Abstract
Background
Stimulus parameters, in particular pulse shape, are an important consideration in the application of electrical stimulation when experimentally testing a visual prosthesis. We changed the biphasic pulse shape of several asymmetric charge-balanced pulses to investigate their effect on optic nerve (ON) stimulation and the recorded cortical response.
Methods
Monopolar platinum–iridium electrodes were implanted into the rabbit’s ON behind the eyeball. Electrical evoked potentials (EEPs) were recorded with silver ball electrodes placed on the cortex, and the results quantified.
Results
Our results indicate that changing the shape of cathodic-first charge-balanced biphasic pulse (CA) while maintaining charge balance could reduce the current thresholds for stimulation. When stimulated at the same charge density, the stimulus having high-amplitude short-duration (HASD) cathodic phase produced a higher amplitude response, with a larger spatial spread but with a lower current threshold compared with other stimuli. Adding an inter-phase gap between the two phases of the stimulus increased the EEP amplitude, but was saturated at a gap of ∼0.2 ms; this was most obvious with CA stimulation, which was able to elicit a larger cortical response than that elicited by asymmetrical charge-balanced stimulus pulses with HASD cathodic phase, in contrast to CA without a gap. As the stimulating frequency increased, the amplitudes of the EEP components elicited by CA monotonically decreased. The fastest component (P0) was present with stimulating frequencies as high as 80 Hz, while the slower P1 and P2 disappeared with stimulating frequencies higher than 40 and 20 Hz, respectively.
Conclusion
A CA stimulus waveform with an inter-phase gap of 0.2 ms was more efficacious for ON stimulation than other stimulus combinations, and therefore should result in less tissue damage, minimal electrode etching, and lower power consumption if used in a visual prosthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A visual prosthesis is a new way in which clinicians hope to restore partial vision for blind patients [1–6]. By stimulating the surviving parts of the visual system and bypassing the impaired regions, it is now possible to reconstruct part of the visual percept for blind patients [7, 8]. The optic nerve (ON) containing the surviving retinal ganglion cell axons was proposed as a suitable location for a visual prosthesis implant by Verrart et al. [9]. They implanted a surface spiral cuff electrode array around the ON of two volunteers suffering from RP. In one patient the prosthesis was placed around the intracranial portion of the ON, and in the second patient the prosthesis was positioned intraorbitally, which proved to be surgically easier for exposing a sufficient length of the ON, and required less invasive surgery [9, 10]. After training and testing, the patient could recognize simple characters or the direction of movement even though only four stimulating electrodes were used [11, 12]. We have proposed a different visual prosthetic approach that consists of penetrating electrodes within the ON [13, 14]. Previous reports concerning cochlear and cortical implant experiments have shown that penetrating electrode stimulation has better spatial resolution than surface stimulation [6, 15].
Stimulus parameters, in particular pulse shape, are an important consideration in the application of electrical stimulation when experimentally testing a visual prosthesis. In addition, safe neural stimulation must be considered in order to prevent damage to the neural tissue and electrode. The stimulating pulse causes chemical reaction and electrode etching at an electrode tip, and must be reversed by a pulse of opposite polarity [16]. Irreversible reactions, such as electrode dissolution or water electrolysis, can occur if the charge imposed on the electrode exceeds its charge injection limit, and cannot be effectively reversed. Brummer et al. [16] reported that a minimum requirement for safe electrical stimulation should stipulate the use of charge-balanced stimulating pulses. Monophasic stimulation can lead to charge being accumulated and, to some degree, results in irreversible chemical reactions eroding the electrodes and tissue lesions [17, 18]. Shepherd et al. [19] chronically stimulated cat auditory nerve fibers by noncharge-balanced stimuli, and reported a severe reduction in ganglion cell density with stimulus intensities as low as 0.4 μA. Fortunately, the damage induced by stimulation can be lessened by the application of charge-balanced stimulus waveforms [20–22].
A symmetric charge-balanced biphasic pulse is a universally used stimulus pulse in visual prostheses and other artificial implants (e.g., a cochlear implant). Recently, research on cochlear implants [23, 24] found that changing biphasic pulse shape and maintaining charge balance could reduce stimulating current threshold and was beneficial in terms of reducing power consumption, channel interactions, and increasing spatial resolution. Accordingly, we changed the biphasic pulse shape of several asymmetric charge-balanced pulses to investigate their effect on ON stimulation. The efficacy of the pulses was assessed by recording multi-peaked electrically evoked cortical potentials (EEPs). This data provides an experimental reference for stimulus parameter design, which can be used to reduce power consumption and increase the efficacy of ON stimulation.
Methods and materials
Animals
Fifteen healthy adult Chinese albino rabbits (Fengxian, Shanghai, China), weighing 2.0–2.5 kg, were used in the experiments. Experimental procedures were in accordance with the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research and the policies in the Guide for the Care and Use of Laboratory Animals issued by the U.S. National Institutes of Health and approved by the Ethics Committee of Shanghai Jiao Tong University.
Surgical procedures
The methods of anesthesia and surgery have been reported previously [14], and will be briefly summarized here. The rabbit was anesthetized with 5 % pentobarbital sodium at an initial dose of 5 mg/kg and maintained at 15 mg/kg/h. Body temperature and heart rate were monitored during the experiment. Craniotomy was used to expose the visual cortex (primary visual cortex, area 17, V1) contralateral to the operated eye. The open area extended from 1 to 10.5 mm lateral to the midline and 5 to 14.5 mm posterior to the bregma suture, and encompassed the entire rabbit V1 [25]. ON exposure was accomplished by making an incision ∼3 mm above the upper eyelid, then separating and retracting the orbicularis oculi and levator muscles from the fascia to expose the cartilage of the upper orbit. An incision was made between the cartilage and the frontal bone and then enlarged along its bony edge (∼14 mm) so that the periorbita could be seen clearly. The orbital fascia was carefully opened until the superior rectus muscle appeared, which was then cut at its insertion point and retracted. In this way, ∼3 mm of the intraorbital ON behind the eyeball was exposed.
Stimulating and recording
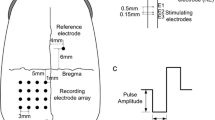
Monopolar electrical stimulation was achieved by a polytetrafluoroethylene-insulated platinum–iridium stimulating electrode inserted to a depth of ∼0.2–0.4 mm into the dorsal–temporal side of the ON. Three electrodes were inserted into the ON 1 mm behind the globe, and were perpendicular to the ON axis with an inter-electrode distance of 0.5 mm (Fig. 1a). The inner and outer diameters (insulation) of the stimulating electrodes were 80 μm and 90 μm respectively, with an uncoated sharpened tip length of 100 μm (area ∼2.7 × 10-4 cm2). The impedance of the electrode ranged from 5–10 kΩ measured by a Precision LCR Meter (E4980A, Agilent Technologies, Santa Clara, CA, USA) using a 100 μA, 1 kHz current. Electrical stimulating current pulses were generated by an isolated and programmable current source stimulator (MS16, Tucker–Davis Technologies, Alachua, FL, USA). A stainless steel needle was inserted into the sclera and used as a return electrode.
A recording electrode array made up of 5 × 6 silver-ball electrodes, 0.3–0.4 mm in diameter with an inter-electrode distance of 2 mm (from the electrode center), was placed on the exposed V1 contralateral to the operated eye in Fig. 1a. The impedance of each silver-ball electrode ranged from 500–800 Ω measured as described above. A stainless-steel needle reference electrode was inserted into the scalp of the forehead ipsilateral to the stimulated eye, and a subcutaneous ground electrode was placed in the ear tip. EEPs in response to ON stimulation were recorded on 30 channels by a TDT system (System3, Tucker–Davis Technologies, Alachua, FL, USA). The 30 EEPs were compared to find the channel with the maximal recorded EEP amplitude, and this was designated as the M-channel and then used to determine ON current threshold.
Visually evoked potentials (VEPs) to a full-field, bright-flash white light were recorded before the electrical stimulation (Fig. 2a) and served as control data. The light source (LS1130-Pulsed Xenon FlashPac; Tucker–Davis Technologies, Alachua, FL, USA) was placed 2.5 cm in front of the operated eye and had a flash luminance of 1.5 cd·s/m2 with a duration of 10 μs, and was repeated at a frequency of 1 Hz.
a An averaged visually evoked cortical waveform elicited by full-field, bright white-light flashed stimuli (flash luminance 1.5 cd·s/m2, duration of 10 μs, 1 Hz, n = 50). The arrow indicates the onset of light stimulus. b An averaged evoked cortical waveform elicited by stimulation with a penetrating ON electrode using a cathodic first charge balanced biphasic pulse (CA: current intensity 50 μA, pulse duration 0.2 ms, stimulating frequency 1 Hz; n = 50). The stimulus artifact and three components (P0, P1 and P2) in the EEP are indicated
Stimulating current pulse shapes
An overview of the stimulating pulse shapes are presented in Fig. 1b. The stimuli were coded according to the following system: (1) CA — cathodic-first, charge-balanced biphasic pulse, (2) AC — anodic-first, charge-balanced biphasic pulse, (3) CA-IPG — cathodic-first, charge-balanced biphasic pulse with inter-phase gap (IPG), (4) Ca — cathodic-first, charge-balanced anterior pseudomonophasic pulse, (5) Ac — anodic-first, charge-balanced anterior pseudomonophasic pulse, (6) Ca-IPG — cathodic-first, charge-balanced anterior pseudomonophasic pulse with IPG, (7) cA — cathodic-first, charge-balanced posterior pseudomonophasic pulse, (8) aC — anodic-first, charge-balanced posterior pseudomonophasic pulse, and (9) aC-IPG — anodic-first, charge-balanced posterior pseudomonophasic pulse with IPG.
In order to determine the current threshold for the EEP responses, the current amplitude was reduced gradually by half until reproducible averaged EEPs were undetectable in the M-channel. The EEP response was deemed to be present only when the second positive EEP peak (P1; see Fig. 2b) was two times greater than the root mean square (RMS) of the baseline fluctuation. After that, the current amplitude was increased in 1 μA or 2 μA steps, until the minimal current to elicit the cortical responses was determined. This minimal current was defined as the threshold. The current threshold of P1 elicited by a CA with a pulse duration of 0.2 ms was 24.2 ± 2.6 μA (seven M-channels, seven rabbits). The stimulating current intensity for the high-amplitude short-duration (HASD) phase of the different stimulus pulse shapes was then set to 50 μA (about two times threshold) and pulse duration 0.2 ms (which corresponds to a stimulating charge density of 37 μC/cm2/phase) in order to ensure a stimulation of a significant portion of the ON fiber population.
Data analysis
Multi-channel signals were recorded by a TDT system at a 6 kHz sampling rate per channel, amplified, and filtered with a band-pass filter of 1–2000 Hz. The cortical response elicited by stimulus pulse with frequency 1Hz was analyzed to evaluate the effects of different stimulus waveforms on the EEP. Fifty consecutive responses to stimuli with 1 s interval were averaged. The frequency of the stimulus pulse was varied from 1 to 80 Hz to evaluate the effect of stimulus frequency on the EEP. Cortical responses to 50 stimulus pulses were averaged. To analyze the stimulation efficacy of different stimulus pulse shapes, only the amplitudes of the EEP components at the M-channel (as defined previously) were analyzed; however, the P1 (the most stable and largest component in EEP ) amplitudes at the other 29 recording electrodes were also measured to determine the spatial spread of the cortical response (Fig. 3). 2-D data interpolation with a 0.01 step was used to redefine the 30 P1 amplitudes. The recording area over which the P1 amplitudes of the 30 electrodes were higher than 50 % of the maximal amplitude was defined as the spatial extent of the response to the stimulus waveform, which was self- normalized to reduce the variations among different animals.
Spatial spread extent of EEP. a Original EEP waveforms from 30 recording channels. b Color-coded map of P1 amplitudes. c Color-coded map of P1 amplitudes with 2-D interpolation. d Contour map of P1 amplitudes with 2-D interpolation. The area inside bold line (the amplitudes represent those that were half of the M-channel response) was defined as the spatial extent of the P1 of EEP
Results were analyzed for statistical significance by a one-way ANOVA and paired t-tests; p ≤ 0.05 was considered significant for both tests. Data is presented as the mean ± SE. In general, stable responses could be obtained for 6 h of continuous stimulation for a particular stimulus pattern. Due to the number of different stimulus combinations used overall and the relatively limited duration of each experiment, different protocols were conducted in different experiments. This also ensured that the same stimulus regime was not consistently used towards the end of the experiments when the animal may not show optimal responses (e.g., due to extended periods of anaesthesia). It should also be noted that in each animal the ON was stimulated by up to three implanted electrodes, and thus for each stimulus regime could be up to three M-channels per animal; this plus the different protocols per animal is reflected in the different n (i.e., number of recording electrodes and animals) within the Results section.
Results
VEP and EEP waveforms
VEP and EEP waveforms are shown in Fig. 2. The EEP waveform had a similar shape as the VEP, but with a much shorter latency due to duration of retinal processing included in the latency of the VEP. The EEP waveform consisted of three positive components, P0, P1, and P2. The implicit times for P0, P1, and P2 elicited by a CA with a 50 μA current intensity and 0.2 ms pulse duration at a stimulating frequency of 1Hz were 4.4 ± 0.01 ms, 11.0 ± 0.3 ms, and 21.3 ± 1.7 ms respectively (16 M-channels, six rabbits). The amplitudes of the P0 and P1 peaks (measured from the immediately preceding trough to the peak of each component) were used to analyze the effects of different stimulus pulse shapes and parameters on the cortical responses. The P2 amplitude was not shown because it was sometimes influenced by the decline phase of P1.
Effects of pulse duration on cortical responses
The effect of pulse duration in the long-duration phase of a pseudomonophasic stimulus pulses was examined. The pulse duration ratio between the short-duration phase (with fixed stimulus intensity and phase duration) and the long-duration phase of the Ca and aC (produced by changing the pulse shape of CA and AC) was set at 1:1, 1:2, 1:5, and 1:10. The current intensity of the long-duration phase was varied accordingly in order to maintain charge balance (Fig. 4). The amplitudes of the P0 and P1 in response to Ca and aC were normalized against a response elicited by stimulus pulses with a 1:1 pulse duration ratio. The ANOVA of the P0 amplitudes showed the pulse duration ratio of Ca or aC to be significant (p < 0.05; ten M-channels, six rabbits). However, there were no significant differences between the P0 amplitudes with pulse duration ratios greater than 1:1. P1 amplitudes were significantly higher, with pulse duration ratios of 1:5 and 1:10 compared with a ratio of 1:1 and 1:2 (p < 0.05), although a ratio of 1:5 vs 1:10 was not significant. Therefore, the pulse duration ratio between the short-duration phase and the long-duration phase for an asymmetrical charge balance stimulus pulse of 1:5 was used for the following experiments.
Effects of changing the duration of the anodic phase of the biphasic stimulus pulse on V1 EEP amplitudes. Two kinds of stimulus waveforms were delivered to ON; a cathodic first charge-balanced anterior pseudomonophasic pulse (Ca), and b anodic first charge-balanced posterior pseudomonophasic pulse (aC). The cathodic phase duration was fixed at 0.2 ms with current intensity 50 μA (∼ 2x threshold of CA). The charge amount of the two phases was fixed to 10 nC and P0 and P1 peak potential amplitudes were normalized to that elicited by a stimulus pulse with a 1:1 duration ratio. *p < 0.05 vs P0, # p < 0.05 vs P1 (ten M-channels, six rabbits, mean ± SE)
Effects of stimulus pulse shape on cortical threshold, amplitude, and spatial responses
Six different stimulus pulse shapes (CA, AC, cA, aC, Ca, and Ac; see Fig. 1b) were used to stimulate the ON fibers, in order to determine which stimulus would evoke the largest V1 response when using the same stimulus intensity and charge density (37 μC/cm2/phase). The results are shown in Fig. 5a, and the P1 current thresholds are shown in Fig. 5b. The current thresholds were normalized to that of CA. aC and Ca had significantly lower current thresholds compared to the CA, while AC, cA, and Ac had significantly higher current thresholds (p < 0.05; eight M-channels, five rabbits). Current thresholds for aC vs Ca and cA vs Ac were not significantly different. The P0 and P1 amplitudes elicited by the same stimulating charge density are shown in Fig. 5c. Similarly to current threshold responses, significant changes were seen in the P0 and P1 amplitudes between each of the stimulus patterns, although P0 vs P1 comparisons within a stimulus pattern were not significantly different. Examples of the spatial extent of the cortical responses (based on P1 amplitudes) elicited by the six stimuli for one animal are shown in Fig. 5d, and their quantification shown in Fig. 5e. Stimulus pulses with a lower current threshold elicited a larger spatial spread across the cortex. In addition, the M-channel for each different stimulus was recorded at the same location. The spatial spread elicited by an AC stimulus was slightly smaller than elicited by CA (P > 0.05); Ca and aC stimuli elicited larger spatial spreads than that of CA, while Ac and cA elicited a significantly smaller spatial spread compared with CA (p < 0.05).
Effects of pulse shape on the EEP. a EEP waveform series elicited by different stimulus pulse shapes with the HASD phase intensity 50 μA, pulse duration 0.2 ms and stimulating frequency 1 Hz. All the data were from the same M-channel of one animal. b Current thresholds for P1 elicited by the six kinds of charge-balanced stimulus pulse shapes. The duration of the HASD phase was 0.2 ms. The ratio between the duration of short phase and long phase was 1:5 (eight M-channels, five rabbits, mean ± SE). c The P0 and P1 EEP amplitudes elicited by the six stimulus pulses to that elicited by CA in the same animals; amplitudes were normalized to that elicited by CA. d Spatial distribution maps of P1 amplitudes across the 30 recording channels of the array using different stimulus pulses; amplitudes were normalized to the maximal one (data from the same animal). e The P1 spatial spread in the same animals elicited by the six stimulus pulses was quantified and normalized to that elicited by CA. (*p < 0.05 vs P0 , # p < 0.05 vs P1)
Effects of an inter-phase gap between stimulus pulses
Figure 6 shows the effect of changing the inter-phase gap on the P0 and P1 amplitudes when using the same stimulus charge density. Increasing the gap from 0 to 0.2 ms caused a significant increase in the magnitude of both EEP peaks when using a CA stimulus (p < 0.05; 16 M-channels, seven rabbits), although further gap increases had no additional effect; P0 amplitudes were slightly lower than P1 amplitudes but not significantly so. Similarly, an inter-phase gap of 0.2 ms with Ca or aC stimuli caused a significant increase in peak P0 and P1 amplitudes (p < 0.05; larger gaps had no additional effect), but these were significantly lower than that seen with CA stimulation (p < 0.05), in spite of the fact that the recorded P0 and P1 amplitudes in response to a gap =0 were significantly higher compared with CA stimulation (p < 0.05). Thus, the amount of the amplitude increase is significantly less when using Ca or aC stimuli with larger inter-phase gaps. There were no significant differences between Ca and aC and changes in the inter-phase gap (p > 0.05). Similarly to that seen with CA stimulation, changes in the P1 amplitudes were all slightly higher than the changes seen in the P0 amplitudes.
Effect of an inter-phase gap on the P0 and P1. Different stimulus pulse shapes were used; a CA b Ca c aC (HASD phase intensity 50 μA and pulse duration 0.2 ms). The gap ranged from 0.0 ms to 1.0 ms. The ratio between the duration of short phase and long phase was 1:5. The amplitudes were normalized to CA without an inter-phase gap. *P < 0.05 vs P0 , # P < 0.05 vs P1 in a. (16 M-channels, seven rabbits, mean ± SE)
Effects of stimulating frequency on cortical responses
An example of averaged EEP series from V1 elicited by a CA-IPG stimulus (current intensity 50 μA, pulse duration 0.2 ms and inter-phase gap of 0.5 ms, as determined in the previous sections) over frequency range of 1 to 80 Hz is shown in Fig. 7a (range of frequencies used are those shown in the figure). The P0 and P1 amplitudes clearly decreased as stimulating frequency increased, although the P1 amplitudes declined much more steeply than P0 amplitudes did. The P0 response was still obviously present at 80 Hz, while measurable responses were not recorded with frequencies higher than 40 Hz for P1 component (Fig. 7b). Furthermore, P0 and P1 had a relatively linear decline with frequencies, demonstrating an exponential decrease in amplitude as stimulus frequencies increase.
a An example of EEP waveforms recorded from V1 as stimulating frequency increased from 1 to 80 Hz. The stimulus pulse was a CA-IPG (current intensity 50 μA, pulse duration 0.2 ms, and inter-phase gap 0.5 ms). b Effect of stimulating frequency (log scale) on the P0 and P1 amplitudes using the same stimulus pulse shape as a (11 M-channels, eight rabbits, mean ± SE). The amplitudes elicited by different stimulating frequencies were normalized to that elicited by stimulating frequency at 1 Hz. *P < 0.05 vs P0 , # P < 0.05 vs P1
Discussion
Stimulation parameters are an important aspect when considering the design and function of a visual prosthesis. An optimal stimulating parameter may not only reduce tissue injury but also reduce the power requirement for activating the target tissue, consequently minimizing power consumption. Tissue injury and power considerations are of paramount importance in prosthetic design. Our work shows that various changes to the stimulus can maximize the recorded EEP amplitude at the level of the cortex, and suggests that sufficient cortical activation has been achieved to induce a phosphene percept. Our data provide a starting point for manipulating the effects of pulse shape and parameters when using a visual prosthesis implanted within the ON.
There were three positive components (P0, P1, and P2) in the V1 EEP elicited by ON stimulation, which have distinctive current thresholds and temporal characteristics. Our previous study showed that components with shorter implicit time had lower current thresholds and faster temporal variation compared to longer implicit time components [14]. The faster components might reflect the inputs through faster fibers with large diameters, while the slower components are less readily explained. Slower components may be elicited following activation of slower ON fibers, or influenced by intracortical signaling, as well as collicular input via thalamic connections [26, 27].
The current threshold needed to elicit cortical responses was polarity-dependent, and cathodic pulses were more effective than anodic pulses for ON stimulation. This is in agreement with previous studies of monopolar stimulation of single cat auditory-nerve fibers or epiretinal stimulation in the rabbit [28–30]. CA stimulation had a lower current threshold than AC; moreover, an asymmetrical charge-balanced stimulus pulse with a HASD cathodic phase had significantly lower current thresholds than a CA stimulus. In contrast, an asymmetrical charge-balanced stimulus pulse with a HASD anodic phase had significantly higher current thresholds than a CA stimulus. In addition, the EEP amplitudes elicited by different stimulus pulse shapes (using the same charge density) showed an opposite relationship to the current thresholds.
By electrically stimulating the cochlea of the guinea pig and cat and recording responses from the auditory nerve trunk, Miller et al. reported that a pseudomonophasic (PS) stimulus with HASD cathodic phase first and low-amplitude long-duration anodic phase second can present some advantages of monophasic stimuli, such as producing lower thresholds relative to CA stimuli [31]. This implied that pseudomonophasic stimuli approached the function of monophasic stimuli. Macherey et al. [24] investigated the relative contributions of the two different phases of a pseudomonophasic stimulus with an inter-phase gap in patients fitted with a cochlear implant. They demonstrated that the short/high phase dominated at threshold, but that the long/low one still had a significant effect. In our study, Ca stimulation greatly reduced the current threshold compared to CA, which was consistent with the theory of van den Honert and Mortimer [32], in which they proposed that delaying the charge recovery of the second phase of a pulse will increase the stimulating effect of the first phase. Pseudomonophasic stimuli were demonstrated to have lower thresholds and a higher dynamic range than biphasic stimuli [23, 31]. Stimulation with aC also significantly reduced the current threshold, but there was no statistical difference between the current thresholds of aC vs Ca. Grill et al. [33] reported that a subthreshold hyperpolarizing pre-pulse could induce a transient increase in excitability by reducing the threshold of the following depolarizing phase and an increase in spatial selectivity. Because the trans-membrane voltage of ON fiber was anodic outside and cathodic inside, an aC stimulus waveform pattern should hyperpolarize the ON fibers with the first low-amplitude long-duration anodic phase, and then depolarize the ON fibers with the HASD cathodic phase to promote the excitability of ON fibers. However, our study did not detect a noticeable effect arising from the hyperpolarizing pre-pulse. This may be due to a nonoptimal current intensity and duration of the hyperpolarizing pre-pulse. Van Wieringen et al. [34] using bipolar intra-cochlea stimulation also investigated the effect of aC in humans, and found no obvious transient increase in excitability produced by a hyperpolarizing pre-pulse. However, this may be a result of the bipolar stimulating configuration, because the function of the hyperpolarizing pulse delivered by an electrode can be influenced by the depolarizing pulse on the other electrode near by.
The effects of stimulus pulse shapes on spatial selectivity can help optimize the strategy of electrical stimulation. The spatial distributions of P1 amplitudes in response to different stimulus waveforms at same electrode were slightly different, but the M-channel position remained stable. Grill and Mortimer [35] suggested that short pulse widths were more spatially selective than long widths. Accordingly, increasing the effect of the HASD phase in a charge-balanced stimulus (e.g., Ca and aC) may result in a more spatially selective stimulation of ON fibers, although this will require further study. However, long pulses were demonstrated to produce phosphenes more ‘substantial’ than short pulses in cortical prosthesis [6].
Adding an inter-phase gap between the phases of a biphasic pulse was another way to reduce the counteracting effect of the phases. It was evident that adding a gap significantly increased the EEP amplitude, but that was saturated after a ∼0.2 ms gap. CA elicited the largest EEP amplitudes after adding a gap, and was consistent with our previous study (inter-phase gap of 50 μs); however, in that study bipolar aC stimulation elicited the lowest EEP amplitude, in contrast to monopolar stimulation [13]. Increasing the inter-phase gap of a biphasic pulse led to a lower current threshold (a minimum threshold was achieved with ∼0.1 ms) in cat auditory nerve fibers [36]. The effect of an inter-phase gap should lower ON thresholds and thus increase EEP amplitudes (as seen in the present study) for the same current pulse. Prado-Guitierrez et al. [37] found the magnitude of the gap effect was related to the neural survival.
As the stimulus frequency increased, the amplitudes of the cortical responses elicited by CA monotonically decreased, and is in agreement with previous results for the rabbit ON, showing that stimulation of 1–10 Hz results in a decline in EEP amplitudes [13]. Rizzo et al. [28] electrically stimulated the rabbit epi-retina at 0.5–16 Hz, and reported that the EEP amplitude responses also monotonically declined at higher frequencies. The reduction in the slope of normalized EEP amplitudes with epi-retinal stimulation (2–16 Hz; ∼3 %/Hz) [28] and ON stimulation (2–10 Hz; ∼3 %/Hz) [13] were similar to our P1 data (∼4 %/Hz). Burke et al. [26, 27] suggested that inhibition within the dorsal lateral geniculate nucleus (either feed-forward or feed-back inhibition) could account for a decreased cortical response when the cat ON was electrically stimulated by repeated pulses at short intervals or a train of stimuli. In contrast, the thresholds for phosphene perception produced by ON electrical stimulation in RP patients decreased as the stimulating frequency increased over a range of 10–320 Hz [38]. The P0 and P1 potentials differed in their responses to the frequency increases, indicating that the fast P0 potential had better temporal resolution and could respond to higher frequencies compared to the slower P1 potential. The different cortical potentials may represent the response of different diameter ON fibers carrying distinct visual signals [27]; thus, differential usage of stimulus frequency could potentially be selective for different visual channels, and add a further dimension to the percepts of the blind prosthetic user.
This study provides experimental data on the effects of stimulus pulse shape and other parameters that affect cortical responses. The results showed that a pseudomonophasic pulse with either a previous HASD cathodic phase or after a low-amplitude long-duration anodic phase (Ca and aC), could markedly reduce voltage requirements to obtain an EEP amplitude that was similar to or higher than that obtained with CA stimuli. Adding an inter-phase gap of 0.2 ms significantly increased the M-channel response when using CA stimulation compared to a weaker response when using Ca and aC. In addition, the current threshold of elicited cortical responses was polarity-dependent, and a stimulus waveform with a HASD cathodic pulse was more effective than an anodic pulse. EEP amplitudes decreased monotonically as the stimulating frequency increased, and the components in EEP had different frequency limits. A ON stimulus pulse with lower threshold has a larger spatial spread across the cortex, but the focus of the responses remains at the same V1 location. Overall, CA stimuli with 0.2 ms pulse gap could be beneficial to electrical stimulation of ON visual prosthesis.
Reference
Schmidt EM, Bak MJ, Hambrecht FT, Kufta CV, O'Rourke DK, Vallabhanath P (1996) Feasibility of a visual prosthesis for the blind based on intracortical micro stimulation of the visual cortex. Brain 119:507–522
Humayun MS, Weiland JD, Fujii GY, Greenberg R, Williamson R, Little J, Mech B, Cimmarusti V, Van Boemel G, Dagnelie G (2003) Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res 43:2573–2581
Rizzo JF 3rd, Wyatt J, Loewenstein J, Kelly S, Shire D (2003) Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. Invest Ophthalmol Vis Sci 44:5355–5361
Kanda H, Morimoto T, Fujikado T, Tano Y, Fukuda Y, Sawai H (2004) Electrophysiological studies of the feasibility of suprachoroidal–transretinal stimulation for artificial vision in normal and RCS rats. Invest Ophthalmol Vis Sci 45:560–566
Kim ET, Kim C, Lee SW, Seo JM, Chung H, Kim SJ (2009) Feasibility of microelectrode array (MEA) based on silicone–polyimide hybrid for retina prosthesis. Invest Ophthalmol Vis Sci 50:4337–4341
Yamauchi Y, Franco LM, Jackson DJ, Naber JF, Ziv RO, Rizzo JF, Kaplan HJ, Enzmann V (2005) Comparison of electrically evoked cortical potential thresholds generated with subretinal or suprachoroidal placement of a microelectrode array in the rabbit. J Neural Eng 2:S48–S56
Zrenner E, Bartz-Schmidt KU, Benav H, Besch D, Bruckmann A, Gabel VP, Gekeler F, Greppmaier U, Harscher A, Kibbel S (2011) Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci 278:1489–1497
da Cruz L, Coley B, Christopher P, Merlini F, Wuyyuru V, Sahel JA, Stanga P, Filley E, Dagnelie G (2010) Patients blinded by outer retinal dystrophies are able to identify letters using the ArgusTM II retinal prosthesis system. Invest Ophthalmol Visual Sci 51: ARVO E-Abstract #2023
Brelén ME, De Potter P, Gersdorff M, Gersdorff M, Cosnard G, Veraart C, Delbeke J (2006) Intraorbital implantation of a stimulating electrode for an optic nerve visual prosthesis. J Neurosurg 104:593–597
Veraart C, Raftopoulos C, Mortimer JT, Delbeke J, Pins D, Michaux G, Vanlierde A, Parrini S, Wanet-Defalque MC (1998) Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res 813:181–186
Duret F, Brelén ME, Lambert V, Gérard B, Delbeke J, Veraart C (2006) Object localization, discrimination, and grasping with the optic nerve visual prosthesis. Restor Neurol Neurosci 24:31–40
Veraart C, Wanet-Defalque MC, Gérard B, Vanlierde A, Delbeke J (2003) Pattern recognition with the optic nerve visual prosthesis. Artif Organs 27:996–1004
Li L, Cao P, Sun M, Chai X, Wu K, Xu X, Li X, Ren Q (2009) Intraorbital optic nerve stimulation with penetrating electrodes: in vivo electrophysiology study in rabbits. Graefes Arch Clin Exp Ophthalmol 247:349–361
Sun J, Lu Y, Cao P, Li X, Cai C, Chai X, Ren Q, Li L (2010) Spatiotemporal properties of multipeaked electrically evoked potentials elicited by penetrative optic nerve stimulation in rabbits. Invest Ophthalmol Vis Sci 52:146–154
Rosahl SK, Mark G, Herzog M, Pantazis C, Gharabaghi F, Matthies C, Brinker T, Samii M (2001) Far-field responses to stimulation of the cochlear nucleus by microsurgically placed penetrating and surface electrodes in the cat. J Neurosurg 95:845–852
Brummer SB, Turner MJ (1977) Electrochemical considerations for safe electrical stimulation of the nervous system with platinum electrodes. IEEE Trans Biomed Eng 24:59–63
Rowland V, MacIntyre WJ, Bidder TG (1960) The production of brain lesions with electric currents. II. Bidirectional currents. J Neurosurg 17:55–69
Loucks RB, Weinberg H, Smith M (1959) The erosion of electrodes by small currents. Electroencephalogr Clin Neurophysiol 11:823–826
Shepherd RK (1999) Chronic electrical stimulation of the auditory nerve using non-charge-balanced stimuli. Acta Otolaryngol 119:674–684
Lilly JC, Hughes JR, Alvord EC Jr, Galkin TW (1955) Brief, noninjurious electric waveform for stimulation of the brain. Science 121:468–469
Lilly JC, Cherry RB (1955) Surface movements of figures in spontaneous activity of anesthetized cerebral cortex: leading and trailing edges. J Neurophysiol 18:18–32
Lilly JC (1961) Injury and excitation by electric currents. In: Electrical stimulation of the brain. University of Texas Press, Austin, pp 60–66
Van Wieringen A, Macherey O, Carlyon RP, Deeks JM, Wouters J (2008) Alternative pulse shapes in electrical hearing. Hear Res 242:154–163
Macherey O, Van Wieringen A, Carlyon RP, Deeks JM, Wouters J (2006) Asymmetric pulses in cochlear implants: Effects of pulse shape, polarity, and rate. J Assoc Res Otolaryngol 7:253–266
Choudhury BP (1987) Visual cortex in the albino rabbit. Exp Brain Res 66:565–571
Burke W, Cottee LJ, Garvey J, Kumarasinghe R, Kyriacou C (1986) Selective degeneration of optic nerve fibres in the cat produced by a pressure block. J Physiol 376:461–476
Burke W, Burne JA, Martin PR (1985) Selective block of Y optic nerve fibres in the cat and the occurrence of inhibition in the lateral geniculate nucleus. J Physiol 364:81–92
Rizzo JF 3rd, Goldbaum S, Shahin M, Denison TJ, Wyatt J (2004) In vivo electrical stimulation of rabbit retina with a microfabricated array: strategies to maximize responses for prospective assessment of stimulus efficacy and biocompatibility. Restor Neurol Neurosci 22:429–443
Miller CA, Abbas PJ, Robinson BK, Rubinstein JT, Matsuoka AJ (1999) Electrically evoked single-fiber action potentials from cat: responses to monopolar, monophasic stimulation. Hear Res 130:197–218
Ranck JB (1975) Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 98:417–440
Miller CA, Robinson BK, Rubinstein JT, Abbas PJ, Runge-Samuelson CL (2001) Auditory nerve responses to monophasic and biphasic electric stimuli. Hear Res 151:79–94
van den Honert C, Mortimer JT (1979) The response of the myelinated nerve fiber to short duration biphasic stimulating currents. Ann Biomed Eng 7:117–125
Grill WM, Mortimer JT (1995) Stimulus waveforms for selective neural stimulation. IEEE Eng Med Biol Mag 14:375–385
Van Wieringen A, Carlyon RP, Laneau J, Wouters J (2005) Effects of waveform shape on human sensitivity to electrical stimulation of the inner ear. Hear Res 200:73–86
Grill WM, Mortimer JT (1996) The effect of stimulus pulse duration on selectivity of neural stimulation. IEEE Trans Biomed Eng 43:161–166
Shepherd RK, Javel E (1999) Electrical stimulation of the auditory nerve: II. Effect of stimulus waveshape on single fibre response properties. Hear Res 130:171–188
Prado-guitierrez P, Fewster LM, Heasman JM, Mckay CM, Shepherd RK (2006) Effect of interphase gap and pulse duration on electrically evoked potentials is correlated with auditory nerve survival. Hear Res 215:47–55
Delbeke J, Oozeer M, Veraart C (2003) Position, size and luminosity of phosphenes generated by direct optic nerve stimulation. Vision Res 43:1091–1102
Acknowledgements
This research is supported by The National Basic Research Program of China(973 Program, 2011CB707502/3); The National Natural Science Foundation of China (60971102, 31070981, 61171174, 91120304). The authors thank Dr. T. FitzGibbon for comments on earlier drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, J., Chen, Y., Chai, X. et al. Penetrating electrode stimulation of the rabbit optic nerve: parameters and effects on evoked cortical potentials. Graefes Arch Clin Exp Ophthalmol 251, 2545–2554 (2013). https://doi.org/10.1007/s00417-013-2449-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2449-6