Abstract
Background
This retrospective study investigated the efficacy of tocilizumab (TCZ), a fully humanized antibody that binds both to soluble and membrane bound IL-6 receptors, for the treatment of uveitis-related cystoid macular edema (CME) refractory to immunomodulatory therapy.
Methods
Five refractory patients with uveitis-related CME who received TCZ between January and August 2012 were included. All patients received 8 mg/kg TCZ at 4-week intervals. Data regarding patient demographics, use of immunosuppressive drugs, biologic agents or intravitreal therapies prior to TCZ infusions were collected. Main outcome measure was central foveal thickness (CFT) measured by optical coherence tomography at 6 months. Secondary outcome measures were degree of anterior and posterior chamber inflammation (Standardization of Uveitis Nomenclature Working Group criteria) and visual acuity (logarithm of the minimum angle of resolution [log-MAR]) at month 6. Adverse events (AEs) related to TCZ therapy were also assessed.
Results
Eight eyes from five patients (all females) were included. Mean age was 49.4 years (range, 30–68). Mean follow-up was 8.4 months (range, 6–12). Before TCZ, all patients received and failed conventional immunosuppressive therapy and had received at least another biologic agent. Uveitis diagnoses were Birdshot chorioretinopathy (n = 3), juvenile idiopathic arthritis (JIA)-associated uveitis (n = 1), and idiopathic panuveitis (n = 1). Mean evolution of CME was 13.4 years (range, 2–30). Mean baseline CFT (95 % confidence interval) was 602 ± 236 μm at baseline, 386 ± 113 μm at month 1 (p = 0.006), 323 ± 103 μm at month 3 (p = 0.026), and 294.5 ± 94.5 μm at month 6 (p = 0.014). Median best-corrected visual acuity (BCVA) improved from 0.66 ± 0.57 at baseline to 0.47 ± 0.62 at month 6 (p = 0.035). After 6 months, an improvement of ≥ 2 lines of BCVA was observed in 50 % of eyes (p = 0.028) remained stable in 25 % and worsened in none of the patients. Sustained uveitis remission was achieved in all patients. No AEs were reported.
Conclusions
These data suggest that TCZ is effective for treating CME in otherwise treatment-refractory cases of uveitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystoid macular edema (CME) is the most frequent structural complication of uveitis and is responsible for a significant amount of visual morbidity [1]. Once developed, CME may either spontaneously subside or respond to treatment intended to reduce local immune and inflammatory mediators. In recurrent and chronic forms, systemic treatment should be started, usually consisting on corticosteroids and immunesuppressors [2]. Our group recently reported that dexamethasone intravitreal implant can be effective for treating non-infectious uveitic CME [5]. In cases of lack of efficacy or intolerance, biologic agents such as monoclonal anti-tumor necrosis factor (TNF) antibodies (infliximab and adalimumab) may also be used [3, 4]. Despite these treatment options, a number of patients remain refractory and CME may lead to serious visual impairment [6]. Tocilizumab (TCZ), a fully humanized antibody that binds both to soluble and membrane-bound IL-6 receptors, is currently approved for the treatment of rheumatoid arthritis refractory to one or more anti-TNF drugs [7]. We describe here a case series of five patients treated with TCZ after being diagnosed of CME secondary to uveitis.
Patients and methods
All patients who received tocilizumab (RoActemra®, Hoffmann-La Roche, Basel,Switzerland,Roche) at the Ophthalmology Institute of the Hospital Clinic of Barcelona, Spain from January 2012 to August 2012 were included in this report. In all cases, patients referred progressive visual loss for several months due to CME. Data were collected retrospectively. Written informed consent was obtained from patients for off-label use of TCZ. Institutional review board approval for the record review was granted before the start of the study.
Collected data included demographic data such as age, gender, and type of uveitis. Previous and concomitant treatments and follow-up time were also noted. Data on prior and concomitant treatment included use of oral corticosteroids, conventional immunosuppressive drugs, biologic agents, and intravitreal therapies.
TCZ was administered intravenously at a dose of 8 mg/kg body weight at 4-week intervals. The drug was infused for 1 hour. Potential side-effect monitoring, including hemogram, liver function tests, and vital signs, was performed in all patients before initiating the treatment and was repeated before each infusion. All patients were tested for latent tuberculosis by tuberculin skin test before initiation of TCZ.
Primary outcome measure was the decrease in Central Foveal Tickness (CFT) measured with optical coherence tomography (OCT) from baseline. Secondary outcomes were changes in best-corrected visual acuity (BCVA) and evidence of active inflammation. Treatment results were assessed at 1, 3, and 6 months after initiating TCZ infusions.
Baseline examination included BCVA, slit lamp examination, ophthalmoscopy, and measurement of CFT by spectral OCT (Cirrus HD, Model 4000. Carl Zeiss, CA, USA). The cube 512 × 128 protocol was used to obtain the SD-OCT analysis. Inflammatory activity was graded according to the Standardization of Uveitis Nomenclature (SUN) Working Group grading schemes for the anterior chamber (cells and flare) from grade 0 to 4, representing the level of active inflammation [8]. A modified version of the National Eye Institute system for grading vitreous haze as proposed by the SUN working group was used [9]. Clinical activity of uveitis, including binocular indirect ophthalmoscopy score in the anterior and vitreous chamber, was <0.5+ in all eyes at the time of initiating TCZ treatment. BCVA was measured with Snellen charts, and was converted to logarithm of the minimum angle of resolution (log-MAR) values for statistical analysis.
Statistical analysis
The effect of TCZ was evaluated by changes in CFT and BCVA. Continuous and two measurement interval variables were analyzed with the Wilcoxon signed-rank test, and three-measurement or more interval variables were tested with the Friedman test. Statistical analysis was performed with the SPSS software (PASW for Windows 18.0, SPSS Inc, Chicago, IL), and we considered a Type I error of 0.05. Quantitative variables were described by median and standard deviation and qualitative variables with absolute frequencies and percentages. Baseline values before treatment were used as covariate. Inferential analyses were performed using the same methodology by means of a nonparametric approach using a rank transformation of dependent variables. Because this is a retrospective, observational non-comparative study, all p values were considered for descriptive purposes.
Results
Demographics and clinical characteristics of the study population are summarized in Table 1. Five patients with uveitis-related CME were included in this report. All patients had bilateral disease. Nevertheless, eight eyes from five patients (all females) were included. Two eyes (of patients 1 and 2) were not included in the study, because their visual acuity was only light perception. Mean age was 49.4 years (range, 30–68 years). Uveitis diagnoses were: Birdshot chorioretinopathy (n = 3), juvenile idiopathic arthritis (JIA)-associated uveitis (n = 1), and idiopathic panuveitis (n = 1). Before TCZ therapy, patients had been refractory to high dosages of previous systemic corticosteroid, immunosuppressive drugs and biological therapy. Dexamethasone implants were previously used in seven eyes. Failure was defined as macular edema progression despite different therapeutic approaches (systemic and intravitreal). Macular edema progression was defined as CFT > 350 μm determined by OCT. Adalimumab and infliximab were withdrawn and patients had a washout period of at least 3 months before initiating TCZ treatment.
Evolution of CME was 13.4 ± 13.1 years (mean ± SD). The mean baseline CFT (95 % confidence interval) was 602 ± 236 μm at baseline, decreased to 386 ± 113 μm at month 1 (p = 0.006), continued decreasing to 323 ± 103 μm at month 3 (p = 0.026) and remained low at month 6 (294.5 ± 94.5 μm, p = 0.014) (Fig. 1).
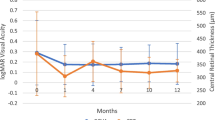
BCVA (logMAR) improved from 0.66 ± 0.57 at baseline to 0.47 ± 0.62 at final follow-up (p < 0.05). After 6 months, an improvement of ≥ 2 lines of BCVA was observed in 50 % of eyes (p = 0.028), remained stable in 25 % and worsened in none (Fig. 2). Sustained uveitis remission was maintained in all patients. Any remarkable side effects after 6 months of follow-up were reported.
Selected case report
Case 5
A 39-year-old-woman diagnosed with birdshot chorioretinopathy was treated with prednisone (7.5 mg per day) in combination with cyclosporine A (CsA) (150 mg per day) for 6 months. In April 2012, her inflammatory condition relapsed with severe CME; intravenous infusion with 5 mg/kg of infliximab was given, but she developed a hypersensitivity reaction. Biologic therapy was switched from infliximab to 40 mg every 2 weeks of adalimumab in order to maintain her uveitis and macular edema under control. Nevertheless, CME persisted in both eyes. In May 2012, an intravitreal injection of dexamethasone implant was placed in her right eye without a significant decrease in CFT. In June 2012 bilateral severe CME occurred in both eyes (Fig. 3a and b). Her visual acuity was 20/100 in the right eye and 20/50 in the left eye. Then, intravenous TCZ (8 mg/Kg monthly) was initiated with oral prednisone at a daily dose of 7.5 mg. Adalimumab was stopped prior starting TCZ. After six TCZ infusions, the patient remained asymptomatic, ophthalmic examination revealed no signs of active inflammation, and CFT decreased in both eyes (Fig. 3c, d, e, f, g and h). CFT in the right eye decreased from 974 to 267 μm and in the left eye from 644 to 334 μm. Visual acuity improved to 20/32 in her right eye and 20/25 in her left eye. No remarkable side effects after 12 months of follow-up were reported.
(Case 5): a Baseline in right eye showing CME and subfoveal retinal detachment (RD) by OCT. b Baseline in left eye showing CME and subfoveal RD in OCT. c Right eye. OCT 1 month after initiating TCZ therapy showing persistence of CME and subfoveal RD. d Left eye. OCT 1 month after initiating TCZ therapy. CFT decreased with residual subfoveal RD. e Right eye. OCT 3 months after initiation of TCZ. CFT decreased with residual subfoveal RD. f Left eye. OCT 3 months after initiation of TCZ. Subfoveal RD resolved. g Right eye. OCT 6 months after initiation with TCZ. CME was absent. h Left eye. OCT 6 months after initiation with TCZ. CME was absent. Epiretinal membrane was observed
Discussion
IL-6 is a proinflammatory cytokine which is mainly produced by T cells and monocytes/macrophages inducing proliferation and differentiation of T cells and terminal differentiation of B cells [10]. IL-6 contributes to the host defense against acute environmental stress, while dysregulated, persistent IL-6 production has been demonstrated to play a pathological role in various autoimmune and chronic inflammatory diseases. The increase in systemic IL-6, as well as its possible local production, may be associated with the properties of this cytokine itself, which affects different stages of the immune response. IL-6 increases vascular permeability early in acute disease, leading to vascular leakage and an influx of cytokines and inflammatory cells. It plays an important role in cell proliferation and differentiation and increases the secretion of acute phase reactants by the liver. IL-6 is also involved in the homeostasis of the immune response at a later stage and can remain elevated during remission.
Serum levels of IL-6 have been shown to be significantly elevated in patients with active non-infectious uveitis disease and decrease during remission, playing an active role in the modulation of inflammation in the chronic disease [11]. In addition, elevated intraocular levels of IL-6 have been observed in patients with active intermediate or posterior uveitis and probably plays a key role in uveitis and in the pathogenesis of macular edema [12–16]. Targeting IL-6 is thus a rational approach for the treatment of non-infectious uveitis. Furthermore, inhibition of the IL-6 receptor diminishes Th17 responses and ameliorates experimental autoimmune uveitis [17]. Anti-interleukin 6 receptor (anti- IL-6R) antibodies have proven to be effective in experimental models of autoimmune arthritis, encephalomyelitis, and also uveitis [18]. TCZ is a fully humanized antibody that binds both to soluble and membrane bound IL-6 receptors. Anti-IL-6 receptor monoclonal antibody (anti-IL-6R mAb) is available for clinical use and has demonstrated its therapeutic efficacy in the treatment of severe rheumatoid arthritis. Thus, TCZ may represent a treatment option for refractory uveitic macular edema.
This work reports the efficacy of TCZ in five uveitis patients with CME refractory to local steroids, traditional immunosuppressive therapy and biologic therapy. In the present study, all patients had clearly visible cystic changes in the macula at baseline, and a complete or partial resolution of the CME was found in all eyes after 6 months of TCZ therapy, with a significant change in the mean CFT. To the best of our knowledge, the effectiveness of TCZ in uveitis has been reported only in a few small case series. Muselier found that TCZ was effective in two refractory uveitis patients [19]. They included one patient with birdshot chorioretinopathy. Tappeiner reported three patients with refractory uveitis associated with JIA and demonstrated that TCZ was effective for the treatment of ocular inflammation [20]. Efficacy of TCZ in patients with uveitis accompanied with Behçet’s and Castleman disease has also been reported [21–23]. However, the efficacy of TCZ in cases of refractory uveitis-related CME has not been specifically studied. We previously reported a case of idiopathic panuveitis with severe CME refractory to systemic and local treatments that favourably responded to TCZ [24].
In the present study, five patients had clearly visible cystic changes in the macula at baseline, and a complete resolution of the CME was found in all eyes after 6 months, with a significant change in the mean CFT. It is important to highlight the rapid effect of TCZ infusions in our series, leading to good anatomical results. Improvement of CME was seen at first month in six eyes (75 %) that showed a reduction of more than 100 μm in CFT. In addition, BCVA improved in 50 % of eyes. The improvement in visual acuity in our cases was limited due to the long duration of the edema. Early administration of TCZ in selected cases could lead to better functional results.
In the present data, control of inflammation was achieved in all patients at 1, 3, and 6 months of follow-up. TCZ treatment leaded to suppression of uveitis and macular edema in patients whose disease had been refractory to previous treatments, including anti-TNF drugs. This suggests that TCZ can also maintain inactivity of the disease. Therefore, TCZ seems to prove to be effective in reducing CME and controlling inflammation, maintaining or improving visual acuity. TCZ was well tolerated in most patients, although this study provided limited information about side effects due to the small sample size and limited follow-up.
Although preliminary, our results seem to be very promising, and further studies on IL-6 inhibition in uveitis are warranted. This study had a retrospective design with a relatively small number of patients and a short follow-up period. Long-term effects of TCZ treatment remain unknown. These promising results suggest that IL-6 may play an important pathogenic role in the perpetuation of chronic CME associated with uveitis. Thus, controlled trials will be very useful to further elucidate the therapeutic benefit of IL-6 inhibitors in CME associated to uveitis.
References
Lardenoye CW, van Kooij B, Rothova A (2006) Impact of macular edema on visual acuity in uveitis. Opthalmology 113:1146–1149
Rothova A (2002) Medical treatment of cystoid macular edema. Ocul Immunol Inflamm 10:239–246
Díaz Llopis M, Salom D, Garcia de Vicuña C, Cordero Coma M, Ortega G, Ortego N, Suarez de Figueroa M, Rio Pardo MJ, Fernandez-Cid C, Fonollosa A, Blanco R, Garcia-Aparicio AM, Benitez del Castillo JM, Olea JL, Arevalo JF (2012) Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology 119:1575–1581
Markomichelakis NN, Theodossiadis PG, Pantelia E, Papaefthimiou S, Theodossiadis GP, Sfikakis PP (2004) Infliximab for chronic cystoid macular edema associated with uveitis. Am J Ophthalmol 138:648–650
Adán A, Pelegrín L, Rey A, Llorenç V, Mesquida M, Molins B, Ríos J, Keller J (2013) Dexamethasone intravitreal implant for treatment of uveitic persistent cystoid macular edema in vitrectomized patients. Retina. 2013 Mar 19. PMID:23514796
Taylor SR, Lightman SL, Sugar EA, Jaffe GJ, Freeman WR, Altaweel MM, Kozak I, Holbrook JT, Jabs DA, Kempen JH (2012) The impact of macular edema on visual function in intermediate, posterior, and panuveitis. Ocul Immunol Inflamm 20:171–181
Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, Alecock E, Lee J, Kremer J (2008) IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis 67:1516–1523
Jabs DA, Nussenblatt RB, Rosenbaum JT (2005) Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 140:509–516
Nussenblatt RB, Palestine AG, Chan CC, Roberge F (1985) Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 92:467–471
Tanaka T, Kishimoto T (2012) Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int J Biol Sci 8:1227–1236
Kramer M, Monselise Y, Bahar I, Cohen Y, Weinberger D, Goldenberg-Cohen N (2007) Serum cytokine levels in active uveitis and remission. Curr Eye Res 32:669–675
van Kooij B, Rothova A, Rijkers GT, de Groot-Mijnes JD (2006) Distinct cytokine and chemokine profiles in the aqueous of patients with uveitis and cystoid macular edema. Am J Ophthalmol 142:192–194
Valentincic NV, de Groot-Mijnes JD, Kraut A, Korosec P, Hawlina M, Rothova A (2001) Intraocular and serum cytokine profiles in patients with intermediate uveitis. Mol Vis 17:2003–2010
Kuiper JJ, Mutis T, de Jager W, de Groot-Mijnes JD, Rothova A (2011) Intraocular interleukin-17 and proinflammatory cytokines in HLA-A29-associated birdshot chorioretinopathy. Am J Ophthalmol 152:177–182
Curnow SJ, Falciani F, Durrani OM, Cheung CM, Ross EJ, Wloka K, Rauz S, Wallace GR, Salmon M, Murray PI (2005) Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Invest Ophthalmol Vis Sci 46:4251–4259
Ongkosuwito JV, Feron EJ, van Doornik CE, Van der Lelij A, Hoyng CB, La Heij EC, Kijlstra A (1998) Analysis of immunoregulatory cytokines in ocular fluid samples from patients with uveitis. Invest Ophthalmol Vis Sci 39:2659–2665
Yoshimura T, Sonoda KH, Ohguro N, Ohsugi Y, Ishibashi T, Cua DJ, Kobayashi T, Yoshida A, Yoshimura A (2009) Involvement of Th17 cells and the effect of anti-IL-6. Therapy in autoimmune uveitis. Rheumatology 48:347–354
Hohki S, Ohguro N, Haruta H, Nakai K, Terabe F, Serada S, Fujimoto M, Nomura S, Kawahata H, Kishimoto T, Naka T (2010) Blockade of interleukin-6 signaling suppresses experimental autoimmune uveoretinitis by the inhibition of inflammatory Th17 responses. Exp Eye Res 91:162–170
Muselier A, Bielefeld P, Bidot S, Vinit J, Besancenot JF, Bron A (2011) Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis. Ocul Immunol Inflamm 19:382–383
Tappeiner C, Heinz C, Ganser G, Heiligenhaus A (2012) Is tocilizumab and effective option for treatment of refractory uveitis associated with juvenile idiopathic arthritis? J Rheumatolol 39:1294–1295
Oshitari T, Kajita F, Tobe A, Itami M, Yotsukura J, Baba T, Yamamoto S (2012) Refractory uveitis in patient with castleman disease successfully treated with tocilizumab. Case Rep Ophthalmol Med. Epub 2012 Nov 6. PMID:23198204
Urbaniak P, Hasler P, Kretzschmar S (2012) Refractory neuro-Behçet treated by tocilizumab: a case report. Clin Exp Rheumatol 30(3 Suppl 72):S73–S75
Hirano T, Ohguro N, Hohki S, Hagihara K, Shima Y, Narazaki M, Ogata A, Yoshizaki K, Kumanogoh A, Kishimoto T, Tanaka T (2012) A case of Behçet’s disease treated with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Mod Rheumatol 22:298–302
Adán A, Llorenç V, Mesquida M, Pelegrín L (2012) Tocilizumab treatment for recalcitrant uveitic macular edema. Graefes Arch Clin Exp Ophthalmol. Dec 28 PMID:23271335
Financial support
None.
Conflict of interest
No conflicting relationship exists for any author.
Author information
Authors and Affiliations
Corresponding author
Additional information
The material of the manuscript is not under consideration for presentation.
Rights and permissions
About this article
Cite this article
Adán, A., Mesquida, M., Llorenç, V. et al. Tocilizumab treatment for refractory uveitis-related cystoid macular edema. Graefes Arch Clin Exp Ophthalmol 251, 2627–2632 (2013). https://doi.org/10.1007/s00417-013-2436-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-013-2436-y