Abstract
Purpose
To compare visual and anatomic outcomes in eyes with type 2 idiopathic macular telangiectasia (Mactel) treated with either intravitreal bevacizumab (IVB), observation, or pars plana vitrectomy (PPV) with internal limiting membrane removal.
Methods
Retrospective, consecutive, interventional case series of phakic patients with Mactel. Best-corrected Snellen visual acuity (BCVA) and complete ophthalmic exam was obtained prior to treatment and at subsequent 3-month intervals for a minimum of 6 months. Fluorescein angiographic and spectral-domain optical coherence tomography features were examined, and compared to BCVA at treatment initiation and follow-up.
Results
Fifty-six eyes of 28 patients were evaluated. Mean age was 65 ± 12 years, and mean follow-up was 24 ± 13 months. Patients were treated with either observation (n = 33), IVB (n = 15), or PPV (n = 8). Mean number of treatments for the IVB group was 2.5 ± 3.5 intravitreal injections. No significant differences in BCVA change were observed between treatment groups via one-way ANOVA (p = 0.49). Presence of inner retinal cysts was not correlated to BCVA (p > 0.05). Discontinuous outer nuclear layer was significantly related to worse initial and final vision, but not to BCVA change.
Conclusion
IVB and PPV with ILM removal appear ineffective in improving visual outcome in eyes with non-proliferative Mactel. SD-OCT evidence of disrupted foveal outer nuclear layer is related to decreased BCVA, but not related to BCVA change following treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Idiopathic macular telangiectasia type 2 (Mactel) is a relatively rare [1], bilateral retinal disorder involving telangiectatic perifoveal retinal capillaries, of unknown etiology, typically diagnosed in mid to late adult life. Originally termed idiopathic juxtafoveal telangiectasis, Mactel was described by Gass in 1968 [2] and was subsequently classified further by both Gass [3, 4] and Yannuzzi [5]. The original classification differentiated forms of idiopathic retinal telangiectasis based on angiography, laterality, morphology, and age at diagnosis. A simplified classification was later proposed based on vascular morphology (aneurysmal vs telangiectatic vs occlusive) [5]. The telangiectatic form, or type 2, has since been reported to commonly occur in the 5th to 6th decade of life, typically presenting bilaterally, often with asymmetric involvement, and has no apparent gender predilection. The most common clinical findings in Mactel involve non-proliferative changes including degenerative cystic spaces, perifoveal telangiectatic vessels most prominent in the temporal macula, and and loss of transparency of the central macula. Proliferative changes can occur rarely, and include intra-retinal and sub-retinal neovascularization [4, 5]}.
Despite many recent investigations into the clinical and morphologic characteristics of Mactel, the underlying cause of the disorder remains obscure. Common fluorescein angiographic features of the disorder include dilated, right-angle branching perifoveal vessels crossing the horizontal raphe immediately temporal to the fovea, with intraretinal leakage [2–5]. Frequent optical coherence tomography findings include inner lamellar foveal and parafoveal cysts or “empty spaces”, hyper-reflectant flecks, and outer retinal atrophy [6–8]. Various treatment strategies including laser photocoagulation, intravitreal triamcinolone acetate, and intravitreal anti-VEGF agents have been attempted for treatment of cystic spaces and vascular leakage associated with Mactel with mixed results [9–16], but no clear visual benefit. The purpose of the present study was to compare visual outcomes among patients with non-proliferative Mactel treated with either observation, intravitreal bevacizumab, or pars plana vitretomy with internal limiting membrane (ILM) removal.
Materials and methods
This retrospective interventional case series conformed to the tenets set forth in the Declaration of Helsinki. All patients were recruited from a single vitreoretinal referral center (Charles Retina Institute, Memphis, TN, USA). The study period, including follow-up, was from 1/2007 to 12/2012. All patients completed informed consent for ophthalmic imaging and study participation.
Consecutive patients with non-neovascular Mactel were included. Only phakic patients with no history of ocular surgery, diabetes mellitus, or other eye disease were included. Two patients were initially excluded due to history of focal laser photocoagulation. All patients with unknown diabetes status were referred for appropriate systemic testing. Two patients were excluded prior to data analysis due to concomitant systemic disease, one each for uncontrolled hypertension and diabetes mellitus. Consecutive patients were imaged using Spectralis SD-OCT (Heidelberg Engineering, Heidelberg, Germany) 12-line radial scans centered on the foveola. Fundus photography (FP), fluorescein angiography (FA), and fundus autofluorescence (FAF) were also obtained at the initial visit by a single operator.
Two subgroups of eyes were treated with either intravitreal Bevacizumab (IVB, Genentech, South San Francisco, CA, USA) 2.5 mg/0.1 ml or pars plana vitrectomy (PPV) and ILM peeling. The decision to treat with IVB was based upon subjective visual decline combined with documented decrease in BCVA of at least one Snellen line. IVB treatment was performed at 4–6 week intervals for all treated eyes receiving multiple treatments. Re-treatment was performed in conjunction with patient counseling and in response to stabilization of vision combined with subjective or objective visual improvement. The decision to perform surgery in the PPV subgroup was made in conjunction with extensive counseling with guarded prognosis in patients with relatively poor vision, no neovascular process, and evidence of lamellar macular hole, epimacular membrane, or vitreo-foveal attachment on SD-OCT. No additional treatment was performed in eyes treated with PPV, and no ILM stains were used during surgery. Treatment category was recorded (observation vs IVB vs PPV), and number of IVB were recorded for the treatment group. Best-corrected Snellen visual acuity (BCVA) was tested and converted to log of minimum angle of resolution (log MAR).
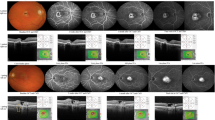
Initial SD-OCT morphology was recorded into categories as depicted in Fig. 1. Eyes were categorized as inner cystic spaces only, combined inner and outer cystic spaces, or outer nuclear layer disruption within 500 μm of the foveola. Eyes were additionally broadly categorized by the presence or absence of a continuous outer nuclear layer (ONL) and the presence or absence of an intact inner segment-outer segment line (IS/OS) within 500 μm of the foveola as measured by SD-OCT. Vascular leakage pattern was categorized as focal temporal, incomplete circumfoveal, or annular perifoveal, and was consistent with previously described patterns in Mactel in all cases. Time to resolution of leakage in response to IVB and time to recurrence of leakage after IVB cessation were similarly recorded.
Spectral domain optical coherence tomography categories of eyes with type 2 macular telangiectasia. a Inner lamellar hyporeflectant cystic spaces only. b Inner lamellar spaces with disrupted IS/OS line within 500 μm of foveola. c, d Combined inner and outer cystic spaces. e, f Discontinuous outer nuclear layer in the sub-foveal region
Data analysis
All data analysis was performed using JMP 9 (SAS, Cary, NC, USA). Category distributions were obtained. One-way analysis of variance was used to compare final visual acuity and change in visual acuity between treatment categories. One-way ANOVA was used to compare BCVA among SD-OCT morphologic categories and angiographic categories. Bivariate analysis was used to compare age and BCVA. Logistic regression was used to compare categorical with continuous variables.
Results
Fifty-six eyes of 28 patients were included. All patients were Caucasian, and consisted of 13 males and 15 females. Mean age at diagnosis was 65 ± 9.2 years. Mean BCVA at presentation was 0.29 ± 0.39. Mean overall follow-up was 24 ± 13 months. There was no difference in visual change between gender categories (p = 0.46) among all eyes. No significant correlations were observed between age and initial vision (p = 0.23), final vision (p = 0.52), or visual change (p = 0.21) among all eyes.
Overall mean change in vision was 0.04 ± 0.20. Clinical characteristics of eyes by sub-group are demonstrated in Table 1. There were no statistically significant differences in change in vision among eyes treated with observation, IVB, or PPV. Mean number of treatments for the IVB group was 2.5 ± 3.5.
The presence of a continuous foveal ONL was significantly associated with better initial (p = 0.02) and final visual acuity (p = 0.01) but not to visual change (p = 0.31) via one-way ANOVA. IS/OS line disruption was not statistically related to BCVA (p = 0.18 for final vision). Angiographic leakage pattern was not significantly related to visual acuity (p > 0.10 for all patterns). Leakage decreased, but did not completely resolve, in all eyes in treated with IVB within 1 month following treatment initiation as depicted in Fig. 2. Leakage recurred in all treated eyes in 1–3.5 months following treatment cessation (mean 2.2 ± 0.63 months).
Fluorescein angiographic (FA) response to intravitreal Bevacizumab in eyes with type 2 macular telangiectasia. a, b 2-min recirculation phase FA in a patient with focal temporal telangiectatic retinal capillaries and late angiographic leakage. c, d One month after a single intravitreal bevacizumab (IVB) in each eye, there is marked reduction in capillary leakage but not complete resolution. e, f Three months after the final treatment, in each eye leakage has returned. The patient was treated with a total of three IVB in each eye
Discussion
The results of the present study indicate that intravitreal bevacizumab or PPV with internal limiting membrane removal are ineffective treatment strategies for visual improvement in non-proliferative Mactel. To date, there have been at least seven reports [7, 12, 13, 16–20] involving 36 eyes demonstrating mixed and inconsistent visual outcomes in non-proliferative Mactel after IVB. These reports have consistently described transient decreased angiographic leakage with mixed results on OCT examination, and no definite visual benefit. While the overall visual change in the IVB group was marginally better in the present study, we found no statistically significant visual benefit compared to a relatively large observed control group. The decision to treat based on decreased vision may account for this marginal observed difference. Similar to our findings for IVB, ranibizumab (Genentech, South San Francisco, CA, USA) has recently been evaluated in prospective, randomized trials [9, 10] and has shown no functional benefit for patients with non-neovascular Mactel, despite some morphologic response.
We included a group of eyes with poor vision and no neovascular process that were treated with PPV with internal limiting membrane removal for lamellar macular hole configuration, epimacular membrane, or vitreofoveal traction evident on SD-OCT. While the visual change was marginally worse in this group, we found no statistically significant difference between this group and either the observation or IVB groups. This is in agreement with a previous small unpublished series of eyes operated by the authors, in which the majority of eyes experienced stabilized or marginally improved vision, with one eye developing a full-thickness macular hole and worse visual outcome. Mixed results have previously been reported in eyes with macular hole undergoing PPV in Mactel [21–23], and these patients are generally considered poor surgical candidates due to the underlying neurodegenerative process. To our knowledge, the present report represents the only current experience involving PPV and ILM removal in Mactel without macular hole. Caution must be used when considering a surgical option for Mactel treatment, as vitrectomy and ILM peeling does not improve visual outcomes and may actually have deleterious effects in eyes with a large cystic retinal spaces.
The SD-OCT morphologic features present in our investigation are similar to those previously described [6–8]. As expected, the presence of foveal disruption of the ONL seen on OCT correlates with decreased BCVA, probably corresponding to photoreceptor cell death. This appears to hold true for all eyes, whether undergoing treatment or not, but did not predict treatment response in the present study. We did observe decreased fluorescein leakage in all treated eyes identical to previous reports [9, 10], that recurred in all cases over a mean 2-month time period. This appears to support the notion that abnormal vascular permeability in Mactel is partially VEGF-dependent, but does not completely resolve in response to a series of monthly IVB.
The pathogenesis of Mactel remains elusive despite a relatively large body of clinical research. The presence of a transient angiographic response to anti-VEGF agents, but without visual change or long-term resolution of leakage, lends support to a chronic, non-ischemic, incompetent vascular etiology. The lack of response noted to intravitreal triamcinolone [14, 24], lack of increased microglia in pathologic specimens [25], and the presence of disease in eyes without previous surgery or other ocular disease points away from an inflammatory pathogenesis. One hypothesis that would explain the findings and clinical course of Mactel is a primary developmental anomaly of the perifoveal neurovascular ring. While the average onset of symptoms in the 5th or 6th decade of life may seem incompatible with this hypothesis, there is a paucity of clinical data on Mactel patients prior to the development of visual symptoms. In addition, Mactel patients appear to have a relatively small foveal avascular zone [26]. We hypothesize that an altered foveal development underlies a chronic, life long, waxing and waning of retinal extravascular fluid which manifests as visual symptoms only after 4–6 decades. The primary defect within this hypothesis may be vascular in origin. Because deficient blood–retina barrier is primary under this hypothesis, the extracellular fluid remains nutritive to the surrounding neural tissue until additional factors, such as chronicity and the development of intraretinal spaces, lead to secondary photoreceptor and glia damage, rarely inducing intraretinal neovascularization. This would explain the laterality, temporal predominance of telangiectasis, and slow progression with predominantly non-proliferative clinical course. While previous specimens have demonstrated intact desmosomes in Mactel blood vessels [27, 28], there does appear to be decreased type IV collagen [25], indicating defects in basement membrane and perhaps also pericytes. The available histopathologic data in Mactel is limited to a small number of cases, however. The primary Muller cell etiology suggested by some [4, 25, 29] is also compelling, and the notion that glial cell support dysfunction may lead to increased vascular permeability is possible. This theory may also be supported by a developmental defect, as glial precursor cells lay the framework for the subsequent angiogenesis that underlies perifoveal capillary maturation. However, a primary Muller cell dysfunction does not explain the foveal and especially the temporal predominance of lesions in Mactel.
The present study may be limited by the all-Caucasian study population, retrospective design, and potentially unrecognized confounding variables. Particularly, re-treatment criteria for the IVB group was less well-defined due to the retrospective design. Angiographic leakage patterns were variable, as commonly encountered in Mactel, but this variability may further complicate interpretation of outcome measures in the present study. While we used strict inclusion criteria, such as evaluating only phakic eyes and excluding diabetics in an effort to ensure the evaluation of only idiopathic Mactel patients, this may also limit the applicability of the findings to additional patient populations. Nevertheless, we conclude, in agreement with previous reports [9, 10], that there is currently no clear beneficial treatment for patients with non-proliferative Mactel. The results of this retrospective study reveals lack of efficacy of both intravitreal bevacizumab or PPV with ILM peeling for non-proliferative Mactel, and helps confirm the results of currently existing reports.
References
Klein R, Blodi BA, Meuer SM, Myers CE, Chew EY, Klein BE (2010) The prevalence of macular telangiectasia type 2 in the Beaver Dam eye study. Am J Ophthalmol 150:55–62
Gass JD (1968) A fluorescein angiographic study of macular dysfunction secondary to retinal vascular disease. V. Retinal telangiectasis. Arch Ophthalmol 80:592–605
Gass JD, Oyakawa RT (1982) Idiopathic juxtafoveolar retinal telangiectasis. Arch Ophthalmol 100:769–780
Gass JD, Blodi BA (1993) Idiopathic juxtafoveolar retinal telangiectasis. Update of classification and follow-up study. Ophthalmology 100:1536–1546
Yannuzzi LA, Bardal AM, Freund KB, Chen KJ, Eandi CM, Blodi B (2006) Idiopathic macular telangiectasia. Arch Ophthalmol 124:450–460
Albini TA, Benz MS, Coffee RE, Westfall AC, Lakhanpal RR, McPherson AR, Holz ER (2006) Optical coherence tomography of idiopathic juxtafoveolar telangiectasia. Ophthalmic Surg Lasers Imaging 37:120–128
Charbel Issa P, Holz FG, Scholl HP (2007) Findings in fluorescein angiography and optical coherence tomography after intravitreal bevacizumab in type 2 idiopathic macular telangiectasia. Ophthalmology 114:1736–1742
Surguch V, Gamulescu MA, Gabel VP (2007) Optical coherence tomography findings in idiopathic juxtafoveal retinal telangiectasis. Graefes Arch Clin Exp Ophthalmol 245:783–788
Charbel Issa P, Finger RP, Kruse K, Baumuller S, Scholl HP, Holz FG (2011) Monthly ranibizumab for nonproliferative macular telangiectasia type 2: a 12-month prospective study. Am J Ophthalmol 151:876–886
Toy BC, Koo E, Cukras C, Meyerle CB, Chew EY, Wong WT (2012) Treatment of nonneovascular idiopathic macular telangiectasia type 2 with intravitreal ranibizumab: results of a phase II clinical trial. Retina 32:996–1006
Park DW, Schatz H, McDonald HR, Johnson RN (1997) Grid laser photocoagulation for macular edema in bilateral juxtafoveal telangiectasis. Ophthalmology 104:1838–1846
Moon SJ, Berger AS, Tolentino MJ, Misch DM (2007) Intravitreal bevacizumab for macular edema from idiopathic juxtafoveal retinal telangiectasis. Ophthalmic Surg Lasers Imaging 38:164–166
Kovach JL, Rosenfeld PJ (2009) Bevacizumab (avastin) therapy for idiopathic macular telangiectasia type II. Retina 29:27–32
Wu L, Evans T, Arevalo JF, Berrocal MH, Rodriguez FJ, Hsu M, Sanchez JG (2008) Long-term effect of intravitreal triamcinolone in the nonproliferative stage of type II idiopathic parafoveal telangiectasia. Retina 28:314–319
Matt G, Sacu S, Ahlers C, Schutze C, Dunavoelgyi R, Prager F, Pruente C, Schmidt-Erfurth U (2010) Thirty-month follow-up after intravitreal bevacizumab in progressive idiopathic macular telangiectasia type 2. Eye 24:1535–1541, quiz 1542
Roller AB, Folk JC, Patel NM, Boldt HC, Russell SR, Abramoff MD, Mahajan VB (2011) Intravitreal bevacizumab for treatment of proliferative and nonproliferative type 2 idiopathic macular telangiectasia. Retina 31:1848–1855
Charbel Issa P, Finger RP, Holz FG, Scholl HP (2008) Eighteen-month follow-up of intravitreal bevacizumab in type 2 idiopathic macular telangiectasia. Br J Ophthalmol 92:941–945
Schulze S, Mennel S (2007) Treatment of idiopathic juxtafoveolar retinal telangiectasis with bevacizumab (avastin). Klin Monatsbl Augenheilkd 224:787–790
Gamulescu MA, Walter A, Sachs H, Helbig H (2008) Bevacizumab in the treatment of idiopathic macular telangiectasia. Graefes Arch Clin Exp Ophthalmol 246:1189–1193
Matsumoto Y, Yuzawa M (2010) Intravitreal bevacizumab therapy for idiopathic macular telangiectasia. Jpn J Ophthalmol 54:320–324
Sandhu SS, Steel DH (2010) Comment on macular full-thickness and lamellar holes in association with type 2 idiopathic macular telangiectasia. Eye 24:1119
Shukla D, Venkatesh R (2011) Spontaneous closure of full-thickness macular hole in type 2 idiopathic macular telangiectasia. Graefes Arch Clin Exp Ophthalmol Sep 22 [Epub ahead of print]
Gregori N, Flynn HW Jr (2010) Surgery for full-thickness macular hole in patients with idiopathic macular telangiectasia type 2. Ophthalmic Surg Lasers Imaging 41(Online):1–4
Maia OO Jr, Takahashi WY, Bonanomi MT, Nascimento VP, Melo CS (2006) Intravitreal triamcinolone injection in the treatment of idiopathic juxtafoveal telangiectasis. Arq Bras Oftalmol 69:941–944
Powner MB, Gillies MC, Tretiach M, Scott A, Guymer RH, Hageman GS, Fruttiger M (2010) Perifoveal muller cell depletion in a case of macular telangiectasia type 2. Ophthalmology 117:2407–2416
Mansour AM, Schachat A (1993) Foveal avascular zone in idiopathic juxtafoveolar telangiectasia. Ophthalmologica 207:9–12
Green WR, Quigley HA, De la Cruz Z, Cohen B (1980) Parafoveal retinal telangiectasis. Light and electron microscopy studies. Trans Ophthalmol Soc U K 100:162–170
Eliassi-Rad B, Green WR (1999) Histopathologic study of presumed parafoveal telangiectasis. Retina 19:332–335
Gass JD (1999) Muller cell cone, an overlooked part of the anatomy of the fovea centralis: hypotheses concerning its role in the pathogenesis of macular hole and foveomacualr retinoschisis. Arch Ophthalmol 117:821–823
Conflict of interest
The authors have no conflict of interest, financial interest, or sources of support for the material presented in this report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sigler, E.J., Randolph, J.C., Calzada, J.I. et al. Comparison of observation, intravitreal bevacizumab, or pars plana vitrectomy for non-proliferative type 2 idiopathic macular telangiectasia. Graefes Arch Clin Exp Ophthalmol 251, 1097–1101 (2013). https://doi.org/10.1007/s00417-012-2150-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-012-2150-1