Abstract
Background
Intrapapillary hemorrhage with adjacent peripapillary subretinal hemorrhage (IHAPSH) is a clinical syndrome most commonly affecting myopic eyes with tilted discs that usually resolves spontaneously without treatment. Subretinal hemorrhage usually occurs peripapillary on the nasally adjacent side near the optic disc. The etiology of this condition is still unknown. The purpose of this study was to determine if a crowded optic nerve head and small scleral canal are involved in the pathogenetic mechanisms of IHAPSH.
Methods
Twelve subjects with IHAPSH diagnosed at the Affiliated Ophthalmology Hospital of the First Clinical College of Harbin Medical University and 24 control subjects were examined. The size of the inner aspect of the scleral canal and level of nerve fiber crowding of the optic nerve head were analyzed with optic nerve head analysis software packet of the Stratus Optical Coherence Tomography software and manual segmentation software. The Mann–Whitney U test and multiple comparisons (with the Bonferroni correction method) were performed. p values less than 0.002 (two-sided) were considered statistically significant. The area, perimeter, and the perimeter/area ratio of the optic disc, vertical and horizontal diameter of the inner aspect of the scleral canal, vertical integrated rim area (VIRA), and the rim area were calculated.
Results
The area and perimeter of the optic disc and the horizontal diameter of the inner aspect of the scleral canal were significantly lower in the affected and contralateral eyes of the subjects with IHAPSH than in the eyes of the controls. Conversely, the IHAPSH-affected and contralateral eyes had significantly higher perimeter/area ratio of the optic disc, VIRA, and rim area values than the control eyes. The VIRA and rim area were greater in the IHAPSH-affected eyes than in the contralateral eyes.
Conclusions
Patients with IHAPSH have smaller optic discs and scleral canals than control subjects, with a higher level of nerve fiber crowding.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A clinical syndrome characterized by the spontaneous onset of IHAPSH due to an unknown cause has been reported [1–4]. In the eyes that are affected by this condition, there is significant bleeding within the optic disc that extends into the peripapillary subretinal space and often into the vitreous [4]. It most commonly affects myopic eyes with tilted discs and usually resolves spontaneously without treatment. In terms of prognosis, there is an excellent chance of a complete recovery of vision, and IHAPSH usually does not recur. Subretinal hemorrhage usually occurs peripapillary on the nasally adjacent side near the optic disc. Although this syndrome seemed to be benign, which affects patients of both genders and was reported mostly in young and otherwise healthy Asian individuals of various ethnicities [1–4], the etiology of this condition is still uncertain.
The purpose of this study was to determine if a crowded nerve head and a small scleral canal may be involved in the pathogenesis of IHAPSH. The scleral canal size was measured in fundus images by the use of scleral analysis software packet of the Stratus optical coherence tomography (OCT). Optic Nerve Head analysis software, as well as manual segmentation software, was used to analyze the characteristics of the optic nerve head (ONH) in patients with idiopathic IHAPSH.
Materials and methods
This study was a prospective, observational case–control study. A series of 12 consecutive patients from February 2005 to July 2012 who suffered from IHAPSH were reviewed in the Department of Ophthalmology at the First Affiliated Hospital of Harbin Medical University. Inclusion criteria for the study were ophthalmoscopic evidence of both intrapapillary and adjacent peripapillary subretinal hemorrhage or isolated peripapillary subretinal hemorrhage. Exclusion criteria included disc hemorrhage without adjacent peripapillary subretinal hemorrhage, disc hemorrhage secondary to optic nerve head drusen, peripapillary subretinal neovascularization, ischemic optic neuropathy, Terson’s syndrome, Leber’s idiopathic stellate neuroretinitis, polypoidal choroidal vasculopathy, and bleeding diatheses.
A control group of age- and sex-matched subjects were recruited from the myopic patients in our hospital. These participants had no personal history of IHAPSH or any other ocular disease. All control subjects had best-corrected Snellen visual acuities of 20/20 or better at the time of the study.
All participants provided informed consent before participation according to the Declaration of Helsinki. The study was approved by the ethics committee of Harbin Medical University. Stratus OCT scanning with a Zeiss Stratus 3000 (Carl Zeiss Meditec, Inc. Dublin, CA) and fundus photography with a TOPCON IMAGE net 2000 TM (TOPCON, Inc. Japan) were performed.
Manual segmentation software for the analysis of fundus images based on a VC++ and Open CV library was utilized. First, the optic disc in the fundus photography and fluorescein angiography images was objectively and accurately segmented by two experts using this software. Second, the area, perimeter, and the perimeter/area ratio of the optic disc (a reflection of the smoothness of the rim of the optic disc) were calculated by the program automatically.
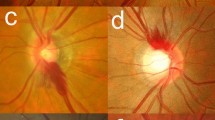
The scleral canal was measured and the characteristics of the ONH was analyzed using Stratus OCT Optic Nerve Head analysis software. Photographers were masked to the disease status of all subjects. Image acquisition was performed with Fast Optic Disc protocols. Good-quality scans (signal score of 4 or higher) were saved as soon as they appeared on the screen. Before recording these values, the results of the OCT software analysis was checked by Dr. Teng and another photographer to ensure that the limits of the optic nerve had been correctly identified. If the limitations had not been correctly recognized, they would be manually readjusted immediately. The inner aspect of the scleral canal was set based on determinations shown in Fig. 1a, c. The individual scan analysis values recorded were the vertical and horizontal diameters of the inner aspect of the scleral canal and the rim area (the vertical cross-sectional area). The ONH analysis results were compiled by making six radial scans through the optic nerve. The schematic outline of the optic disc area and the optic cup was plotted (Fig. 1b, d). The vertical integrated rim area (the volume and VIRA) were calculated by the analysis of the ONH using the OCT software (Fig. 1b, d). The VIRA is an estimate of the total volume of retinal nerve fiber layer tissue in the rim and was calculated by multiplying the average individual rim area with the circumference of the disc [5, 6]. Hemorrhage might block the disc and make measurement of parameters somewhat difficult to reproduce and define. The OCT scans of the optic disc were repeated once the hemorrhage resolved.
Optical coherence tomography (OCT) of optic nerve head (ONH) analysis report (180° radial scan). a The left eye of a control subject. The OCT radial scan analysis of the eye shows a normal optic nerve cup. The inner retinal layers are depicted in red and yellow. The black space below these layers represents the photoreceptor outer segments and the subretinal space. The inferior red and yellow layers represent the retinal pigment epithelium and Bruch’s membrane. The outer limit of this layer on both sides of the optic nerve corresponds to both the optic disc area and the inner aspect of the scleral canal. The limits manually identified are signed by blue circles. A straight line connects the edges, and a second line is drawn 150 μm anterior and parallel to the first one. Below this last line is defined as the disc cup, and above this line as the neuroretinal rim (which is shaded in red).b The OCT printout can give individual scan values. The optic nerve head analysis results were compiled by making six radial scans through this optic nerve. The schematic outline of the optic disc area has been plotted in red, and a schematic outline of the optic cup has been plotted in green. c An affected left eye of a patient with IHAPSH. The individual radial scan analysis of the eye reveals a nasally elevated optic nerve. Below this elevation, the termination of the retinal pigment epithelium and Bruch’s membrane can be observed. d The optic nerve head analysis results show a schematic outline of the optic disc area, plotted in red
Nonparametric statistical analysis was performed using the Kruskal–Wallis test where appropriate using the SPSS v13.0 program (SPSS, Inc.). The mean and standard deviation were calculated for each group. As multiple comparisons were performed according to the Bonferroni correction method, the final level of significance was set at 0.002 (two-sided).
Results
Demographics of study population
The demographic characteristics of the study population are shown in Table 1. The time from onset to initial examination was less than or equal to 1 week in seven patients, between 1 and 2 weeks in three patients, and between 2 weeks and 1 month in one patient (for one patient, the time of onset was not known, thus a time from onset to initial examination could not be determined). All patients were Asian (Han Chinese). The right eye was affected in seven of the patients, whereas five patients demonstrated IHAPSH in the left eye. Eleven patients had an acute onset of visual symptoms at presentation, including blurred vision and floaters. None of the patients had a history of significant prior medical problems.
All the affected eyes were myopic. The best-corrected Snellen visual acuity scores of the affected eyes of the patients were 20/20 or better in eight of the affected eyes at the time of the study, 20/25 or better in two affected eyes, and 20/40 or better in two affected eyes. All contralateral unaffected eyes had scores of 20/20 or better.
The optic discs of all the affected eyes and unaffected contralateral eyes were crowded with small or absent cups, exhibited variable degrees of tilting with elevation and blurring of the nasal, and/or superonasal regions of the nerve head (Fig. 2a, b). Temporal or inferotemporal crescents were visible in both eyes in 11 patients (Fig. 2a, b). Many types of hemorrhage appeared in a variety of combinations in the affected eyes, including intrapapillary, adjacent peripapillary, intraretinal, vitreous, and subretinal hemorrhages (found in five eyes) (Fig. 2b), intrapapillary, adjacent peripapillary, subretinal, and vitreous hemorrhages (three eyes), intrapapillary, adjacent peripapillary, intraretinal, and subretinal hemorrhages (two eyes), and an isolated peripapillary subretinal hemorrhage (two eyes).
Treatment-naive intrapapillary hemorrhage with adjacent peripapillary subretinal hemorrhage (IHAPSH). Fundus photograph of IHAPSH-contralateral eye (a) and IHAPSH-affected eye (b) showing tilted, crowded optic discs with small or absent variable optic cups and signs of elevation and blurring of the nasal or superonasal region of the nerve head. b Intrapapillary hemorrhages located in the cup. There were peripapillary subretinal hemorrhages, vitreous hemorrhages, and hemorrhages that extended from the nasal edge of the optic disc to beyond the disc margin intraretinally. c, d Fluorescein angiogram of retinal arterio-venous-phase of IHAPSH-affected eye and fluorescein angiogram of late-phase showing hypofluorescence due to intrapapillary, intraretinal, peripapillary subretinal, and vitreous hemorrhages. A retinal temporal aspect with a temporal crescent in the eye was also observed
Intrapapillary hemorrhage was extended from the center of the optic cup in five eyes. Three of these five intrapapillary hemorrhages were found to extend directly from the center of the cup into the vitreous, with microscopic amounts of vitreous hemorrhage limited to the superior region over the disc (Fig. 2b). Hemorrhage was observed in the center of the optic cup and at the nasal edge of the optic disc in five eyes. Crescent-shaped peripapillary subretinal hemorrhages were observed in nine eyes along the nasal and superonasal margin (Fig. 2a, b) and along the nasal inferonasal margin in three eyes.
Two patients showed no abnormalities upon Humphrey visual field testing. In four patients, the contralateral eye showed no abnormalities, but the affected eye showed a mild enlargement of the blind spot. Further, there was a mild loss of the superior visual field in the affected eyes of two of the patients, and other patients showed mild losses in the nasal and superior fields (one patient), the nasal field (one patient), and the inferior field (one patient) of their affected eye. In one patient, the affected and contralateral eyes showed a mild enlargement of the blind spot with mild losses in the nasal and superior fields, and another patient’s contralateral eye had mild superonasal and superotemporal field losses, with the affected eye only showing mild enlargement of the blind spot and a mild superior field loss.
All 12 patients underwent fluorescein angiography. In the initial angiograms, all the affected eyes showed hypofluorescence due to intrapapillary, intraretinal, peripapillary subretinal, and vitreous hemorrhages (Fig. 2c, d). Late-phase fluorescein angiograms demonstrated focal disc staining on the nasal aspect of the optic disc in one of the IHAPSH-affected eyes. Two of the affected eyes showed staining in the center of the optic disc. None of the patients’ eyes showed diffuse leakage of the optic disc or telangiectasia of the disc vessels, and none of the studies showed abnormal intraretinal, intrapapillary, or choroidal vasculature.
All patients underwent Stratus OCT, and five patients underwent Spectralis OCT. OCT showed an elevation of the nasal side of the optic disc that was accompanied by a widening of the adjacent nasal peripapillary subretinal space. Further, affected eyes showed either a small cup or no physiological cup, a sharp nasal edge of the scleral canal, and rounded edge of the temporal side of the canal. None of the affected eyes showed any evidence of vitreopapillary traction, and detachment of the posterior vitreous was not found (Fig. 1. a, b; Fig. 3a, b).
Contralateral and affected eye of intrapapillary hemorrhage with adjacent peripapillary subretinal hemorrhage (IHAPSH). a Section of an optical coherence tomography (OCT) image of IHAPSH contralateral eye revealing an elevation of the nasal side of the optic disc and a sharp nasal edge of the scleral canal (the rounded edge of the temporal side). b Section of an optical coherence tomography(OCT) image of IHAPSH affected eye showing an elevated optic nerve head accompanied by a widening of the adjacent nasal peripapillary subretinal space, a sharp nasal edge of the scleral canal. c B-scan ultrasonography image showing the absence of any evidence of the complete or partial detachment of the posterior vitreous. An elevated optic disc was found. d Orbital and brain computed tomographic image showing retrobulbar optic nerve and the brain were normal
B-scan ultrasonography was performed in nine patients. The absence of any evidence of the complete or partial detachment of the posterior vitreous was noted. An elevated optic disc was found in all patients, and a substantial increase in the vitreous cavity link with the optic nerve was observed in two of the affected eyes (Fig. 3c).
Four patients underwent orbital computed tomographic (CT) (Fig. 3d) and brain scanning. The ONHs, retrobulbar optic nerves, and brains were normal in all the patients.
By the time of follow-up, the intrapapillary, intraretinal, and subretinal hemorrhages observed in the affected eyes of five patients at the initial examination had resolved spontaneously. Further, by 1 month after the initial examination, the subretinal hemorrhages could only be seen by FFA, and these and all the other hemorrhages resolved spontaneously within 6 months. Visual acuities maintained or improved to 20/25 or better in all eyes.
Results of the prospective, observational case–control study
Comparisons between the right and left eyes of the control group in terms of ONH parameters did not show any statistically significant differences (data not shown). For the comparisons of ONH parameters between controls and patients, the means of the left and right eye values of each control were utilized in the comparisons between control eyes and both IHAPSH-affected eyes and the contralateral eyes of IHAPSH patients. There were no statistically significantly differences in refractive error between the control group eyes and both the affected and contralateral eyes of the subjects with IHAPSH (Table 2).
The manual segmentation software analysis of the fundus images indicated that the area, perimeter, of the optic disc were significantly lower, whereas the perimeter/area ratios were significantly higher in the affected and contralateral eyes of the subjects with IHAPSH compared to the eyes of the controls (Table 2).
Analysis of the OCT results by Stratus OCT Optic Nerve Head Analysis software indicated that the IHAPSH-affected and contralateral eyes had a significantly smaller horizontal diameter of the inner aspect of the scleral canal than controls. There were no significant differences in the vertical diameter parameters between the three groups. Significantly higher values for VIRA and rim area were found in the IHAPSH-affected and contralateral eyes compared to the controls. The IHAPSH-affected eyes had significantly higher VIRA and rim area (the vertical cross-sectional area) values than the contralateral eyes (Table 2).
Discussion
Proposed pathogenetic mechanisms of IHAPSH include vitreopapillary traction, the hemorrhage of anatomically vulnerable prelaminar blood vessels in crowded optic discs, the hemodynamic effects of the Valsalva maneuver, and complications of optic disc edema [1–4]. However, vitreopapillary traction was not observed in reports by Kokame [3, 4] and also not in our study. In our patients, Valsalva maneuvers were not found, and the typical signs of optic disc edema—dilated telangiectatic vessels of optic disc and diffuse late leakage into the optic disc as observed by fluorescein angiography—were not observed. Thus, we believe pseudopapilledema is present in IHAPSH.
Kokame et al. [4] suggested that the unique structural architecture of the elevated superior and nasal margins of a tilted myopic disc results in the dragging of the retinal and choroidal tissues over and around the elevated edge. Capillaries in such dragging area may suffer an increased risk of bleeding. Thus, the choroidal blood supply of the prelaminar optic nerve may predispose patients to bleeding from the optic disc, which may be spontaneous or may be precipitated by an acute event. In our study, nasal optic disc elevation, a tilting disc, and a widening of the peripapillary subretinal space on the nasal side in our patients was found in OCT results, and our findings are consistent with the conclusions of Kokame et al. In addition, we found that the edge of the nasal scleral canal was sharp, but the edge of the temporal side was rounded. Further, we speculate that there might be other triggers for IHAPSH.
Sibony et al. [7] described a benign syndrome of isolated spontaneous monocular, nasal, or superonasal peripapillary subretinal hemorrhage (PSH) (but not intrapapillary) in crowded and tilted optic discs in myopic eyes of Caucasian women that may mimic papilledema. This suggested that isolated PSH and IHAPSH are variants of the same underlying process, and proposed that the optic disc hemorrhages are the result of an interplay of ocular motor forces, scleral thinning, and vitreopapillary traction acting on a morphologically vulnerable crowded and tilted optic disc predisposed to hemorrhage. The relative contribution of these three forces in an individual case may influence the location of the optic disc hemorrhage. Vitreopapillary traction is more likely to result in intrapapillary, vitreous, or nerve fiber hemorrhages as described by Katz and Hoyt [2], whereas biomechanical stress due to eye movements and peripapillary thinning at the choroidal and/or scleral levels may be predominant in patients with isolated PSH. Temporal crescents in myopic eyes may also represent an early sign of globe expansion. Although our patients had mild to moderate myopia, most had visible choroidal atrophy at the temporal optic disc arc spot. Further, thinning of the sclera was confirmed. Because all the peripapillary subretinal hemorrhages observed in IHAPSH and PSH were located on the nasal, rather than temporal, side of the optic disc, we believe that IHAPSH might not be associated with scleral thinning. Although one of the causes of isolated peripapillary subretinal hemorrhage was spontaneously resolved, there was still residual bleeding in the subretinal hemorrhage.
The three-dimensional architecture of the border tissue of Elschnig combined with the presence of the overhanging Bruch’s membrane makes an important contribution to disc margin anatomy [8]. Although the inner aspect of the sclera cannal can be recognized directly by the OCT, the outer aspect of the sclera canal cannot be directly measured with currently available OCT technology. We have minimized any optical effect by selecting a control group that has a mean refractive error similar to that of the IHAPSH group. Further, we manually corrected the limits of the optic disc. It was found that the horizontal diameter of the inner aspect of scleral canal of the affected eyes of subjects with IHAPSH were smaller than those in the eyes of control groups. In addition, we used fundus images and manual segmentation software to compare the area and perimeter of the optic discs of the two groups. The optic disc area and perimeter of the affected eyes of subjects with IHAPSH were smaller, whereas the perimeter/optic ratio of disc area were higher than those of the control group eyes. These results showed a relatively smaller inner aspect of the scleral canal may be involved in the pathogenesis of IHAPSH.
No differences were found in ONH parameters between the right and left eyes of the control subjects. This is in accordance with the presumption that before an episode of IHAPSH, both eyes have similar ONHs and confirms the validity of evaluating changes in the ONH in an IHAPSH eye by comparing it with that of the contralateral eye. Thus, our results indicating that the contralateral eyes have a small canal size is very strong evidence that a small canal size is associated with IHAPSH.
The level of nerve fiber crowding in the ONH was evaluated by VIRA, rim area (the vertical cross-sectional area), as measured by OCT. Higher VIRA and rim area values were observed in the affected and contralateral eyes of IHAPSH patients compared to the eyes of control subjects. Additionally, the IHAPSH-affected eyes had higher VIRA and rim area values than the contralateral eyes of IHAPSH patients. These results support the notion that there is more crowding in the eyes in which IHAPSH develops.
The main limitation of this study was the relatively small number of patients. However, to the best of our knowledge, this is the first prospective, controlled study to compare ONH parameters in IHAPSH with a quantitative and objective test. In summary, we are confident in stating that patients with IHAPSH have smaller optic disc canals than controls and that IHAPSH-affected eyes have a higher level of nerve fiber crowding than the contralateral eyes of IHAPSH patients and control eyes. Therefore ,it is speculated that compression may be of great importance in the etiology of IHAPSH because of possible effect of the small scleral canal and especially the higher level of nerve fiber crowding.
Based on the following clinical manifestation and optic papilla anatomical features in IHAPSH patients, analysis of the hemorrhage mechanisms revealed: (1) A variety of fundus hemorrhage phenomena coexisted, namely the intrapapillary, adjacent peripapillary, intraretinal, vitreous, and subretinal hemorrhages, originating from the center of the cup and/or the nasal edge of the optic disc. In all patients, crescent-shaped peripapillary subretinal hemorrhages were located along the nasal margin of the optic disc. Some patients with fluorescein angiography exhibited advanced visible optic papilla central or nasal partial fluorescein staining in our patients and others [4]. We inferred that the bleeding in IHAPSH may originate from optical disc nasal rim deep vessels rather than the superficial vessels of the optic disc; (2) we observed a sharp edge on the nasal side and a rounded edge on the temporal side of the scleral canal OCT. We also confirmed that IHAPSH patients have smaller optic discs and a higher level of nerve fiber crowding than controls. The nerve fibers enter through a small scleral ring along an acutely angled pathway into the nasal retina, and temporal fibers gently bend over the scleral rim [9]. The scleral canal forms a section of a cone. A smaller inside diameter of the scleral canal formed inside the mouth edge protruding and attached to the optic nerve can lead to circulatory disorders involving the optic nerve [10]. It is speculated that the bleeding was associated with compression of the scleral canal and was most likely to occur in the prelaminar region adjacent to the inner aspect of the scleral canal; (3) vision mild depression, normal visual field, or only a mild enlargement of the blind spot with mild losses in the peripheral fields in some of the affected and contralateral eyes of IHAPSH patients, shows that the reason may be because of hemorrhage of the optic disc, or nerve fiber was compressed, while rather than nerve fiber itself pathological changes; (4) the vascular anatomy of the prelaminar portion of the ONH has unique architecture [11, 12]. The arterial supply of the ONH is derived from peripapillary choroid and posterior short ciliary arteries, whereas the venous system of the prelaminar portion almost exclusively drains to the central retinal vein with minor contributions to the peripapillary choroidal veins. Vessels from the peripapillary choriocapillaris, choroidal branches that supply the prelaminar nerve head, and branches of the posterior ciliary artery that traverse the border tissue of Elschnig [13–15] in the nasal region of the disc, may be prone to stretching, kinking, and/or compression in crowded and tilted optic discs [8]; (5) a defect in the permeability of the blood–retina barrier in the ONH region has been demonstrated at the junction between the subretinal space and the border tissue of Elschnig [16]. Optic papilla nasal bleeding can come to the subretinal space through this junction; in patients with IHAPSH due to uplift of the neural tissue that resulted in stretching of the width of the subretinal space, bleeding can easily reach the subretinal space.
We inferred that the pathogenetic mechanisms of IHAPSH were as follows. In myopic eyes with an anatomy characterized by a specific optic disc of unique structural architecture, the elevated superior and nasal margin of the crowded tilted disc and the crowding of optic nerve fibers due to their compression by a small scleral canal that has a sharp nasal edge predispose to a circulatory compromise of the ONH in IHAPSH. Bleeding originates from capillaries of the peripapillary choriocapillaris and branches of the posterior ciliary artery origin that traverse the border tissue of Elschnig, between the nasal side of the optic nerve and the adjacent inner aspect of the scleral canal. The onset of bleeding could be spontaneous, but could also be the result of an acute event (a Valsalva maneuver or movement), reaching the nasal edge of the optic nerve adjacent to the subretinal space, peripapillary intraretinal, and/or penetrating the nerve tissues in the prelaminar portion of the optic disc, reaching the optic cup and spreading to the disc surface, the shallow portion of the retina around the optic disc, or vitreous.
References
Cibis GW, Watzke RC, Chua J (1975) Retinal hemorrhages in posterior vitreous detachment. Am J Ophthalmol 80:1043–1046
Katz B, Hoyt WF (1995) Intrapapillary and peripapillary hemorrhage in young patients with incomplete posterior vitreous detachment. Signs of vitreopapillary traction. Ophthalmology 102:349–354
Kokame GT (1995) Intrapapillary, peripapillary, and vitreous hemorrhage. Ophthalmology 102:1003–1004
Kokame GT, Yamamoto I, Kishi S, Tamura A, Drouilhet JH (2004) Intrapapillary hemorrhage with adjacent peripapillary subretinal hemorrhage. Ophthalmology 111:926–930
Contreras I, Rebolleda G, Noval S, Munoz-Negrete FJ (2007) Optic disc evaluation by optical coherence tomography in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci 48:4087–4092
Floyd MS, Katz BJ, Digre KB (2005) Measurement of the scleral canal using optical coherence tomography in patients with optic nerve drusen. Am J Ophthalmol 139:664–669
Sibony P, Fourman S, Honkanen R, El Baba F (2008) Asymptomatic peripapillary subretinal hemorrhage: a study of 10 cases. J Neuroophthalmol 28:114–119
Strouthidis NG, Yang H, Downs JC, Burgoyne CF (2009) Comparison of clinical and three-dimensional histomorphometric optic disc margin anatomy. Invest Ophthalmol Vis Sci 50:2165–2174
Apple DJ, Rabb MF, Walsh PM (1982) Congenital anomalies of the optic disc. Surv Ophthalmol 27:3–41
Bellezza AJ, Rintalan CJ, Thompson HW, Downs JC, Hart RT, Burgoyne CF (2003) Deformation of the lamina cribrosa and anterior scleral canal wall in early experimental glaucoma. Invest Ophthalmol Vis Sci 44:623–637
Hayreh SS (2001) The blood supply of the optic nerve head and the evaluation of it - myth and reality. Prog Retin Eye Res 20:563–593
Onda E, Cioffi GA, Bacon DR, Van Buskirk EM (1995) Microvasculature of the human optic nerve. Am J Ophthalmol 120:92–102
Lieberman MF, Maumenee AE, Green WR (1976) Histologic studies of the vasculature of the anterior optic nerve. Am J Ophthalmol 82:405–423
Hayreh SS (1974) Anatomy and physiology of the optic nerve head. Trans Am Acad Ophthalmol Otolaryngol 78:OP240–OP254
Teng Y, Yu XH, Dong L, Teng YF, Su Y (2012) Clinical characteristics and pathogenesis of intracapillary hemorrhage with adjacent peripapillary subretinal hemorrhage. Zhonghua Yan Ke Za Zhi 48:131–136
Flage T, Ringvold A (1980) Demonstration of a diffusional pathway between the subretinal space and the juxtapapillary connective tissue. An in vitro experiment using horseradish peroxidase as a tracer. Acta Ophthalmol (Copenh) 58:899–907
Financial disclosure
This study was supported by the International Science & Technology Cooperation Project of Heilongjiang Province, China (WB08B02), Harbin, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have full control of all primary data and they agree to allow Graefes Archive for Clinical and Experimental Ophthalmology to review their data upon request.
Yan Teng, Xuhui Yu, and Yufei Teng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Teng, Y., Yu, X., Teng, Y. et al. Evaluation of crowded optic nerve head and small scleral canal in intrapapillary hemorrhage with adjacent peripapillary subretinal hemorrhage. Graefes Arch Clin Exp Ophthalmol 252, 241–248 (2014). https://doi.org/10.1007/s00417-013-2459-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2459-4