDI) of one female patient with unilateral NAION in the affected eye after the first and last EECP treatments. a CDI of the OA of the NAION eye after the first hour EECP; b CDI of the OA of the NAION eye after the last EECP treatment. Arrow indicates that the blood flow velocity was augmented by EECP; c CDI of the CRA in the NAION eye after the first hour EECP; d CDI of the CRA in the NAION eye after the last EECP treatment. Arrow indicates that the blood flow velocity was augmented by EECP
IOP
IOP measured before EECP ranged from 13 to 20 mmHg (mean 17.31 ± 2.41 mmHg). There were no significant differences of IOP measurements between NAION eyes and normal fellow eyes before EECP (P = 0.65). The effect of EECP on IOP of the patients is shown in Table 5. IOP of both eyes was progressively decreased from the first EECP to the last EECP treatment, and differences between IOP before and after EECP were statistically significant (NAION eyes, P < 0.001; normal eyes, P < 0.01). No significant differences were found between IOP of NAION eyes and IOP of normal fellow eyes after EECP (P = 0.58).
For IOP changes and duration between symptom onset and beginning of treatment, their correlations with the variability of blood flow parameter were not statistically significant (P > 0.05).
Visual acuities
The median VA in NAION eyes before EECP was 20/120, ranging from hand movements to 20/30. The median logMAR VA in NAION eyes before EECP was 0.92 (±0.94) [0.32; 1.92] (median ± standard deviation and interquartile range P25–P75 [IQR]) and the median logMAR VA in NAION eyes after the last EECP treatment was 0.40 (±0.49) [0.22; 0.90]. Statistical analysis showed a significant difference in median change in VA in NAION eyes between before EECP and right after 12 h EECP (P = 0.003). Median change in VA in NAION eyes was significantly improved right after 12 h EECP. A significant increase in VA from finger counting to above 20/40 in NAION eyes occurred in three patients. Of the total 16 NAION eyes, ten eyes (62.5 %) obtained improvement of three or more Snellen lines and none was observed with decrease in VA.
Visual field
Median VF mean deviation (MD) in NAION eyes before EECP was −15.90 dB (SD 5.81), and median VF pattern standard deviation (PSD) was 10.55 dB (SD 2.73). Median VF MD after the 12 h EECP was −15.14 dB (SD 5.02) and median VF PSD was 10.99 dB (SD3.40). Statistical analysis showed a significant difference in median MD in NAION eyes between before EECP and right after 12 h EECP (P = 0.049). Median change in PSD was not statistically different between before EECP and right after 12 h EECP (P = 0.877).
VA was significantly associated with the variability of the blood flow parameter in the affected eyes. (P < 0.05) The correlation of PSD and MD with the variability the of blood flow parameter was not statistically significant (P > 0.05).
Discussion
A study reported that EECP could improve the cerebral perfusion in ischemic stroke patient with intracranial arterial occlusive disease [7]. The therapeutic effects of EECP have been documented in increasing shear stress of blood flow, resulting in the release of vasodilating factors (e.g., nitric oxide, prostacyclin) and antithrombotic factors from the vascular endothelial cells and platelets, contributing to reopening of the occluded arteries and increasing collateral perfusion [5, 11–14]. However, reports of the retrobulbar haemodynamics and IOP alterations in patients with NAION during EECP are rare. In this prospective study for elderly patients with acute unilateral NAION, we demonstrated that EECP progressively increased blood flow velocities of the OA and CRA, and progressively decreased IOP in both eyes. Furthermore, the visual function in the affected eyes of NAION patients was significantly improved after 12 h EECP in our study.
Werner et al., reported a greater increase in EDV and a smaller reduction in PSV in the OA of elderly patients with atherosclerosis before and during EECP [8]. Our results of flow velocities in the OA of elderly patients with acute unilateral NAION before and after EECP are consistent with their report. Werner et al., also reported that the flow pattern of the OA showed a characteristic second diastolic wave in all subjects during EECP [8]. However, this pattern was not found immediately after EECP in our patients.
The cause of progressive reduction of IOP after EECP in our study is unclear. EECP augmented the ocular blood flow velocities, which may lessen the venous blood stasis and reduce the outflow resistance of aqueous humor (flows through trabecular meshwork and sclera venous), eventually resulting in reduced IOP. However, although in a previous report, EECP may augment cerebral arterial blood flow [7], no evidence was found to support that EECP increased cerebral venous outflow, which requires further investigation. Another possible mechanism is the reduced systemic systolic pressure after EECP [15], which is associated with reduced IOP [16–18]. However, further study is also necessary for the correlation, especially long-term correlation, between IOP and blood pressure.
In the present study, we found that EECP progressively increased blood flow velocities of the OA and CRA and decreased IOP in both eyes. Reduction of IOP and improvement of ocular blood flow were also reported in the literature [19, 20] that assessed the effect of IOP-lowering eye drops on eyes of primary glaucoma patients. However, the correlation between reduction of IOP and ocular blood flow was rarely studied. It has been reported that ocular perfusion pressure (OPP) increases with the decrease of IOP [21]. The blood flow velocities of the CRA (e.g., PSV) could be increased when OPP was elevated due to the positive correlation between them [22], which therefore may reveal the association between blood flow and changes of IOP. Although the ocular blood flow in our study was not correlated with the reduction of IOP, their correlation is worth further investigation.
Although flow velocities of the CRA in both eyes were progressively increased by EECP, more significant increases were found in the change of flow velocities of the CRA in NAION eyes than that of the normal fellow eyes. This may be explained by the blood perfusion in ischemic eyes that was significantly increased by EECP compared to that in normal eyes. In a previous study, an increase in blood flow occurred in ischemic retinal tissue after EECP, but no change of perfusion was found in the surrounding nonischemic retinal tissue [9]. Therefore, the results from our study and Werner et al., indicate that ischemia and/or impaired blood vessel autoregulation may make the ischemic tissue a priority region for rapid reperfusion after EECP.
EECP is a noninvasive, safe, and well-tolerated procedure with very few contraindications. In the literature, only minor EECP device-related side effects (e.g., pain in the legs or skin abrasion) were reported [6]. No retinal bleeding caused by the procedure was observed [9]. In our study, all patients wore fitted cotton trousers and air bags were wrapped properly with bone salient placed above pads during the treatment. Only one patient complained of the pain in the lower legs, which was caused by a skin abrasion induced by EECP. No retinal bleeding induced by EECP was observed in our study, suggesting that EECP is safe in these patients. Furthermore, there was significant improvement in VA in NAION eyes of the patients after EECP treatment, and the VA improvement was correlated positively with the blood flow parameter. We also found a significant difference in median MD in NAION eyes after 12 h EECP. These findings may further indicate the effect of EECP on NAION eyes. However, the clinical significance of these changes, especially the change in VF, is still minimal. Further studies are required to verify these findings, and to justify the clinical impact of such changes in NAION patients.
The pathogenic concept of an acute NAION includes the acute hypoperfusion of short posterior ciliary arteries (SPCAs) [1, 2, 23]. However, it is impossible for CDI to differentiate the individual paraoptic branches of SPCAs. The values obtained from CDI represent “acceptable” waveforms from bundles of vessels rather than individual vessels [24]. The anatomy of SPCAs is highly variable between different individuals, and anastomoses and narrowing are commonly observed [25]. Therefore, SPCAs were not included in our study since these data cannot be measured individually by using CDI. Since EECP therapy could produce the vibration of body and head, measurements of IOP and ocular blood flow velocities during EECP were not recorded in the present study. In the future, more indices for blood flow comparison other than blood flow velocities alone should provide more insights into this treatment. The treatment duration was based on prior clinical studies in chronic angina (thirty-five 1-h daily treatment sessions) [5, 6]. However, considering that EECP is a long-term treatment method, in the present study, which is an initial trial of EECP therapy on NAION, we used a less but effective amount of treatment with 12 sessions. In future study, long-term treatment and randomly controlled clinical trials should be performed on a large sample size, and the research period may also be extended to days or weeks after EECP so as to investigate the sustained effect of EECP on NAION patients.
In conclusion, in the present study, EECP progressively increases the blood flow velocities of the OA and CRA in both eyes of acute unilateral NAION patients. The extent of ocular blood flow velocities augmentation immediately after EECP appears to be more significant in NAION eyes than that in fellow eyes. EECP could be a clinically effective and safe procedure for patients with NAION.
References
Hayreh SS, Podhajsky P, Zimmerman MB (1999) Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica 213(2):76–96
Newman NJ, Scherer R, Langenberg P et al (2002) The fellow eye in NAION: report from the ischemic optic neuropathy decompression trial follow-up study. Am J Ophthalmol 134(3):317–328
Kaup M, Plange N, Arend KO, Remky A (2006) Retrobulbar haemodynamics in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol 90(11):1350–1353
Sanjari MS, Falavarjani KG, Mehrabani M, Ghiasian L, Zamani B (2009) Retrobulbar haemodynamics and carotid wall thickness in patients with non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol 93(5):638–640
Braith RW, Conti CR, Nichols WW, Choi CY, Khuddus MA, Beck DT, Casey DP (2010) Enhanced external counterpulsation improves peripheral artery flow-mediated dilation in patients with chronic angina: a randomized sham-controlled study. Circulation 122(16):1612–1620
Rohit R, Tony M, Jain D et al (1999) The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol 33(7):1833–1840
Lin W, Xiong L, Han J et al (2012) External counterpulsation augments blood pressure and cerebral flow velocities in ischemic stroke patients with cerebral intracranial large artery occlusive disease. Stroke 43(11):3007–3011
Werner D, Michelson G, Harazny J, Michalk F, Voigt JU, Daniel WG (2001) Changes in ocular blood flow velocities during external counterpulsation in healthy volunteers and patients with atherosclerosis. Graefes Arch Clin Exp Ophthalmol 239(8):599–602
Werner D, Michalk F, Harazny J, Hugo C, Daniel WG, Michelson G (2004) Accelerated reperfusion of poorly perfused retinal areas in central retinal artery occlusion and branch retinal artery occlusion after a short treatment with enhanced external counterpulsation. Retina 24(4):541–547
Gosling RG, King DH (1974) Arterial assessment by Doppler-shift ultrasound. Proc R Soc Med 67(6 Pt 1):447–449
Akhtar M, Wu GF, Du ZM, Zheng ZS, Michaels AD (2006) Effect of external counterpulsation on plasma nitric oxide and endothelin-1 levels. Am J Cardiol 98(1):28–30
Lu L, Zheng ZS, Zhang MQ, Lawson WE, Hui JCK (2001) Beneficial effects of EECP on the renin-angiotensin system in patients with coronary artery disease. Eur Heart J 22(Suppl):538
Wu GF, Zheng QS, Zheng ZS, Lawson WE, Hui JCK (1999) A neurohumoral mechanism for the effectiveness of enhanced external counterpulsation. Circulation 100(Suppl 1):832
Zhang Y, He X, Chen X et al (2007) Enhanced external counterpulsation inhibits intimal hyperplasia by modifying shear stress responsive gene expression in hypercholesterolemic pigs. Circulation 116(5):526–534
Bondesson S, Pettersson T, Ohlsson O, Hallberg IR, Wackenfors A, Edvinsson L (2010) Effects on blood pressure in patients with refractory angina pectoris after enhanced external counterpulsation. Blood Press 19(5):287–294
Klein BE, Klein R, Knudtson MD (2005) Intraocular pressure and systemic blood pressure: longitudinal perspective: the Beaver Dam Eye Study. Br J Ophthalmol 89(3):284–287
He Z, Vingrys AJ, Armitage JA, Bui BV (2011) The role of blood pressure in glaucoma. Clin Exp Optom 94(2):133–149
Caprioli J, Coleman AL (2010) Blood flow in glaucoma discussion. Blood pressure, perfusion pressure, and glaucoma. Am J Ophthalmol 149(5):704–712
Vetrugno M, Cantatore F, Gigante G, Cardia L (1998) Latanoprost 0.005 % in POAG: effects on IOP and ocular blood flow. Acta Ophthalmol Scand Suppl 76(227):40–41
Agarwal HC, Gupta V, Sihota R (2003) Effect of changing from concomitant timolol pilocarpine to bimatoprost monotherapy on ocular blood flow and IOP in primary chronic angle closure glaucoma. J Ocul Pharmacol Ther 19(2):105–112
Harris A, Kagemann L, Cioffi GA (1998) Assessment of human ocular hemodynamics. Surv Ophthalmol 42(6):509–533
Riva CE, Hero M, Titze P, Petrig B (1997) Autoregulation of human optic nerve head blood flow in response to acute changes in ocular perfusion pressure. Graefes Arch Clin Exp Ophthalmol 235(10):618–626
Arnold AC (2003) Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol 23(2):157–163
Hayreh SS (2001) The blood supply of the optic nerve head and the evaluation of it—myth and reality. Prog Retin Eye Res 20(5):563–593
Olver JM, Spalton DJ, McCartney AC (1994) Quantitative morphology of human retrolaminar optic nerve vasculature. Invest Ophthalmol Vis Sci 35(11):3858–3866
Acknowledgments/disclosure
All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest, and no conflicts were reported. Funding support was provided from the Medical Research Foundation of Guangdong, China (grant B2011081).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, W., Liao, R., Chen, Y. et al. Effect of enhanced extracorporeal counterpulsation in patients with non-arteritic anterior ischaemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol 253, 127–133 (2015). https://doi.org/10.1007/s00417-014-2823-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2823-z