Abstract
Background
Previously we have shown that acute exposure to thimerosal (Thi) can induce oxidative stress and DNA damage in a human conjunctival cell line. However, the long-term effect of Thi on Chang conjunctival cells is not clear. Therefore, the aim of this study was to further investigate the fate of the cells after acute exposure to Thi.
Method
Cells were first exposed to various concentrations of Thi (0.00001 % ∼ 0.001 %) for 30 min, and then cells were assessed after a 24-h recovery period. Morphologic changes were observed under a light microscope and cell viability was evaluated. Cell apoptosis, cell cycle distribution and mitochondrial membrane potential (MMP) (rhodamine 123 assay) were detected by flow cytometry analysis. Poly (ADP-ribose) polymerase (PARP), activation of caspase-3 and microtubule-associated protein light chain 3 (LC-3) were examined by western blot analysis.
Results
DNA strand breaks were significantly increased in a dose-dependent manner with 30 min exposure to Thi, although no significant cell death was detected. However, after 24-h recovery, the ratio of apoptotic cells was significantly increased to 0.0005 % and 0.001 % in Thi treated groups (p < 0.001 compared to the control group). Apoptosis was confirmed by the cleavage of PARP and caspase-3 activation. In addition, G2/M cell cycle arrest and decrease of MMP were recorded. Finally, the LC-3 results indicated the occurrence of autophagy in Thi-treated cells.
Conclusion
Acute exposure to Thi can induce DNA damage, and eventually can lead to cell death, probably through the caspase-dependent apoptosis pathway, while autophagy might also be involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thimerosal (Thi), an organic mercury-containing antibacterial and antifungal agent, has been widely used as an antiseptic and preservative in various formulations including cosmetics, topical medications, and eye lens cleaners since the 1930s. Its usual concentration in ocular drugs ranges from 0.001 % to 0.004 %, and at a concentration of 0.0005 %, it functions as a disinfectant in contact lens solutions. Recently, however, there is growing concern regarding the safety of Thi in several formulations.
Several in vitro studies have shown its cytotoxic effects on the ocular surface. Thi (0.001 %) can cause corneal epithelial cell retraction, cessation of mitotic activity, and total cell destruction [1]. In vivo reports of toxic and immunoallergic effects on the ocular surface that are induced by Thi include a punctate coarse keratopathy, pseudodendritic lesions, superior limbic keratoconjunctivitis, and a more diffuse keratoconjunctivitis [2–6].
As a component of Thi, organomercury is known to induce membrane and DNA damage [7]. Since both prokaryotic and eukaryotic cells are constantly exposed to exogenous and endogenous agents that cause DNA damage, cells have evolved an intricate web of signaling pathways known as the DNA damage response (DDR) to deal with the many types of DNA damage. The extent of the DNA damage determines a cell’s fate: cell cycle arrest to allow the damaged DNA to be repaired, or, if the damage cannot be repaired, cell death [8]. Consistent with this, mercurial substances have also been reported to cause apoptosis in cultured neurons, although the signaling pathways resulting in cell death have not been well characterized [9].
The above information points to the possibility that Thi might be also genotoxic, i.e., can induce DNA damage. Therefore, we first examined the genotoxic effect of Thi, and the results showed that indeed Thi was a strong oxidative agent that could induce ROS production and DNA damage in conjunctival cells following short-term exposure (30 min) at micromolar and nanomolar concentrations [10]. However, the long term effects on cells after the acute exposure of Thi were not clear, such as whether cell death was induced, and if so, what type of cell death was involved.
Therefore, in this study, we examined the fate of the cells which were first exposed to Thi for 30 min, and then were allowed to recover for 24 h. Apoptosis, cell cycle and mitochondrial membrane potential (MMP) were analyzed and key proteins involved in apoptosis and autophagy were also examined.
Materials and Methods
Cell Culture
A human conjunctival cell line (Wong Kilbourne derivative of Chang conjunctiva, clone 1-5c-4, CCL–20.2; American Type Culture Collection [ATCC], Manassas, VA) was cultured under standard conditions (humidified atmosphere of 5 % CO2 at 37 °C) in Medium 199 (Gibco, Grand Island, NY) supplemented with 10 % fetal bovine serum (Gibco, Grand Island, NY), 1 % glutamine, 0.1 % ampicillin, and 2 % kanamycin. Confluent cultures were removed by 0.25 % trypsin (Sigma Aldrich, St. Louis, MO) incubation.
Cell Incubation
Cells were exposed to various concentrations of Thi (0.00001 %,0.00005 %,0.0001 %, 0.0005 %, 0.001 %) for 30 min to determine whether DNA repair occurred in cells after Thi exposure. Cells were also assessed after a 24-h cell recovery period in normal cell culture. Thi was dissolved in culture medium; thus, culture medium (0.0 % Thi) was used as the control. The 0.001 % BAC was used as a positive control drug in trypan blue staining and apoptosis analysis.
Morphological observations and trypan blue staining
After every treatment, morphological changes of cells were observed by using a Nikon phase contrast microscope. For cell counting, 1 × 105 cells were seeded into 12-well plates (Corning Glass, Corning, NY) and grown in 2 ml of media. After 30 min incubation of Thi at different concentration and a 24 h recovery period later, cells were harvested with 0.25 % trypsin. The number of trypan blue-excluding cells was determined using a hemacytometer and the culture viability was calculated as percentage of total cell density.
Flow Cytometry
Apoptosis analysis
The annexin V fluorescein isothiocyanate (FITC)/propidium iodide (PI) kit (Becton Dickinson, Franklin Lakes, NJ) was used to assess modifications of the cell membrane that are associated with programmed cell death. Experiments were conducted according to the manufacturer’s instructions. Cells were collected and centrifuged, then resuspended to 5 × 105 cells in 500 μl of 1 × binding buffer. Annexin V-FITC (5 μl) and 5 μl PI were added to each sample. The samples were incubated in the dark at room temperature for 15 min. Samples were then examined immediately on the flow cytometer (Cytomics FC 500, Beckman Coulter Inc., USA) using the CXP software for data analysis. The percentage of early apoptotic cells was estimated by counting cells that were Annexin V positive but PI negative (B4 quadrant), whereas the percentage of late apoptotic plus necrotic cells was estimated by counting cells that were both Annexin V and PI positive (B2 quadrant).
Cell Cycle Assay
At the end of a 24-h recovery, approximately 2 × 106 cells were washed twice with PBS, then fixed in 75 % ethanol for 12 h at 4 °C. After three washes with cold PBS, cells were stained with 50 μg/ml PI (Sigma, St. Louis, MO) and 10 μg/ml RNase (Sigma). After incubation at 37 °C for 15 min, cells were analyzed by flow cytometry using CellQuest 3.1f software (BD Biosciences, San Jose, CA).
Measurement of mitochondrial membrane potential
MMP were detected by measuring the fluorescent intensity of rhodamine123 in the mitochondria according to the method described previously [11]. Rhodamine123 can be selectively taken up by mitochondria, and the amount of rhodamine123 in mitochondria is proportional to MMP [12]. Briefly, immediately after 30 min incubation of Thi and at the end of the 24 hr recovery period, a total of 1 × 106 cells were harvested and incubated with rhodamine 123 (10 μg/μl) at 37 °C for 30 min in the dark. After washed and suspended in Medium 199 at 37 °C for another 60 min, the cells were resuspended in PBS and analyzed directly by flow cytometry to monitor the formation of the fluorescence at an emission wavelength of 525 nm and an excitation wavelength of 488 nm. Statistical analysis was performed using CXP software. The MMP was represented as the mean fluorescence intensity (MFI) of rhodamine123 in a treated sample/the MFI in the control group.
Western blotting analysis
Expression levels of Caspase-3, and poly (ADP-ribose) polymerase (PARP), microtubule-associated protein light chain 3 (LC3) were analyzed by western blotting. In short, at the end of each treatment, cells were lyzed with an RIPA buffer (1 M Tris–HCl, 5 M NaCl, 1 % Nonidet P-40, 1 % sodium deoxycholate, 0.05 % SDS, 1 mM phenylmethyl sulfonyl fluoride). Protein concentrations were determined using the Pierce bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Inc., Rockford, IL, USA). The lysates were subjected to electrophoresis on sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and then transferred onto polyvinylidene fluoride membrane (PVDF) membranes (Millipore, USA). The PVDF membranes were blocked with 5 % BSA in Tris-buffered saline (TBS) containing 0.1 % Tween-20 (TBST) for 1 h at 37 °C, followed by incubation with the respective antibodies overnight at 4 °C, then with the corresponding IRDye-conjugated secondary antibodies at room temperature for 1 h.
The primary antibodies used were anti-PARP antibody, anti-LC3 antibody (1:1000; Cell Signaling Technology, Inc., Beverly, MA, USA), anti-Caspase-3 antibody, β-actin antibody (Santa Cruz, CA, USA), as well as IRDye-conjugated anti-rabbit and anti-mouse secondary antibodies (Bioworld Technology, St. Louis Park, MN, USA), anti-LC3 antibody detects endogenous levels of total LC3-I and LC3-II proteins and all antibodies were diluted by PBS. Membranes were visualized using the Odyssey Infrared Imaging System and Odyssey v1.2 (LI-COR, NE, USA). The relative densities of the protein bands were analyzed using Quantity One software (Bio-Rad, CA, USA). The relative expressions of target proteins were normalized to the corresponding intensities of β-actin.
Statistical analysis
Statistical analysis was performed with one-way ANOVA followed by the Dunnett multiple comparison tests (GraphPad Prism 5 software; GraphPad Software, San Diego, CA). A p value of <0.05 was considered to be statistically significant. Results were expressed as the mean ± standard deviation from more than three independent experiments.
Results
Morphological analysis and cell viability assay
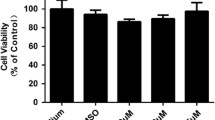
Cells were visualized and continuously photographed with a Nikon phase contrast microscope. After 30 min treatment, only cells treated with 0.001 % Thi represent a shrunken alteration of membranes when compared to the control. On the other hand, after removing Thi and letting cells recover for 24h, it was found that the density of adherent cells was diminished, and cell shrinkage and detachment were also observed in the 0.0005 % and 0.001 % Thi treatment groups. (Fig. 1A-1H) Cell viability at concentrations ranging from 0.00001 % to 0.001 % was measured as 99.7 % ± 0.3 %, 99.4 % ± 0.45 %, 98.43 % ± 1.47 %, 98.37 % ± 1.05 % and 94.87 % ± 1.67 %, respectively, indicating no decrease of viability compared to the control group after 30 min incubation, while the relative cell survival rate of cells treated with 0.001 % BAC was significantly decreased to 76.47 % ± 6.02 % (p < 0.001). However, after 24-h recovery time the cell viability was 80.33 % ± 0.88 %, 51.23 % ± 5.64 % with a concomitant decrease in density of adherent cells in the 0.0005 % and 0.001 % Thi treated groups (p < 0.01, Fig. 1I).
Phase contrast microscopy of Chang conjunctival cells with Thi treatment. Magnification, ×400 a-b: cells treated with Thi for 30 min. a, control cells; b, 0.001 % Thi caused alterations of membranes (arrow); c-h: cells treated with Thi for 30 min, and then changed to fresh medium and allowed to recover for 24 h. c, control; d, 0.00001 %; e, 0.00005 %; f, 0.0001 %; g, 0.0005 %; h, 0.001 %, cell shrinkage and detachment (arrow) occurred in groups exposed to 0.0005 % and 0.001 %; I: The cytotoxic effect of Thi and 0.001 % BAC on cell survival. **p < 0.001 ***p < 0.001 compared with control group
Flow cytometry analysis of cell apoptosis
By staining cells with annexin V-FITC and PI, early apoptotic cells (annexin V-positive, PI-negative) can be distinguished from later apoptosis plus necrotic cells (annexin V positive, PI positive) and viable cells (annexin V negative, PI negative). After 30 min incubation with Thi, no significant difference in the ratio of apoptotic cells was found in every concentration (p > 0.05, compared to the control), while 12.56 % ± 0.78 % early apoptosis and15.9 % ± 0.46 % late apoptosis plus necrosis stage cells were found in 0.001 % BAC treated cells. However, after the 24 h recovery period, 7.87 ± 1.03 % and 15.65 ± 0.48 % of cells were found in early apoptosis, while 8.89 ± 0.69 % and 23.3 ± 3.03 % of cells were found in later apoptosis plus necrosis stage for those treated with 0.0005 % and 0.001 % Thi, respectively, representing a significant increase in cell death (p < 0.001, compared to the control group, Fig. 2B).
Flow cytometry analysis of the cell apoptosis a Representative flow cytometry charts of cells treated with 0.001 % BAC, various concentrations of Thi (0.00001 % to 0.001 %) for 30 min and followed by 24 h recovery. b. Quantitative analysis of apoptosis of cells treated with various concentrations of Thi and 0.001 % BAC for 30 min. c. Quantitative analysis of apoptosis of cells treated with various concentrations of Thi for 30 min followed by 24 h recovery. *p < 0.05, ***p < 0.001, compared to the control group
Thi blocks cell cycle in G2/M phase
Figure 3 shows the distribution of cell cycle of cells treated with Thi followed by 24 h recovery, as determined by flow cytometry. As seen from Fig. 3, although at lower concentrations Thi treatment did not affect cell cycle progression, 0.0005 % and 0.001 % of Thi caused significant changes in cell cycle distribution, with 36.12 % ± 1.9 % and 40.02 % ± 7.62 % cells accumulated in the G2/M phase, respectively, compared to only 14.85 % ± 1.77 % in control cells (P < 0.01, Fig. 3).
Flow cytometry analysis of the cell cycle distribution of Chang conjunctival cells treated with various concentrations (0.0001 %-0.001 %) of Thi for 30 min followed by 24 h recovery a: Representative flow cytometry images of cell cycle distribution.b: Quantitative analysis of cell cycle distribution from a. **p < 0.01, ***p < 0.001, compared to the control group
Effect of Thi on MMP
Rhodamine 123 is a sensitive and specific probe of MMP in isolated mitochondria. Using this agent, it was shown that even after 30 min exposure to Thi, disruption in MMP could be observed, the MIF for 0.0005 % and 0.001 % of Thi treatment groups was 88.8 % ± 1.33 % and 87.57 % ± 0.74 %, respectively, compared to the control. After a 24 h recovery time, the MIF in 0.0005 % and 0.001 % Thi-treated groups was further decreased to 76.78 % ± 3.63 % and 66.71 % ± 1.53 %, respectively, significantly lower than the corresponding untreated groups (P < 0.001, Fig. 4).
Thi induces Caspase-3 activation in Chang conjunctical cells
Caspase-3 activation was a sensitive marker for apoptosis, and the Caspase-3-dependent mechanism constitutes one of the most frequent apoptotic pathways. We examined Caspase-3 activation in cells treated with Thi at concentrations ranging from 0.00001 % to 0.001 % for 30 min, followed by a 24 h recovery. As shown in Fig. 5, as the concentration increased, the amount of pro-Caspase-3 was slightly decreased, while the active Caspase-3 (17KD and 12KD) were gradually increased. Significant changes were found in 0.0005 % and 0.001 % Thi-treated groups.
a: Western blotting analysis showing that the expression of active Caspase-3 was increased in cells treated with 0.0005 % and 0.001 % Thi 30 min followed by a 24 h recovery time. b-c: Densitometry data of three independent experiments, standardized by β-actin were presented below the band. *p < 0.05, **p < 0.01, ***p < 0.001 compared to the control group
Thi induces PARP cleavage in Chang conjunctical cells
PARP is a DNA nick sensor that is subjected for cleavage by activated Caspase-3. Using 0.001 % Thi treated cells as an example, we examined the cleavage of PARP in such cells. It was shown that 30 min exposure did not lead to the cleavage of PARP, however, as the recovery time prolonged, degradation of the 116 kDa parental PARP protein and the subsequent generation of an 89 kDa immunoreactive cleavage product was observed (Fig. 6). The relative density of the bands was calculated and significant changes in parental PARP and cleavage product were found beginning at 8 h after the 30 min exposure (p < 0.05).
Western blotting analysis of PARP in Chang conjunctival cells treated with 0.001 % Thi. a. Representative western blot image of PAPR protein expression. After treated with 0.001 % Thi for 30 min, cells were harvested at the time points indicated, PARP was detected with anti-PARP antibody, β-actin was used as control. b-c: Densitometry data of PARP expression from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, compared to control group
Thi induces LC3 activation in Chang conjunctical cells
LC3 is a mammalian homolog of yeast Atg8, and it is the only reliable marker of autophagosomes. It exists in two forms, LC3-I and LC3-II. LC3-I is an 18-kDa polypeptide normally found in the cytosol, whereas the product of its proteolytic maturation (LC3-II, 16 kDa) resides in the autophagosomal membranes [13]. Thus, monitoring LC3-I and LC3-II by western blotting is essential for investigating the mechanism of mammalian autophagy. In our study, LC3-II accumulated in higher amounts in 0.00005 % ∼ 0.001 % Thi-treated groups compared with controls (p < 0.05, Fig. 7), indicating a build up of autophagosomes and an increase in the autophagic flux.
a: Western blot analysis showing the expression of LC3-I and LC3-II proteins representing a dose-dependent processing treated with every concentration of Thi 30 min followed by a 24 h recovery time. b-c: Densitometry data of three independent experiments, standardized by β-actin were presented below the band. *p < 0.05, **p < 0.01, ***p < 0.001 compared the to control group
Discussion
The eyes are one of the most susceptible organs to damage agents. Preservatives are an indispensable constituent of ocular formulations; however, the potential cytotoxic effects of preservatives associated with long-term therapy, especially when multiple preserved drugs are used, are often under-recognized. Thi is one of the most commonly used preservatives in ophthalmic drugs and contact lens solutions, and recently, the antifungal activity of Thi was found to be significantly superior to that of amphotericin B and natamycin against ocular pathogenic fungi in vitro, which makes it a more useful drug than just as a preservative [14]. Still, there have been reports showing the cytotoxic effects of Thi on the ocular surface; Thi might cause structural and functional damage to the endothelium with prolonged direct exposure [15]. In addition, it has been shown that Thi might be responsible for delayed hypersensitivity, which can cause conjunctival hyperemia and corneal infiltrates [16]. Therefore, there is also growing concern for the clinical use of Thi. Previously we have shown that acute exposure to Thi could induce oxidative stress and DNA damage in a human conjunctival cell line [10]. In this paper, we found that DNA damage induced by acute exposure to Thi eventually can lead to cell death, probably through the caspase-dependent apoptosis pathway, while autophagy might also be involved.
Besides the cytotoxic effect, genotoxic damage includes DNA single-strand breaks (SSBs), double-strand breaks (DSBs), alkali labile sites (ALSs), and DNA cross-linking occurring in all prokaryote or eukaryote cells with exposure to exogenous and endogenous agents. DNA damage can be transmitted to differentiated daughter cells, thereby compromising tissue integrity and function [17, 18]. It is unknown whether Thi has genotoxic effects on Chang conjunctival cells at clinical concentrations. In our previous study, we used two classic methods, the alkaline comet assay and the phosphorylated form of the histone variant H2AX (γH2AX) foci detection, to investigate DNA damage induced by Thi after 30 min incubation. The results indicated that Thi induced DNA single-strand and double-strand breaks in Chang conjunctival cells and that the damage was correlated with Thi concentrations [10]. In addition, compared to Benzalkonium chloride (BAC), the most frequently used preservative in ocular drugs that has been proved to be toxic to ocular cells, which could cause notably decreased cell viability and cell apoptosis at 0.001 %, Thi did not induce significant changes in cell viability nor the ratio of apoptotic cells, suggesting that Thi was less toxic than BAC.
Any type of DNA lesion can be rapidly sensed, and then DDR is activated, including the activation of a cell cycle checkpoint that allows damaged DNA to be repaired before reengaging the cell cycle and completing mitosis, thus maintaining the genomic integrity [19]. In this study, a significant increase in G2/M phase cells was observed after the 24-h recovery period (0.0005 % and 0.001 % Thi treatment), indicating that the G2 checkpoint was triggered.
The cell cycle checkpoints include the G1 checkpoint, the S phase checkpoint and the G2 phase checkpoint. The G1 checkpoint depends on increased expression and activation of the p53 gene product [20]. Several carcinogenic agents and chemotherapeutic agents were known to trigger the S phase response [21], and agents such as ionizing radiation and oxidative stress can trigger the G2 checkpoint response [22]. Cells that have a defective G2-M checkpoint enter mitosis before repairing their DNA, leading to death after cell division [23]. Hydrogen peroxide and tert-butyl hydroperoxide have been demonstrated to induce a G2 checkpoint response in eukaryotic cells, and in our previous study we have shown that Thi could induce ROS overproduction after 30 min exposure. Since ROS was found to induce many types of DNA damage [24], it is believed that ROS-induced DNA damage could be responsible for the initiation of G2 checkpoint responses shown in this study.
The cell cycle checkpoint response can either lead to cell survival if DNA is properly repaired or, if not, to cell death [25]. Several studies have indicated that both inorganic and organic mercurials could induce apoptosis in vitro as well as in vivo [26, 27]. Using phase contrast microscopy, we found membrane alterations and cell shrinkage after 30 min of treatment (0.001 %); detachment occurred after 24 h recovery (0.0005 %-0.001 %) followed by decreased cell viability . In addition, a disruption in MMP was observed after 30 min incubation and 24 h later (0.0005 %-0.001 %), indicating impairment of normal mitochondrial function starting after 30 min of incubation. Decreased MMP represents the point of no return in the cascade of events that ultimately leads to the cell’s demise [28], which can cause the release of cytochrome C from mitochondria into cytosol, where it binds to apoptotic protease activating factor (Apaf-)-1 and activates caspases [29, 30]. In addition, ROS appears to be mitochondria-derived and responsible for later mitochondrial events leading to the full activation of the caspase cascade [31].
The cell death labeled by Annexin-V in the case of high concentration Thi treatment deserves additional discussion. PARP is an enzyme involved in a number of cellular processes involving DNA repair and programmed cell death. It can be differentially processed in apoptosis and necrosis; therefore, its activity can potentially be used as a way of distinguishing these two forms of cell death [32]. In apoptosis, PARP undergoes rapid cleavage and inactivation while during necrosis PARP is highly activated. Thus, detection of the cleaved and inactivated PARP is a diagnostic test for apoptosis in cells. In our study, we found that as time lengthened (time dependent), the amount of activated PARP protein decreased, while the cleaved (inactivated form) increased, indicating that the cells underwent apoptosis but not necrosis. In contrast, Caspase-3 exists as an inactive (35KD) form in the cytoplasm [22], while two subunits (17KD and 12KD) dimerize to form the active enzyme when activated. Sequential activation of caspases plays a central role in the execution phase of cell apoptosis, and caspase-3 is responsible for the cleavage of PARP, which occurs when DNA damage is extensive. It is suggested that during apoptosis, the DNA-binding domain of cleaved PARP will attach to a damaged site, thus preventing other, non-cleaved PARP from accessing the damaged site and initiating repair.
Autophagy has been proposed as a third mode of cell death besides apoptosis and necrosis. It is a process in which cells generate energy and metabolites by digesting a cell’s own components through the autophagy machinery [23, 24]. In normal cells, autophagy occurs constitutively at low, basal levels. However, when under various environmental or cellular stresses, such as nutrient deprivation, oxidative stress, and toxic stimuli [25, 26], autophagy can be a tightly regulated adaptive mechanism in order to help cells survive stress. However, in recent years, it has been accepted that autophagy can also lead to cell death in addition to its role in cell survival [33]. The amount of LC3-II correlates well with the number of autophagosomes, so immunoblotting of endogenous LC3 can be used to measure autophagic activity. Increased LC3-II levels can be associated with either enhanced autophagosome synthesis or reduced autophagosome turnover. Inhibitors such as Bafilomycin A1 can inhibit degradation of autolysosome content by inhibiting the Na+H+ pump at the lysosome, increasing lysomal pH and inhibiting acidic lysosomal proteases, respectively [34]. However, it is important to use such inhibitors appropriately, since their activity becomes non-specific and influences protein turnover at the proteasome, as well as at the autolysosome, when used at too high a concentration or for extended periods of time [35]. In our study, we found that the expression of LC3-I and LC3-II both increased after 24 h without inhibitors and was dose-dependent, indicating the occurrence of autophagy in Thi-treated cells.
Although autophagy is considered a nonselective procedure, there is accumulating evidence for selective autophagic processes in response to ROS. Mitophagy, the selective degradation of mitochondria, can be induced by mitochondria functional impairment and/or by decreased MMP. Mitophagy may ensure the removal of damaged and potentially dangerous mitochondria, thus acting as a quality control mechanism [36]. Decrease of MMP induced by oxidative stress can lead to mitochondrial permeability transition, which triggers mitophagy, and could act as either a survival or a death pathway [37, 38]. In our study, cells treated with low concentration of Thi still demonstrated autophagy activation, but cell death was not detected; thus, it was assumed that autophagy provides the front line of defense against oxidative stress. However, programmed death is activated in response to oxidative stress when survival mechanisms fail.
In conclusion, we demonstrated that an additional 24 h of recovery after short-term Thi treatment was associated with toxicity that was not seen during the acute exposure. Thi induced G2-M cell cycle arrest caspase-3-dependent apoptosis in conjunctival cells, and this apoptosis was associated with the depolarization of the mitochondrial membrane. In addition, autophagy also occurred, although its exact physiological significance needs to be further explored.
References
Tripathi BJ, Tripathi RC, Kolli SP (1992) Cytotoxicity of ophthalmic preservatives on human corneal epithelium. Lens Eye Toxic Res 9:361–375
Burstein NL, Klyce SD (1977) Electrophysiologic and morphologic effects of ophthalmic preparations on rabbit cornea epithelium. Invest Ophthalmol Vis Sci 16:899–911
Baines MG, Cai F, Backman HA (1991) Ocular hypersensitivity to thimerosal in rabbits. Invest Ophthalmol Vis Sci 32:2259–2265
Wilson-Holt N, Dart JK (1989) Thiomersal keratoconjunctivitis, frequency, clinical spectrum and diagnosis. Eye (Lond) 3(Pt 5):581–587
Buck SL, Rosenthal RA, Schlech BA (2000) Methods used to evaluate the effectiveness of contact lens care solutions and other compounds against Acanthamoeba: a review of the literature. CLAO J 26:72–84
Wilson LA, McNatt J, Reitschel R (1981) Delayed hypersensitivity to thimerosal in soft contact lens wearers. Ophthalmology 88:804–809
Ferrat L, Romeo M, Gnassia-Barelli M, Pergent-Martini C (2002) Effects of mercury on antioxidant mechanisms in the marine phanerogam Posidonia oceanica. Dis Aquat Organ 50:157–160
Barzilai A, Yamamoto K (2004) DNA damage responses to oxidative stress. DNA Repair (Amst) 3:1109–1115
Geier DA, Sykes LK, Geier MR (2007) A review of Thimerosal (Merthiolate) and its ethylmercury breakdown product: specific historical considerations regarding safety and effectiveness. J Toxicol Environ Health B Crit Rev 10:575–596
Ye J, Zhang H, Wu H, Wang C, Shi X, Xie J, He J, Yang J (2012) Cytoprotective effect of hyaluronic acid and hydroxypropyl methylcellulose against DNA damage induced by thimerosal in Chang conjunctival cells. Graefes Arch Clin Exp Ophthalmol 250:1459–1466
Perez MJ, Cederbaum AI (2002) Antioxidant and pro-oxidant effects of a manganese porphyrin complex against CYP2E1-dependent toxicity. Free Radic Biol Med 33:111–127
Baracca A, Sgarbi G, Solaini G, Lenaz G (2003) Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F(0) during ATP synthesis. Biochim Biophys Acta 1606:137–146
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Xu Y, Pang G, Zhao D, Gao C, Zhou L, Sun S, Wang B (2010) In vitro activity of thimerosal against ocular pathogenic fungi. Antimicrob Agents Chemother 54:536–539
Van Horn DL, Edelhauser HF, Prodanovich G, Eiferman R, Pederson HF (1977) Effect of the ophthalmic preservative thimerosal on rabbit and human corneal endothelium. Invest Ophthalmol Vis Sci 16:273–280
Mondino BJ, Groden LR (1980) Conjunctival hyperemia and corneal infiltrates with chemically disinfected soft contact lenses. Arch Ophthalmol 98:1767–1770
Morgan SE, Kastan MB (1997) p53 and ATM: cell cycle, cell death, and cancer. Adv Cancer Res 71:1–25
Moll UM, Slade N (2004) p63 and p73: roles in development and tumor formation. Mol Cancer Res 2:371–386
Kaufmann WK, Paules RS (1996) DNA damage and cell cycle checkpoints. FASEB J 10:238–247
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res 51:6304–6311
Cistulli CA, Kaufmann WK (1998) p53-dependent signaling sustains DNA replication and enhances clonogenic survival in 254 nm ultraviolet-irradiated human fibroblasts. Cancer Res 58:1993–2002
Lock RB, Ross WE (1990) Possible role for p34cdc2 kinase in etoposide-induced cell death of Chinese hamster ovary cells. Cancer Res 50:3767–3771
Cuddihy AR, O’Connell MJ (2003) Cell-cycle responses to DNA damage in G2. Int Rev Cytol 222:99–140
Shackelford RE, Kaufmann WK, Paules RS (2000) Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med 28:1387–1404
Castedo M, Perfettini JL, Roumier T, Andreau K, Medema R, Kroemer G (2004) Cell death by mitotic catastrophe: a molecular definition. Oncogene 23:2825–2837
Shenker BJ, Guo TL OI, Shapiro IM (1999) Induction of apoptosis in human T-cells by methyl mercury: temporal relationship between mitochondrial dysfunction and loss of reductive reserve. Toxicol Appl Pharmacol 157:23–35
Shenker BJ, Guo TL, Shapiro IM (2000) Mercury-induced apoptosis in human lymphoid cells: evidence that the apoptotic pathway is mercurial species dependent. Environ Res 84:89–99
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163
Scarlett JL, Sheard PW, Hughes G, Ledgerwood EC, Ku HH, Murphy MP (2000) Changes in mitochondrial membrane potential during staurosporine-induced apoptosis in Jurkat cells. FEBS Lett 475:267–272
Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G (1995) Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182:367–377
Schulze-Osthoff K, Beyaert R, Vandevoorde V, Haegeman G, Fiers W (1993) Depletion of the mitochondrial electron transport abrogates the cytotoxic and gene-inductive effects of TNF. EMBO J 12:3095–3104
Putt KS, Beilman GJ, Hergenrother PJ (2005) Direct quantitation of poly(ADP-ribose) polymerase (PARP) activity as a means to distinguish necrotic and apoptotic death in cell and tissue samples. Chembiochem 6:53–55
Codogno P, Meijer AJ (2005) Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 12(Suppl 2):1509–1518
Yla-Anttila P, Vihinen H, Jokitalo E, Eskelinen EL (2009) Monitoring autophagy by electron microscopy in Mammalian cells. Methods Enzymol 452:143–164
Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC (2008) Does bafilomycin A1 block the fusion of autophagosomes with lysosomes. Autophagy 4:849–950
Priault M, Salin B, Schaeffer J, Vallette FM, di RJP, Martinou JC (2005) Impairing the bioenergetic status and the biogenesis of mitochondria triggers mitophagy in yeast. Cell Death Differ 12:1613–1621
Kissova I, Deffieu M, Manon S, Camougrand N (2004) Uth1p is involved in the autophagic degradation of mitochondria. J Biol Chem 279:39068–39074
Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B (1998) The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1366:177–196
Acknowledgments
The work was supported by project foundation : 1. Zhejiang province Key Innovation Team Project of China (No.2009R50039) 2. Zhejiang Key Laboratory Fund of China (No.2011E10006) 3. Natural Science Foundation of China (81070756) 4. National "Twelfth Five-Year" Plan for Science & Technology Support of China (2012BAI08B01) 5. The Specialized Key Science & Technology Foundation of Zhejiang Provincial S & T Department,China (No.2012C13023-2) 6. Zhejiang Provincial Key Project of Medical and Health (No.2011ZDA014)
The authors have no financial interest in any product or concept discussed in article
Grant: Supported by project foundation: 1. Zhejiang province Key Innovation Team Project of China (No.2009R50039) 2. Zhejiang Key Laboratory Fund of China (No.2011E10006) 3. Natural Science Foundation of China (81070756) 4. National "Twelfth Five-Year" Plan for Science & Technology Support of China (2012BAI08B01) 5. The Specialized Key Science & Technology Foundation of Zhejiang Provincial S & T Department,China (No.2012C13023-2) 6. Zhejiang Provincial Key Project of Medical and Health (No.2011ZDA014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, H., Wu, H., Wang, C. et al. Acute exposure to thimerosal induces antiproliferative properties, apoptosis, and autophagy activation in human Chang conjunctival cells. Graefes Arch Clin Exp Ophthalmol 252, 275–284 (2014). https://doi.org/10.1007/s00417-013-2542-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2542-x