Abstract
Background
Virtual reality surgery simulation training improves resident performance as measured by the simulator itself and wet-lab performance. This study aims to determine whether virtual surgery simulator training improves actual resident cataract surgery performance.
Methods
The first 50 phacoemulsification cases of 20 residents, at a single residency program (Henry Ford Hospital), were retrospectively compared as two groups: before (2007–8) and after (2009–10) introduction of the Eyesi virtual surgery simulator to the surgical training program. Primary outcomes were the incidence of posterior capsule tears and operation duration. All residents received traditional didactic and wet-lab training. Instructor surgeons were surveyed for their impression of the simulator’s contribution to resident surgical training.
Results
The nonsimulator and simulator groups each comprised 500 cases with 40 and 35 posterior capsule tears respectively. Capsular tear rates for the nonsimulator and simulator groups were 8.8 % and 10 % respectively for the first 25 cases, and 7.2 % and 3.6 % (P = 0.11) respectively for cases 26 through 50 . The percentage of long cases (defined as >40 min) for cases 10 through 50 was 42.3 % and 32.4 % (P = 0.005) for the nonsimulator and simulator groups respectively.
Conclusions
Virtual reality surgical simulator training mildly shortens the learning curve for the first 50 phacoemulsification cases. The less adept residents appear to benefit most.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cataract surgery is one of the most important surgical techniques taught in ophthalmic residency. A study on resident-performed phacoemulsification (phaco) between 1999 and 2002, without compulsory wet- lab or virtual simulation training, showed that the phaco learning curve required more than 80 cataract cases to plateau [1]. Up to 25 % of residents perform less than 80 cases during residency; and most between 80 and 140 [2]. This, in addition to the need to decrease surgical complications, has made “outside the operating room” training methods an important aspect of resident cataract surgery training. The Accreditation Council for Graduate Medical Education now requires that ophthalmic residents have access to wet-lab or virtual simulator training [3]. Structured surgical training programs (including wet-lab training, simulator training, and supervision and feedback of resident cases) have been shown to hasten the resident learning curve [4, 5].
Recently, virtual reality cataract surgery simulators, such as the Eyesi (VRmagic, Mannheim, Germany), have become available. Studies on the Eyesi have shown the construct validity of its anterior segment anti-tremor and forceps training modules [6], as well as its usefulness for capsulorhexis [7] and phaco training [8]. Nevertheless, data showing that modern simulators improve “real life” resident surgery performance remains scant [9].
Our institution has had a well-structured resident surgical training program, including extensive wet-lab training and close supervision and feedback on resident cases, for many years. Recently, virtual reality surgical training (Eyesi) was added to the program. The preexisting components of the surgical training program remained unchanged. We therefore had an opportunity to assess the impact of the addition of a virtual reality surgical simulator to a well-structured surgical training program. This study aims to assess this addition.
Methods
This retrospective consecutive comparative review of resident surgical outcomes at a single center (Henry Ford Hospital) was approved by the Institutional Review Board of Henry Ford Health System, Detroit, Michigan. Surgical logs were retrieved from the electronic medical record. The first 50 phacoemulsification cases of 20 residents (five residents completing residency per academic year, 2007 to 2010) were studied. Cataract surgery cases using techniques other than phacoemulsification, combined with other procedures, and those where the resident was not the primary surgeon, were excluded. Surgical technique was divide-and-conquer on an Alcon Infinity platform. No resident had significant phacoemulsification training prior to residency at Henry Ford.
The study population was divided into two groups: nonsimulator group comprised ten residents trained without access to virtual reality surgical simulation (graduating June 2007 and 2008), and the simulator group comprised ten residents trained with the Eyesi surgical simulator (graduating 2009 and 2010). Simulator-group residents were required by the residency program to spend at least 6 h training on the simulator within the first 18 months of residency. All 20 residents received significant wet-lab training (26-hour structured course, including 4 h of wet-lab phaco), and surgical case supervision and coaching. Both the simulator and the wet-lab were easily accessible for residents who wanted additional training.
Outcome measures were (1) incidence of posterior capsule tear with or without vitreous loss, and (2) operating time. The operating time was recorded by the nursing and anesthesia staff, and was considered from the first incision up to speculum removal. Cases in which the operating time was not accurately recorded were excluded. The five senior surgeons who supervised the resident cataract surgery cases were surveyed for improvement in surgical performance between the nonsimulator and simulator groups.
Student t-test for unequal variance was used for comparing means. Chi-square or Fisher’s tests were used for comparing categorical variables. Pearson correlation coefficient was used to assess correlation. All tests were 2-tailed. Statistical significance was considered as P < 0.05.
Results
Table 1 compares the main outcome measures of the two groups. Although no statistically significant differences were seen, when stratified longitudinally in order to compare the learning curves of the two groups of residents, a trend to lower complications was seen in the simulator group. Table 2 compares the surgical time for the two groups. With longitudinal stratification of the surgical times, differences between the two groups are evident. The initial cases of the simulator residents had poorer outcomes than the residents without simulator training. Beyond the first 10 cases, the simulator-group residents had shorter surgical times than the nonsimulator residents.
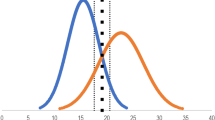
All ten residents of the simulator group fulfilled the program requirement of 6 h of simulator time (mean, 21.2 h; range, 7.1–47.1 h). Figure 1 shows the simulator-group residents’ surgical proficiency parameters plotted against the time that each resident spent training on the simulator. Pearson correlation coefficients for these three surgical proficiency parameters were low (poor correlation) when all ten simulator-trained residents were analyzed, because the resident with the shortest operating times had the longest simulation training time (2,824 min, far greater than any other resident). Excluding this outlying resident from the analysis yielded a trend to correlation between high surgery duration and high simulation time. Pearson correlation coefficients and P values for the median surgery duration, for the percentage of cases with duration greater than 40 min, and for the rate of posterior capsule rupture were 0.58 (P = 0.10), 0.61 (P = 0.08) and −0.40 (P = 0.28) respectively.
Scattergrams of the simulator group residents’ surgical proficiency parameters plotted against the time spent training on the simulator with x = y reference lines (ten residents, each having ∼50 cases). a Median surgery duration. b Percentage of cases with surgical time greater than 40 min. c Number of cases complicated by ruptured posterior capsule
Five senior surgeons supervised most of the 1,000 resident cases studied. These supervising surgeons’ opinions with regard to the improvement in resident surgical proficiency since the simulator was integrated into resident training are shown in Table 3. All five senior surgeons agreed that simulation training decreased the need for instructor intervention during resident phacoemulsification, and improved capsulorhexis performance. Supervising surgeons did not agree that resident surgery was quicker and had fewer complications.
Discussion
Our study showed that simulator-trained residents had shorter phaco learning curves through their first 50 cases. Although surgical time improvement for cases 10 through 50 was significantly better for the simulator group, complication rate was not significantly different. The difference in surgical time between the two groups was most significant for the number of cases longer than 40 min (Table 2), which is a measure of overall surgical proficiency. In addition to the simulator training, all residents received similar wet-lab, didactic and live surgery coaching. Therefore, the quicker surgical time of the simulator-trained residents appears to be due to simulator training.
Comparison between the first cases showed that the nonsimulator group actually performed better (shorter operating times for first ten cases [Table 2], statistically nonsignificant lower incidence of complications [Table 1]). However, further along the learning curve, the simulator residents outperformed the nonsimulator group with regard to operating time, and showed a trend towards fewer posterior capsule ruptures. Possible reasons for better outcomes in the nonsimulator group for the initial cases are that they had a better natural ability than the simulator residents or, on the contrary, the nonsimulator residents required greater instructor surgeon intervention, which resulted in the attending doing most of their initial cases. Because of the retrospective data collection, our study is not able to differentiate between these two possibilities.
Regardless of the reason for poorer outcomes of the initial cases of the simulator group, our data does suggest that simulator-trained residents had shorter learning curves. It appears, for residents that are on the learning curve, that the simulator is useful to practice the surgical techniques and cognitively concentrate on the weak points of past surgical cases. All surgeons appreciate the importance of mentally planning and revising surgical cases, and simulation certainly aids this mental process. Although wet-labs and video review of surgical cases also fulfill this function, the simulator allows for repetitive practice and provides instant feedback. Therefore, residents who struggle with specific steps can focus on these areas, thereby shortening the learning curve. Additional mechanisms by which the simulator may shorten the learning curve is by increasing resident’s surgical confidence, and by aiding the resident to keep focused on cataract surgery during rotations when he or she has few cataract cases. Granted, wet-labs and video review of surgical cases can also fulfill these functions [4, 5].
Upon analyzing the simulator group, an unexpected correlation between real surgical time and simulation time was seen, suggesting that those residents whose cases were taking longer spent more time on simulation training. Considering that the surgical times of the simulator group as a whole were shorter than the nonsimulator group, this correlation, although not statistically significant, is noteworthy. A probable explanation is that the “slower” residents of the simulator group improved their surgical times by additional (all completed 6 compulsory hours) voluntary simulator training, thus shortening their times and decreasing the average surgical time of the simulator group; while the more adept residents used the simulator less. Therefore, the “slower” residents probably benefitted most from the addition of the simulator to the training program.
Supervising senior surgeons felt that the addition of simulator training improved resident surgical proficiency. These senior surgeons were all experienced cataract tutors. Their impression of improvement was possibly limited by their memory of the nonsimulator residents, who formed the two resident classes before the simulator was introduced into the program. Improved capsulorhexis, corroborating the findings of the recent study of Privett et al. [7], and less instructor intervention were the prominent opinions. Less instructor intervention may partly explain the shorter learning curve of the simulator group. Shorter learning curve, improved eye–hand coordination, and improved phacoemulsification technique were agreed on by most instructors, albeit less convincingly.
Although decreased surgical time and fewer complications were apparent in the statistical analysis, these were not the opinions of most of the instructor surgeons. This inconsistency may be explained by the initial poorer outcomes of the simulator group, which may have blunted the impression of better performance further along the learning curve. Alternatively, although the statistical analysis showed that the simulator group performed significantly better in the latter 40 cases, the improvement due to simulator training was nevertheless subtle.
Limitations of our study include its retrospective design, and that it was spread over four resident classes. However, surgical technique and instrumentation, and the dominant surgical instructors were consistent. Although prospective randomization of residents for virtual reality simulator training would make for a more powerful study, this would require preventing, or at least delaying, residents’ access to a possibly useful training tool, and is therefore probably not feasible. Variation in ocular comorbidities and cataract characteristics of patients were not accounted for. Further, variation in resident characteristics could not be accounted for. Since there were 500 cataract cases and ten residents in each group, these variations should be spread more or less evenly amongst the two groups, although including more residents would have strengthened our study.
Our study shows that the addition of a modern virtual reality surgery simulator alongside an organized surgery training program appears to slightly improve resident real surgery performance, with the less adept residents benefitting most. Adding modules that simulate intraoperative complications may further improve the simulator’s impact as a surgery training tool.
References
Randleman JB, Wolfe JD, Woodward M, Lynn MJ, Cherwek DH, Srivastava SK (2007) The resident surgeon phacoemulsification learning curve. Arch Ophthalmol 125:1215–1219
Rowden A, Krishna R (2002) Resident cataract surgical training in United States residency programs. J Cataract Refract Surg 28:2202–2205
Accreditation Council for Graduate Medical Education program requirements for GME in ophthalmology. (Revised 7/1/2009) PDF II.D.4 (page 7) Available at: http://www.acgme.org/acWebsite/RRC_240/240_prIndex.asp Accessed February 7, 2012
Rogers GM, Oetting TA, Lee AG, Grignon C, Greenlee E, Johnson AT, Beaver HA, Carter K (2009) Impact of a structured surgical curriculum on ophthalmic resident cataract surgery complication rates. J Cataract Refract Surg 35:1956–1960
Ezra DG, Aggarwal R, Michaelides M, Okhravi N, Verma S, Benjamin L, Bloom P, Darzi A, Sullivan P (2009) Skills acquisition and assessment after a microsurgical course for ophthalmology residents. Ophthalmology 116:257–262
Mahr A, Hodge DO (2008) Construct validity of anterior segment anti-tremor and forceps surgical simulator training modules: attending versus resident surgeon performance. J Cataract Refract Surg 34:980–985
Privett B, Greelee E, Rogers G, Oetting TA (2010) Construct validity of surgical simulator as a valid model for capsulorhexis training. J Cataract Refract Surg 36:1835–1838
Belyea DA, Brown SE, Rajjoub LZ (2011) Influence of surgery simulator training on ophthalmology resident phacoemulsification performance. J Cataract Refract Surg 37:1756–1761
Ahmed Y, Scott IU, Greenberg PB (2011) A survey of the role of virtual surgery simulators in ophthalmic graduate medical education. Graefes Arch Clin Exp Ophthalmol 249:1263–1265
Acknowledgements
Russell Pokroy received fellowship grants from the American Physicians Fellowship for Medicine in Israel, and from the Israel Ophthalmic Society.
Competing interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Meeting presentations
Presented in part at the Association for Research in Vision and Ophthalmology (ARVO), Fort Lauderdale, FL, USA, May 2011.
Rights and permissions
About this article
Cite this article
Pokroy, R., Du, E., Alzaga, A. et al. Impact of simulator training on resident cataract surgery. Graefes Arch Clin Exp Ophthalmol 251, 777–781 (2013). https://doi.org/10.1007/s00417-012-2160-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-012-2160-z