Abstract
Background
Pathological myopia is a frequent cause of secondary visual disturbance in young individuals worldwide. Myopic maculopathy describes a spectrum of clinical changes that comprise the main cause of visual loss among highly myopic individuals. Our aim is to describe current trends in the medical and surgical management of maculopathy secondary to pathological myopia.
Methods
The epidemiology, natural history, medical and surgical treatment modalities for choroidal neovascular membrane (CNV) and vitreomacular disorders secondary to pathological myopia (PM) are reviewed and evaluated.
Results
The medical and surgical treatment modalities in the management of myopic maculopathy have evolved over time. Laser photocoagulation, photodynamic therapy with verteporfin and other medical treatments have been superseded by the use of anti-vascular endothelial growth factor in the management of CNV secondary to PM. Surgical treatments are beneficial in the treatment of vitreomacular interface disorders such as macular hole retinal detachment and macular traction; however, primary success rates remain lower than those for non-myopic individuals.
Conclusions
This updated clinical perspective demonstrates that CNV and vitreomacular disorders in pathological myopia are treatable conditions. There are numerous medical and surgical interventions that have significantly improved the outcome of myopic maculopathy and several others currently under investigation. Nonetheless, as technology advances, further well-designed studies are necessary to establish a uniform evidence-based approach for classification and treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pathological myopia — prevalence and pathogenesis
Myopia-related visual impairment affects the productivity, mobility, quality of life and activities of daily living of individuals [1]. Although there is no universally agreed definition, pathological or degenerative myopia has been defined in recent studies as a spherical equivalent refractive error of at least −6 dioptres(D) accompanied by characteristic degenerative changes, with complications of pathological myopia being much more common when the refractive error is −8 D or worse [2–4]. As elongation of the globe is a key feature of pathological myopia (PM), an axial length of ≥26.5 mm has been adopted as a biometric definition in clinical trials [5], with recent studies reporting a mean of 29 mm (range 26.8–31.5 mm) [4, 6]. Pathological myopia is one of the leading causes of visual loss in the world [7]. The prevalence of PM is known to differ between ethnicities. In the adult Asian population, its prevalence is 9–21% [8, 9] compared to 2.8–4.6% in the U.S and Australia [10], and the prevalence is thought to be rising globally [1]. The pathogenesis of high myopia is unknown. Twin studies have estimated high trait heritabilities, and genetic and environmental susceptibility factors have been demonstrated to play an important role in its development [11, 12]. Myopic maculopathy describes a spectrum of clinical changes that comprise the main cause of visual loss among highly myopic individuals. The following sections describe the natural history of maculopathy in PM, and we review contemporary trends in the medical and surgical management of common sight-threatening complications related to this condition.
Myopic maculopathy
Long-term studies suggest that approximately 40% of highly myopic eyes develop progressive maculopathy, and over 10 years more than half of these individuals will lose 2 lines of Snellen acuity [13, 14]. Although there is no agreed clinical grading for myopic maculopathy, several authors have used a practical classification originally devised by Avila et al. to describe the natural history [14, 15]. With this method, a scale of increasing severity from 0 to 5 is used to describe the maculopathy as follows: M0: normal-appearing posterior pole, M1: tessellation and choroidal pallor pattern in macular area, M2: M1 and a posterior pole ectasia, M3: M2 and lacquer cracks in Bruch’s membrane and posterior staphyloma, M4: M3 and focal areas of deep choroidal atrophy, and M5: M4 and geographic areas of atrophy and choroidal neovascularisation (CNV). Recent studies have suggested modifications to this classification: Hayashi et al. conducted a comprehensive natural history study of myopic maculopathy, and indicated that lacquer cracks should be classified as an independent lesion, due to the likelihood of progression to CNV as well as diffuse atrophy. The authors also highlight the presence of a posterior staphyloma as an important factor leading to the progression of maculopathy [13]. In the Blue Mountains Eye Study, myopic retinopathy was defined to include: staphyloma, lacquer cracks, Fuchs’ spot and myopic chorioretinal thinning or atrophy [16].
For the purposes of this review, myopic maculopathy will describe both the spectrum of progressive macular degeneration resulting in CNV formation (Fig. 1), and visual loss as well as other causes of visual dysfunction in PM that have been managed surgically, such as macular hole, retinal detachment associated with macular hole, posterior staphyloma, and retinoschisis.
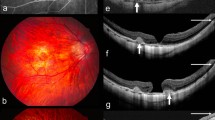
Fundus images of a 75-year-old female (refractive error: −15.0/−0.75 × 98 degrees). This demonstrates the tessellated appearance, attenuated vasculature and peripapillary atrophy with associated retinal hemorrhage (a). The corresponding fluorescein angiogram demonstrates masking due to haemorrhage and intense hyperfluoresce adjacent to the area of juxtapapillary atrophy indicating the presence of a choroidal neovascular membrane (b). Images 4 months post bevacizumab treatment demonstrate significant resolution of the haemorrhage and no leakage (c,d). Corresponding OCT images before (e) and after (f) also show significant improvement of the macular profile to a near-normal appearance
Myopic macular CNV occurs in 4% to 11% of patients with high myopia, and is the most common vision-threatening complication of high myopia [17]. It is subfoveal in 58%, and juxtafoveal in 32% of cases, and is the leading aetiology of CNV among patients younger than 50 years of age [18]. In its advanced stage, CNV appears as a Fuchs’ spot, which is a macular scar with pigment clumping and hyperpigmentation in association with fibrosis and retinal atrophy. Among myopic patients with pre-existing CNV, more than 30% will develop CNV in the fellow eye within 8 years [15].
Although natural history studies of untreated myopic CNV have presented conflicting evidence on visual prognosis, the overall trend suggests that long-term visual prognosis with observation alone is poor. Early studies demonstrated that over a mean follow-up of 3.4 years, 96% of 70 eyes with myopic CNV regressed or stabilised [15]. More recently, however, patients over 50 years with myopic CNV observed for a mean of 4 years had a final visual acuity (VA) of 20/200 or worse in over two-thirds of cases, and a 10-year follow-up observational study noted that 96% of eyes achieve a final VA of 20/200 or worse [6]. Prognostic factors associated with a poor visual outcome included extensive chorioretinal atrophy, an older age of onset and a larger area of CNV [19, 20]. Thus, active interventions are usually recommended for patients with myopic CNV.
Management of CNV secondary to pathological myopia
Myopic CNV has been treated with different approaches including laser photocoagulation, radiotherapy, submacular surgery, photodynamic therapy (PDT), ICG-mediated photothrombosis, macular translocation, combined PDT and intravitreal triamcinolone, transpupillary thermotherapy (TTT), and intravitreal injections of anti- vascular endothelial growth factor (VEGF) agents. We review therapies that have produced significant widespread interest in the practice of most ophthalmologists.
Direct laser photocoagulation
Laser photocoagulation is of limited benefit in myopic CNV [21]. Laser treatment of myopic CNV is difficult because of hypopigmentation of the fundus that reduces laser uptake, difficulties in focusing treatment, enlargement of subsequent photocoagulation scarring (90–100%), and recurrence of CNV, which can occur in up to 72% of treated eyes [22, 23].
Photodynamic therapy (PDT) with verteporfin
PDT causes selective damage to the choriocapillaris, sparing the neurosensory retina and retinal pigment epithelium (RPE), and is used to treat subfoveal CNV without the complications of laser photocoagulation. Verteporfin administered intravenously preferentially binds to CNV endothelial low density lipoprotein (LDL) receptors. Activation of this compound by diode laser produces reactive oxygen species which cause selective endothelial damage that occludes abnormal neovascularisation [24, 25].
The VIP study initially reported the efficacy of PDT in myopic CNV and demonstrated that 72% of treated eyes had visual loss of fewer than 8 letters compared to 44% of placebo-treated eyes, an effect that was significant after year 1, but not at the end of year 2 [5, 26]. There have been several other studies that have demonstrated a significant improvement in VA of 3 or more lines of vision (19–27%) [27, 28] with approximately 55–63% of individuals having stable VA at final follow-up. There are also several reports of successful PDT use in juxta-foveal CNV [29, 30]. In a consecutive case series of 48 patients (49 eyes), with juxtafoveal myopic CNV and a median follow up of 32 months, VA improved by 1 or more Snellen lines in 37%, decreased in 28%, and remained stable in 39%. The size of CNV and the magnitude of refractive error did not influence visual outcomes, and younger patients appeared to respond more favourably to PDT [30]. Table 1 summarises the evidence for the use of PDT in myopic CNV.
Intra-vitreal corticosteroids with PDT have also been used for their anti-angiogenic and anti-inflammatory properties. Several studies describe better outcomes of combination intra-vitreal corticosteroids and PDT compared to standard PDT alone for the treatment of CNV secondary to age-related macular degeneration (ARMD) [31]. A randomised controlled trial in CNV secondary to ARMD demonstrated effective stabilization of VA and reduced treatment frequency at 12 months with combination PDT plus intra-vitreal triamcinolone (IVTA) therapy versus PDT alone; however, 40% of individuals receiving IVTA needed anti-glaucoma treatment [32]. Marticorena et al. evaluated VA changes and safety of combined treatment with PDT and IVTA injection in 12 myopic eyes with CNV in a prospective interventional case series [33]. Although the study showed an improvement in patients with subfoveal myopic CNV, there were significant adverse effects associated with intra-ocular steroid use [34], and subsequent studies have demonstrated the added effect of combination therapy to be insignificant [35].
Anti-VEGF therapy
The role of VEGF (also known as VEGF-A) as a regulator of angiogenesis has been the subject of much investigation. It is clear, however, that ocular angiogenesis is a complex cascade that involves numerous proteins and biochemical interactions, with VEGF playing a central role and its inhibition being an important therapeutic strategy. Anti-VEGF therapy has therefore gained widespread use in the treatment of CNV secondary to ARMD, and has shown much clinical benefit in CNV secondary to other inflammatory and vascular causes. Since then, the evidence for the effective and safe use of anti-VEGF agents in the treatment of myopic CNV have increased dramatically. These reports are largely based on the use of intra-vitreal bevacizumab and ranibizumab, although the successful use of pegaptanib has also been reported [36].
Intra-vitreal bevacizumab
Bevacizumab (Avastin, Genentech Inc. [Roche Group], San Francisco, CA, USA) is a humanised monoclonal anti-VEGF antibody that binds to and neutralises all human isoforms of VEGF-A [37]. Bevacizumab was developed for intravenous administration, and approved for the treatment of colorectal cancer [38]. Bevacizumab has been administered by intravitreal injection in several conditions where VEGF levels are elevated, including CNV, diabetic retinopathy, and central retinal vein occlusion. In 2006, reports began emerging of the beneficial use of bevacizumab in myopic CNV. Many of these reports were initial short-term pilot studies with promising results, some noting an improvement of 2 lines of VA in 70% of cases [2, 39]. By 2009, Cohen stated that based on the collective evidence of 14 pilot studies in over 250 eyes with myopic CNV, anti-VEGF treatment with ranibizumab or bevacizumab can be considered as the first-line recommended therapy for the treatment of CNV secondary to PM [40]. Table 2 summarises the studies to date with a minimum of 1-year follow-up using intra-vitreal bevacizumab for myopic CNV. Overall, between 40 and 72% of cases demonstrated an improvement of more than 3 lines of VA at final follow-up [41]. No adverse effects were noted, and the improvement in VA was seen to persist for up to 2 years. However, the recommended number and time interval for treatments remains inconclusive, with some studies having administered only one injection [42–44], and others using an initial therapeutic treatment of three consecutive monthly injections [41, 45, 46].Younger patients appear to achieve a better final visual outcome, and require fewer injections [44, 46].
Several authors have directly compared PDT and intra-vitreal bevacizumab, and all report the superior efficacy of the latter. Of thirty-nine eyes treated with intra-vitreal bevacizumab, 91% had angiographic closure and 49% had an improvement of >2 lines of VA at 1 year. Patients treated with intra-vitreal bevacizumab had significantly better VA than PDT-treated and control eyes at 1 year [47]. Other authors have similarly demonstrated that the angiographic findings and the VA was significantly better at 6, 12, 18 and 24 months in the intra-vitreal bevacizumab group, with the PDT group showing a worsening of VA at 18 and 24 months [48, 49, 50] Small interventional series have also reported a benefit and reduction in re-treatment following combined PDT and bevacizumb treatment [51, 52].
Intra-vitreal ranibizumab
Ranibuzumab (Lucentis, Genentech Inc. [Roche Group], San Francisco, CA, USA) is a humanised monoclonal anti-VEGF antibody fragment that bind to and neutralises all active isoforms of VEGF-A. It is currently licensed by the U.S. Food and Drug Authority and the Committee for Medicinal Products for Human Use of the European Medicines Agency for the treatment of CNV secondary to age-related macular degeneration. It has, however, been shown to be effective in CNV secondary to PM, ocular histoplasmosis, and angioid streaks [53]. There are several short-term reports of effective ranibizumab use in myopic CNV. Konstantinidis et al. report that after an 8-month follow-up, 64% (9/14 eyes) gained 3 or more lines of vision [54]. In a further study of 26 eyes, 1 month after a single injection, one-third of treated eyes had a 3-line gain in VA [55]. Several 1-year follow-up reports have also recently been published, and show a similar trend. At 1 year after a mean of 1.5 injections in 23 eyes, 69% of patients had improved vision of at least 1 line, and 34.7% achieved 3 or more lines [56]. Similarly, Silva et al. report that out of 34 eyes with myopic CNV treated with a mean of 3.6 injections, at 1 year, 24% of the eyes improved ≥ 3 lines of VA, 44% improved ≥ 2 lines and 65% improved ≥ 1 line. Central retinal thickness also decreased significantly from baseline to the 12-month final follow-up [57]. Other studies also support an improved VA at 12 months after treatment [58, 59]. Cornut et al. report a slightly longer follow-up duration, with a median of 17 months in 32 treated eyes; 47% achieved an improvement of ≥ 3 lines of VA after a median of three injections [59]. There were no serious adverse effects reported in any of the series reported.
To date, one study has compared intra-vitreal bevacizumab versus ranibizumab for the treatment of myopic CNV; however, it found no statistical difference between the two treatment arms [60]. Presently, a phase II multicentre open-label trial of ranibizumab for the treatment of choroidal neovascularisation secondary to pathological myopia: an individualized regimen (REPAIR) (www.clinicaltrials.gov - NCT01037348) is recruiting, and may provide definitive evidence and a suitable regimen for the use of ranibizumab in myopic CNV.
Recent developments
Several pharmacologic agents for the treatment of CNV secondary to ARMD are under investigation, some of which may in turn be of benefit in the treatment of CNV secondary to PM. Interestingly, a phase II clinical trial has reported initial findings of combretastatin A4 phosphate (CA4P), a vascular disrupting agent for the treatment of myopic CNV. However, initial reports were only modest or equivocal: at 3 months, no patients (n = 21) lost >3 lines of VA [61]. VEGF Trap is a pharmacologically engineered protein that binds VEGF with higher affinity than ranibizumab, offering a theoretically longer interval between treatments. A recent phase II study (CLEAR-IT 2) has demonstrated a significant mean improvement in best-corrected visual acuity (BCVA) of 5.3 letters (n = 157) for patients with neovascular ARMD at 1 year [62]. Other experimental approaches to the management of CNV include ingrowth-site treatment of subfoveal CNV using ICG-mediated photothrombosis. A study of six consecutive eyes with subfoveal CNV secondary to PM showed that ICG-mediated photothrombosis obliterated the entire CNV complex, improved VA by at least 5 letters in five of the six eyes, and reduced retinal oedema on OCT [63]. Sirna-027 is a mRNA (siRNA) designed to silence the gene for VEGFR-1 so that it is unable to translate the message to increase vascular production. A phase I study [64] evaluated a single Sirna-027 in 26 patients with wet AMD. Three months after the single injection, 24 patients showed stable VA, with four patients experiencing a clinically significant improvement in vision. Other approaches currently under investigation for exudative ARMD involve nicotinic receptor antagonists, vaccination, immunomodulators and immunosuppressants [65, 66]. However, as yet none of these have been demonstrated to be safe and effective in phase III multi-centre clinical trials.
Surgical approaches to macular disease secondary to pathological myopia
High myopia accompanied by degenerative changes in the posterior segment and visual dysfunction may lend itself to surgical correction in some patients; however, severe axial elongation of the globe, the presence of a posterior staphyloma, and atrophy of the RPE and choroid make these cases challenging. Surgical approaches to the management of myopic macular CNV have involved primarily surgical excision of CNV and macular translocation. Other causes of visual dysfunction in PM that have been managed surgically include myopic macular hole (MMH), retinal detachment associated with macular hole, posterior staphyloma and macular retinoschisis with preretinal traction.
Small uncontrolled studies of surgical CNV excision have shown variable outcomes. The Subretinal Surgery Trial group reported no benefit of surgical excision in patients with ocular histoplasmosis and idiopathic CNV, and marginal benefit for the subgroup of patients with a VA of 20/100 or worse [67]. Overall, recurrence rates of CNV are high, ranging between 18 and 57% in most studies, and visual demise is not uncommon [68–70]. There is at present therefore little evidence to support the surgical excision of CNV in PM. Similarly, surgical rotation of the macula, first described in 1993 [71], showed initial promise in the management of myopic CNV [3, 72], but is now uncommonly performed as there are safer and more effective treatment options.
Myopic macular retinoschisis
Myopic macular retinoschisis (MR) is thought to occur in 9–20% of highly myopic eyes with posterior staphyloma. The clinical presence of macular retinoschisis has been demonstrated with optical coherence interferometry (OCT) by Takano and Kishi [73]. A posterior vitreous detachment (PVD) based on the presence of a Weiss ring is only noted in 7% of patients with MR. The OCT features of MR comprise retinal thickening and splitting of the neurosensory retina into a thin outer outer layer and a thicker reflective inner layer; however, an inner retinoschisis may also be present in some eyes [74] (Figure 2). This can be associated with a foveal cyst, which can be partially de-roofed giving the appearance of a lamellar hole, and in some patients a foveal detachment may be present. Preretinal structures, representing either a thickened posterior hyaloid or epiretinal membrane, may be seen as a hyper-reflective layer indicating the presence of tangential traction. A natural history study of nine eyes with MR without preretinal membranes over at least 1 year demonstrated no significant progression [74]. However, a macular hole may develop spontaneously or possibly after vitreous surgery, as shown in nine of 29 eyes reported by Gaucher et al. [75].
The surgical outcomes of vitreous surgery for MR and foveal detachment without macular hole have resulted in significant anatomical improvements as well as moderate improvements in vision. Kobayashi et al., in a series of nine eyes, had post operative LogMAR visual acuities of 0.4 to 0.6, and similarly Scott et al. reported post operative visual acuities of 20/40 to 20/80 in three eyes [76, 77]. A small risk of post operative macular hole formation has been reported and this is thought to be associated with the presence of premacular traction membranes [77]. In addition, posterior scleral reinforcement with a macular plomb has been reported in a small case series of six patients with a MR and associated retinal detachment. BCVA improved in four eyes, and a subretinal haemorrhage developed in one eye [78]
Furthermore, the possible value of stopping axial elongation in PM using macular buckling surgery has been reported [79], but the true value of this procedure in the context of MR as a prophylactic measure to prevent progressive change to macular hole with an associated retinal detachment has not been fully investigated [80]. In conclusion, well-designed prospective studies with clear case definition are lacking. Nonetheless, limited evidence from case series supports a role for vitreous surgery with separation of the posterior vitreous cortex in eyes with progressive visual loss and MR associated with preretinal tractional membranes. The removal of all tractional forces may underlie the reported benefit; however, the true value in peeling the internal limiting membrane remains unclear. The prophylactic role of macular buckling deserves further investigation.
Macular hole and associated retinal detachment
Macular holes (MH) with associated retinal detachment are rare in the general population. They occur principally in highly myopic eyes, and are thought to be the result of tangential vitreoretinal traction [81]. A foveal detachment often precedes the formation of a MH in highly myopic eyes, and axial elongation, the posterior staphyloma, and a PVD probably all contribute to the formation of a retinal detachment [75, 82, 83]. Whilst the relationship between MR and MH is unknown, it has been reported that MR may be a precursor to MH formation in PM [73, 75, 77]. The reported closure rates for MH associated with RD and PM are disappointing. Pars plana vitrectomy (PPV) in combination with inner limiting membrane (ILM) peel and a long- or short-acting gas tamponade is commonly used to repair MH. Reported closure rates using the above approaches vary between 10% and 44% in studies that have used OCT to evaluate MH closure in high myopes [84, 85]. Poor anatomic outcomes are thought to result from inner retinal shortening, which is not fully compensated for by ILM peeling and does not provide sufficient redundant retina [86]. Reported closure rates also vary significantly if OCT is not used as part of the macular assessment [87–89]. Furthermore, post operative MH enlargement has been reported, supporting the theory that the imbalance between the retina and choroid–sclera complex due to the posterior staphyloma may need to be remedied for the complete relief of traction [85]. Whilst MH closure rates are poor, retinal reattachment rates following vitrectomy surgery are thought to be more successful, varying between 40 and 93% [85, 90].
Most published data on the role of vitrectomy for MMH and retinal detachment have included peeling of preretinal structures and/or the ILM. Most have used SF6 or C3F8 gas, with a few studies reporting success with silicone oil [90–93]. To our knowledge, there are no randomised trials that have evaluated the role of epiretinal membrane or ILM peel, and no studies that have compared types of intraocular tamponade (Table 3). It is, however, intuitive to assume that complete peeling of the thickened posterior hyaloid and all preretinal structures constitutes a logical approach, given the postulated pathogenic forces involved.
Vitrectomy alone, however, does not address the presence of a posterior staphyloma, which has been strongly associated with retinal detachment secondary to MH. In order to treat the posterior staphyloma, macular buckling (MB) was developed. MB is not a new surgical technique, and it has not been widely adopted. Thus, reports of surgical outcomes of MB for myopic MH associated RD are limited. Ando et al. describe a series over 17 years based on a single surgeon’s experience. The authors report a 93% retinal reattachment rate, and MH closure in ten of 12 eyes (83%) where the macular buckle produced an indent over the MH [94]. Similar retinal reattachment rates using MB were reported in two other series [95, 96]. A relatively simple procedure using a T-shaped buckle for the treatment of a myopic retinal detachment associated with MH or MR has been described recently [97]. This case series comprised previous vitrectomy failures and eyes with severe chorioretinal atrophy. Retinal reattachment was observed in 79% of cases.
Based on the available evidence, most of which is based on case series with variable follow-up, it would be difficult to identify the optimal surgical technique for managing retinal detachment secondary to myopic MH. There is significant heterogeneity between different studies, which highlights the need for a well-designed randomised trial to evaluate the role of vitrectomy surgery and ILM peeling for myopic MH and secondary retinal detachment. Furthermore, the role of MB remains inconclusive, and this surgical technique deserves further evaluation for the management of MH and associated retinal detachment in PM.
Conclusions
The natural history and long-term visual prognosis of untreated CNV in high myopia is very poor. The efficacy of laser, PDT, and macular translocation has been superseded by the use of intra-vitreal anti-VEGF agents which are currently considered as first-line therapy for myopic CNV. Nonetheless, large-scale randomised controlled trials are necessary to identify the most suitable agent and to establish an appropriate frequency and dosing regimen. Recent developments for neovascular ARMD may be of value in the management of myopic CNV; however, further evaluation of safety and benefit are needed. Macular retinoschisis associated with epimacular traction and visual decline, as well as macular hole with secondary retinal detachment, are both surgically treatable conditions. Although well-designed studies are lacking, several interventional series highlight a clinical benefit in the role of vitrectomy and peeling of premacular membranes with internal gas tamponade. Macular buckling has been used to treat posterior staphyloma; however, a clear and repeatable benefit is yet to be demonstrated. Well-designed and randomised studies are still necessary to develop a uniform evidence-based approach for management and treatment of this complex condition.
References
Saw SM (2006) How blinding is pathological myopia? Br J Ophthalmol 90:525–526
Yamamoto I, Rogers AH, Reichel E, Yates PA, Duker JS (2007) Intravitreal bevacizumab (Avastin) as treatment for subfoveal choroidal neovascularisation secondary to pathological myopia. Br J Ophthalmol 91:157–160
Tano Y (2002) Pathologic myopia: where are we now? Am. J Ophthalmol 134:645–660
Yoshida T, Ohno-Matsui K, Ohtake Y, Takashima T, Futagami S, Baba T, Yasuzumi K, Tokoro T, Mochizuki M (2002) Long-term visual prognosis of choroidal neovascularization in high myopia: a comparison between age groups. Ophthalmology 109:712–719
Verteporfin in Photodynamic Therapy Study Group (2001) Photodynamic therapy of subfoveal choroidal neovascularization in pathologic myopia with verteporfin. 1-year results of a randomized clinical trial--VIP report no. 1. Ophthalmology 108:841–852
Yoshida T, Ohno-Matsui K, Yasuzumi K, Kojima A, Shimada N, Futagami S, Tokoro T, Mochizuki M (2003) Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology 110:1297–1305
Grossniklaus HE, Green WR (1992) Pathologic findings in pathologic myopia. Retina 12:127–133
Yamada M, Hiratsuka Y, Roberts CB, Pezzullo ML, Yates K, Takano S, Miyake K, Taylor HR (2010) Prevalence of visual impairment in the adult Japanese population by cause and severity and future projections. Ophthalmic Epidemiol 17:50–57
Hsu WM, Cheng CY, Liu JH, Tsai SY, Chou P (2004) Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology 111:62–69
Kempen JH, Mitchell P, Lee KE, Tielsch JM, Broman AT, Taylor HR, Ikram MK, Congdon NG, O’Colmain BJ (2004) The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. Arch Ophthalmol 122:495–505
Hornbeak DM, Young TL (2009) Myopia genetics: a review of current research and emerging trends. Curr Opin Ophthalmol 20:356–362
Jacobi FK, Pusch CM (2010) A decade in search of myopia genes. Front Biosci 15:359–372
Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A, Hayashi W, Yasuzumi K, Nagaoka N, Saka N, Yoshida T, Tokoro T, Mochizuki M (2010) Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology 117(1595–611):1611
Shih YF, Ho TC, Hsiao CK, Lin LL (2006) Visual outcomes for high myopic patients with or without myopic maculopathy: a 10 year follow up study. Br J Ophthalmol 90:546–550
Avila MP, Weiter JJ, Jalkh AE, Trempe CL, Pruett RC, Schepens CL (1984) Natural history of choroidal neovascularization in degenerative myopia. Ophthalmology 91:1573–1581
Vongphanit J, Mitchell P, Wang JJ (2002) Prevalence and progression of myopic retinopathy in an older population. Ophthalmology 109:704–711
Soubrane G, Coscas GJ (1994) Choroidal neovascular membranes in degenerative myopia. In: Ryan SJ (ed) Retina. Mosby, St Louis, pp 1143–1157
Cohen SY, Laroche A, Leguen Y, Soubrane G, Coscas GJ (1996) Etiology of choroidal neovascularization in young patients. Ophthalmology 103:1241–1244
Hayashi K, Ohno-Matsui K, Yoshida T, Kobayashi K, Kojima A, Shimada N, Yasuzumi K, Futagami S, Tokoro T, Mochizuki M (2005) Characteristics of patients with a favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 243:13–19
Kojima A, Ohno-Matsui K, Teramukai S, Yoshida T, Ishihara Y, Kobayashi K, Shimada N, Yasuzumi K, Futagami S, Tokoro T, Mochizuki M (2004) Factors associated with the development of chorioretinal atrophy around choroidal neovascularization in pathologic myopia. Graefes Arch Clin Exp Ophthalmol 242:114–119
Jalkh AE, Weiter JJ, Trempe CL, Pruett RC, Schepens CL (1987) Choroidal neovascularization in degenerative myopia: role of laser photocoagulation. Ophthalmic Surg 18:721–725
Secretan M, Kuhn D, Soubrane G, Coscas G (1997) Long-term visual outcome of choroidal neovascularization in pathologic myopia: natural history and laser treatment. Eur J Ophthalmol 7:307–316
Johnson DA, Yannuzzi LA, Shakin JL, Lightman DA (1998) Lacquer cracks following laser treatment of choroidal neovascularization in pathologic myopia. Retina 18:118–124
Schmidt-Erfurth U, Hasan T (2000) Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol 45:195–214
Schmidt-Erfurth U, Laqua H, Schlotzer-Schrehard U, Viestenz A, Naumann GO (2002) Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol 120:835–844
Blinder KJ, Blumenkranz MS, Bressler NM, Bressler SB, Donato G, Lewis H, Lim JI, Menchini U, Miller JW, Mones JM, Potter MJ, Pournaras C, Reaves A, Rosenfeld P, Schachat AP, Schmidt-Erfurth U, Sickenberg M, Singerman LJ, Slakter JS, Strong HA, Virgili G, Williams GA (2003) Verteporfin therapy of subfoveal choroidal neovascularization in pathologic myopia: 2-year results of a randomized clinical trial–VIP report no. 3. Ophthalmology 110:667–673
Lam DS, Chan WM, Liu DT, Fan DS, Lai WW, Chong KK (2004) Photodynamic therapy with verteporfin for subfoveal choroidal neovascularisation of pathologic myopia in Chinese eyes: a prospective series of 1 and 2 year follow up. Br J Ophthalmol 88:1315–1319
Ergun E, Heinzl H, Stur M (2004) Prognostic factors influencing visual outcome of photodynamic therapy for subfoveal choroidal neovascularization in pathologic myopia. Am J Ophthalmol 138:434–438
Lam DS, Liu DT, Fan DS, Lai WW, So SF, Chan WM (2005) Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization secondary to pathologic myopia—1-year results of a prospective series. Eye 19:834–840
Pece A, Vadala M, Isola V, Matranga D (2007) Photodynamic therapy with verteporfin for juxtafoveal choroidal neovascularization in pathologic myopia: a long-term follow-up study. Am J Ophthalmol 143:449–454
Augustin AJ, Schmidt-Erfurth U (2006) Verteporfin therapy combined with intravitreal triamcinolone in all types of choroidal neovascularization due to age-related macular degeneration. Ophthalmology 113:14–22
Chaudhary V, Mao A, Hooper PL, Sheidow TG (2007) Triamcinolone acetonide as adjunctive treatment to verteporfin in neovascular age-related macular degeneration: a prospective randomized trial. Ophthalmology 114:2183–2189
Marticorena J, Gomez-Ulla F, Fernandez M, Pazos B, Rodriguez-Cid MJ, Sanchez-Salorio M (2006) Combined photodynamic therapy and intravitreal triamcinolone acetonide for the treatment of myopic subfoveal choroidal neovascularization. Am J Ophthalmol 142:335–337
Marticorena J, Gomez-Ulla F, Romano MR, Luna I (2007) Repeated pseudoendophthalmitis after combined photodynamic therapy and intravitreal triamcinolone. Graefes Arch Clin Exp Ophthalmol 245:1403–1404
Chan WM, Lai TY, Wong AL, Liu DT, Lam DS (2007) Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of choroidal neovascularisation secondary to pathological myopia: a pilot study. Br J Ophthalmol 91:174–179
Bennett MD, Yee W (2007) Pegaptanib for myopic choroidal neovascularization in a young patient. Graefes Arch Clin Exp Ophthalmol 245:903–905
Ferrara N, Kerbel RS (2005) Angiogenesis as a therapeutic target. Nature 438:967–974
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Sakaguchi H, Ikuno Y, Gomi F, Kamei M, Sawa M, Tsujikawa M, Oshima Y, Kusaka S, Tano Y (2007) Intravitreal injection of bevacizumab for choroidal neovascularisation associated with pathological myopia. Br J Ophthalmol 91:161–165
Cohen SY (2009) Anti-VEGF drugs as the 2009 first-line therapy for choroidal neovascularization in pathologic myopia. Retina 29:1062–1066
Gharbiya M, Allievi F, Mazzeo L, Gabrieli CB (2009) Intravitreal bevacizumab treatment for choroidal neovascularization in pathologic myopia: 12-month results. Am J Ophthalmol 147:84–93
Ikuno Y, Sayanagi K, Soga K, Sawa M, Tsujikawa M, Gomi F, Tano Y (2009) Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol 147:94–100
Wu PC, Chen YJ (2009) Intravitreal injection of bevacizumab for myopic choroidal neovascularization: 1-year follow-up. Eye 23:2042–2045
Ruiz-Moreno JM, Montero JA, Arias L, Araiz J, Gomez-Ulla F, Silva R, Pinero DP (2010) Twelve-month outcome after one intravitreal injection of bevacizumab to treat myopic choroidal neovascularization. Retina 30:1609–1615
Chan WM, Lai TY, Liu DT, Lam DS (2009) Intravitreal bevacizumab (Avastin) for myopic choroidal neovascularisation: 1-year results of a prospective pilot study. Br J Ophthalmol 93:150–154
Ruiz-Moreno JM, Montero JA, Gomez-Ulla F, Ares S (2009) Intravitreal bevacizumab to treat subfoveal choroidal neovascularisation in highly myopic eyes: 1-year outcome. Br J Ophthalmol 93:448–451
Hayashi K, Ohno-Matsui K, Teramukai S, Shimada N, Moriyama M, Hayashi W, Yoshida T, Tokoro T, Mochizuki M (2009) Comparison of visual outcome and regression pattern of myopic choroidal neovascularization after intravitreal bevacizumab or after photodynamic therapy. Am J Ophthalmol 148:396–408
Ikuno Y, Nagai Y, Matsuda S, Arisawa A, Sho K, Oshita T, Takahashi K, Uchihori Y, Gomi F (2010) Two-year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol 149:140–146
Baba T, Kubota-Taniai M, Kitahashi M, Okada K, Mitamura Y, Yamamoto S (2010) Two-year comparison of photodynamic therapy and intravitreal bevacizumab for treatment of myopic choroidal neovascularisation. Br J Ophthalmol 94:864–870
Yoon JU, Byun YJ, Koh HJ (2010) Intravitreal anti-VEGF versus photodynamic therapy with verteporfin for treatment of myopic choroidal neovascularization. Retina 30:418–424
Ziemssen F, Inhoffen W, Voelcker M, Grisanti S, Szurman P, Bartz-Schmidt KU, Gelisken F; Tuebingen Bevacizumab Study Group (2007) Intravitreous bevacizumab monotherapy vs combined therapy (bevacizumab + PDT) of choroidal neovascularization in pathological myopia: outcome and re-treatment. Invest Ophthalmol Vis Sci 48:3397
Battista C, Nardoni S, Romani A (2009) Combined intravitreal avastin injection and photodynamic therapy for the treatment of macular choroidal neovascularization in pathological myopia. Invest Ophthalmol Vis Sci 50:2266
Heier JS, Brown D, Ciulla T, Abraham P, Bankert JM, Chong S, Daniel PE Jr, Kim IK (2011) Ranibizumab for choroidal neovascularization secondary to causes other than age-related macular degeneration: a phase I clinical trial. Ophthalmology 118:111–118
Konstantinidis L, Mantel I, Pournaras JA, Zografos L, Ambresin A (2009) Intravitreal ranibizumab (Lucentis) for the treatment of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 247:311–318
Silva RM, Ruiz-Moreno JM, Nascimento J, Carneiro A, Rosa P, Barbosaa A, Carvalheira F, Abreu JR, Cunha-Vaz JG (2008) Short-term efficacy and safety of intravitreal ranibizumab for myopic choroidal neovascularization. Retina 28:1117–1123
Mones JM, Amselem L, Serrano A, Garcia M, Hijano M (2009) Intravitreal ranibizumab for choroidal neovascularization secondary to pathologic myopia: 12-month results. Eye (Lond) 23:1275–1280
Silva RM, Ruiz-Moreno JM, Rosa P, Carneiro A, Nascimento J, Rito LF, Cachulo ML, Carvalheira F, Murta JN (2010) Intravitreal ranibizumab for myopic choroidal neovascularization: 12-month results. Retina 30:407–412
Lalloum F, Souied EH, Bastuji-Garin S, Puche N, Querques G, Glacet-Bernard A, Coscas G, Soubrane G, Leveziel N (2010) Intravitreal ranibizumab for choroidal neovascularization complicating pathologic myopia. Retina 30:399–406
Cornut PL, Poli M, Feldman A, El CH, Swalduz B, Burillon C, Denis P (2010) Intravitreal ranibizumab for choroidal neovascularization secondary to pathological myopia: 12-month results. J Fr Ophtalmol 33:327–333
Gharbiya M, Giustolisi R, Allievi F, Fantozzi N, Mazzeo L, Scavella V, Gabrieli CB (2010) Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab–a randomized controlled trial. Am J Ophthalmol 149:458–464
Wong TP, Brown DM, Benz MS (2007) Phase II clinical trial of intravenous combretastatin a4 phosphate in patients with subfoveal choroidal neovascular membranes (CNV) in pathologic myopia. Invest Ophthalmol Vis Sci 48:1457
Heier JS, CLEAR-IT (2009) CLEAR-IT 2: phase 2, randomized, controlled dose-and interval-ranging study of intravitreal VEGF Trap Eye in patients with neovascular age-related macular degeneration: predictive factors for visual acuity outcome at one year. Invest Ophthalmol Vis Sci 50:1255
Costa RA, Calucci D, Teixeira LF, Cardillo JA, Bonomo PP (2003) Selective occlusion of subfoveal choroidal neovascularization in pathologic myopia using a new technique of ingrowth site treatment. Am J Ophthalmol 135:857–866
Kaiser PK, Symons RC, Shah SM, Quinlan EJ, Tabandeh H, Do DV, Reisen G, Lockridge JA, Short B, Guerciolini R, Nguyen QD (2010) RNAi-based treatment for neovascular age-related macular degeneration by Sirna-027. Am J Ophthalmol 150:33–39
Zhang M, Zhang J, Yan M, Luo D, Zhu W, Kaiser PK, Yu DC (2010) A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology 118(4):672–678
Ni Z, Hui P (2009) Emerging pharmacologic therapies for wet age-related macular degeneration. Ophthalmologica 223:401–410
Hawkins BS, Miskala PH, Bass EB, Bressler NM, Childs AL, Mangione CM, Marsh MJ (2004) Surgical removal vs observation for subfoveal choroidal neovascularization, either associated with the ocular histoplasmosis syndrome or idiopathic: II. Quality-of-life findings from a randomized clinical trial: SST Group H Trial: SST Report No. 10. Arch Ophthalmol 122:1616–1628
Uemura A, Thomas MA (2000) Subretinal surgery for choroidal neovascularization in patients with high myopia. Arch Ophthalmol 118:344–350
Ruiz-Moreno JM, De la Vega C (2001) Surgical removal of subfoveal choroidal neovascularisation in highly myopic patients. Br J Ophthalmol 85:1041–1043
Hamelin N, Glacet-Bernard A, Brindeau C, Mimoun G, Coscas G, Soubrane G (2002) Surgical treatment of subfoveal neovascularization in myopia: macular translocation vs surgical removal. Am J Ophthalmol 133:530–536
Machemer R, Steinhorst UH (1993) Retinal separation, retinotomy, and macular relocation: II. A surgical approach for age-related macular degeneration? Graefes Arch Clin Exp Ophthalmol 231:635–641
Kamei M, Tano Y, Yasuhara T, Ohji M, Lewis H (2004) Macular translocation with chorioscleral outfolding: 2-year results. Am J Ophthalmol 138:574–581
Takano M, Kishi S (1999) Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol 128:472–476
Benhamou N, Massin P, Haouchine B, Erginay A, Gaudric A (2002) Macular retinoschisis in highly myopic eyes. Am J Ophthalmol 133:794–800
Gaucher D, Haouchine B, Tadayoni R, Massin P, Erginay A, Benhamou N, Gaudric A (2007) Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. Am J Ophthalmol 143:455–462
Scott IU, Moshfeghi AA, Flynn HW Jr (2006) Surgical management of macular retinoschisis associated with high myopia. Arch Ophthalmol 124:1197–1199
Kobayashi H, Kishi S (2003) Vitreous surgery for highly myopic eyes with foveal detachment and retinoschisis. Ophthalmology 110:1702–1707
Baba T, Tanaka S, Maesawa A, Teramatsu T, Noda Y, Yamamoto S (2006) Scleral buckling with macular plombe for eyes with myopic macular retinoschisis and retinal detachment without macular hole. Am J Ophthalmol 142:483–487
Ward B, Tarutta EP, Mayer MJ (2009) The efficacy and safety of posterior pole buckles in the control of progressive high myopia. Eye 23:2169–2174
Zhu Z, Ji X, Zhang J, Ke G (2009) Posterior scleral reinforcement in the treatment of macular retinoschisis in highly myopic patients. Clin Experiment Ophthalmol 37:660–663
Wolfensberger TJ, Gonvers M (2000) Surgical treatment of retinal detachment owing to macular hole. Semin Ophthalmol 15:122–127
Morita H, Ideta H, Ito K, Yonemoto J, Sasaki K, Tanaka S (1991) Causative factors of retinal detachment in macular holes. Retina 11:281–284
Akiba J, Konno S, Yoshida A (1999) Retinal detachment associated with a macular hole in severely myopic eyes. Am J Ophthalmol 128:654–655
Ikuno Y, Sayanagi K, Oshima T, Gomi F, Kusaka S, Kamei M, Ohji M, Fujikado T, Tano Y (2003) Optical coherence tomographic findings of macular holes and retinal detachment after vitrectomy in highly myopic eyes. Am J Ophthalmol 136:477–481
Ichibe M, Yoshizawa T, Murakami K, Ohta M, Oya Y, Yamamoto S, Funaki S, Funaki H, Ozawa Y, Baba E, Abe H (2003) Surgical management of retinal detachment associated with myopic macular hole: anatomic and functional status of the macula. Am J Ophthalmol 136:277–284
Ikuno Y, Tano Y (2006) Vitrectomy for macular holes associated with myopic foveoschisis. Am J Ophthalmol 141:774–776
Kadonosono K, Yazama F, Itoh N, Uchio E, Nakamura S, Akura J, Sawada H, Ohno S (2001) Treatment of retinal detachment resulting from myopic macular hole with internal limiting membrane removal. Am J Ophthalmol 131:203–207
Seike C, Kusaka S, Sakagami K, Ohashi Y (1997) Reopening of macular holes in highly myopic eyes with retinal detachments. Retina 17:2–6
Tabandeh H, Smiddy WE, Mello M, Alexandrakis G, Flynn HW Jr, Gregor Z, Schiffman J (2001) Surgery for idiopathic macular holes associated with extensive subretinal fluid. Retina 21:15–19
Chen YP, Chen TL, Yang KR, Lee WH, Kuo YH, Chao AN, Wu WC, Chen KJ, Lai CC (2006) Treatment of retinal detachment resulting from posterior staphyloma-associated macular hole in highly myopic eyes. Retina 26:25–31
Lu L, Li Y, Cai S, Yang J (2002) Vitreous surgery in highly myopic retinal detachment resulting from a macular hole. Clin Experiment Ophthalmol 30:261–265
Cheung BT, Lai TY, Yuen CY, Lai WW, Tsang CW, Lam DS (2007) Results of high-density silicone oil as a tamponade agent in macular hole retinal detachment in patients with high myopia. Br J Ophthalmol 91:719–721
Nishimura A, Kimura M, Saito Y, Sugiyama K (2011) Efficacy of primary silicone oil tamponade for the treatment of retinal detachment caused by macular hole in high myopia. Am J Ophthalmol 151:148–155
Ando F, Ohba N, Touura K, Hirose H (2007) Anatomical and visual outcomes after episcleral macular buckling compared with those after pars plana vitrectomy for retinal detachment caused by macular hole in highly myopic eyes. Retina 27:37–44
Sasoh M, Yoshida S, Ito Y, Matsui K, Osawa S, Uji Y (2000) Macular buckling for retinal detachment due to macular hole in highly myopic eyes with posterior staphyloma. Retina 20:445–449
Ripandelli G, Coppe AM, Fedeli R, Parisi V, D’Amico DJ, Stirpe M (2001) Evaluation of primary surgical procedures for retinal detachment with macular hole in highly myopic eyes: a comparison [corrected] of vitrectomy versus posterior episcleral buckling surgery. Ophthalmology 108:2258–2264
Devin F, Tsui I, Morin B, Duprat JP, Hubschman JP (2011) T-shaped scleral buckle for macular detachments in high myopes. Retina 31:177–180
Author information
Authors and Affiliations
Corresponding author
Additional information
No author has any proprietary or financial interest in any product mentioned
Rights and permissions
About this article
Cite this article
Mitry, D., Zambarakji, H. Recent trends in the management of maculopathy secondary to pathological myopia. Graefes Arch Clin Exp Ophthalmol 250, 3–13 (2012). https://doi.org/10.1007/s00417-011-1889-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1889-0