Abstract
Background
Therapy of S. aureus keratitis is increasingly challenging due to emerging resistant strains. Staphylolysin (LasA protease) is a staphylolytic endopeptidase secreted by Pseudomonas aeruginosa. The purpose of the current study was to study the effect of treatment with staphylolysin on experimental keratitis caused by various Staphylococcus aureus strains.
Methods
The therapeutic effect was studied in a keratitis model induced in rabbits by intrastromal injections of 103 S. aureus cells of three different methicillin-resistant S. aureus (MRSA) strains and one methicillin-susceptible S. aureus strain (MSSA). Topical treatment with either staphylolysin or bovine serum albumin (BSA; control) was applied every half hour for 5 h, starting at 4 h after infection. Corneas were removed for bacterial quantification. Histopathological analysis was performed on MSSA-infected rabbits, killed at either one or 84 h after completion of treatment and on uninfected eyes 1 h after treatment termination.
Results
The number of bacteria in the staphylolysin-treated corneas was significantly reduced in all infections with the four S. aureus strains studied as compared to controls: the staphylolysin-treated eyes infected with MRSA strains were either completely sterilized or showed a 3–4 orders of magnitude decrease in the number of cfu/cornea (p = 0.004 to 0.005); all of the staphylolysin-treated MSSA-infected eyes were sterile. Histopathological analysis of the methicillin-sensitive (MSSA) strain-infected eyes at 84 h after completion of treatment showed moderate inflammation in the staphylolysin-treated eyes as compared with extensive abscess formation in the control group. The uninfected corneas showed only mild stromal edema in both the staphylolysin and BSA-treated groups.
Conclusions
Staphylolysin provided long-lasting protection against several strains of S. aureus, evident by both its strong anti-bacterial activity and beneficial histopathological results of treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus keratitis is among the commonest sight-threatening ocular infections either in normal or in compromised corneas [1]. Therefore, timely antimicrobial treatment must be initiated for rapid eradication of the infecting organism. Numerous reports from all over the world indicate a methicillin-resistance rate of up to 74% among S. aureus isolates, which emphasizes the necessity to search for new anti-S. aureus agents as an alternative to currently available antibiotics [2–6]. Enzyme-based therapy has been proposed as an alternative approach to treatment with antibiotics. Lysostaphin, a staphylolytic endopeptidase from Staphylococcus simulans, is a glycylglycine endopeptidase capable of cleaving the cross-linking pentaglycine bridges in the cell walls of staphylococci, and is the first enzyme reported to successfully treat S. aureus experimental keratitis [7]. Staphylolysin (LasA protease) is a 20-kDa staphylolytic endopeptidase secreted by Pseudomonas aeruginosa that similarly to lysostaphin can cleave the pentaglycine bridges of the cell wall peptidoglycan of S. aureus, leading to cell lysis and inhibition of bacterial growth [8–11]. Staphylolysin can cause lysis of a wide range of S. aureus strains and other types of Staphylococci, including S. saprophyticus, S. epidermidis, and S. warneri [12]. We have previously reported that staphylolysin is effective in the treatment of both methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) experimental keratitis [13]. It completely sterilized the infected corneas when treatment was initiated 4 h after infection and reduced the number of viable bacteria (cfu) in both MSSA and MRSA-infected corneas by 3–4 orders of magnitude as compared to controls when treatment was initiated 10 h post-infection. Furthermore, staphylolysin was found to be even more effective than vancomycin in eradicating MRSA cells. In another study, we demonstrated that staphylolysin is also effective in the treatment of experimental endophthalmitis in rats caused by another MRSA strain [14].
The purpose of the current study was to further expand the information regarding the therapeutic potential of staphylolysin in experimental keratitis, focusing on its range of activity against a number of MRSA strains and examination of possible side-effects. Towards this end, we evaluated the protective effect of staphylolysin treatment against three clinical MRSA isolates as compared to the MSSA strain we studied before [13] with respect to both bacterial eradication and reduction of corneal damage as revealed by histopathological analysis. The latter analysis was also used to examine corneal toxicity of staphylolysin in un-infected corneas.
Materials and methods
Animals
New Zealand white rabbits (weight, 2.5 to 3.0 kg) were handled in accordance with the tenets of the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research, and the research was approved by the Tel Aviv University Institutional animal care and use committee. Before induction of infection and clinical assessments, all rabbits were anesthetized by intra-muscular injection of Xylazine (3 mg/kg of body weight; Arendonk, Belgium) and Ketamine hydrochloride (35 mg/kg of body weight; Fort Dodge, IA, USA). At the end of the experiment, prior to enucleation of the eyes, the animals were anesthetized and euthanized by intra-venous injection of excess pentobarbital (60 mg/ml).
Bacteria
Three MRSA strains were used: COL [15] (a kind gift of Dr. Gordon Archer, Virginia Commonwealth University, Richmond, VA) and two clinical isolates, 02/48406, and 02/48103 (a kind gift of Prof. Nathan Keller, The Microbiology Laboratory, Sheba Medical Center, Tel Hashomer, Israel). The MSSA strain used in the study was isolated from a human cornea and was described previously [13].
All strains were propagated on Mueller-Hinton agar (Difco) plates. Fresh cultures were prepared for each experiment by inoculating bacteria onto new plates and incubating the plates at 37°C for 18 h. Several bacterial colonies were pooled and suspended in phosphate-buffered saline to a final concentration of approximately 10,000–20,000 cfu/ml.
In-vitro susceptibility
Inhibitory concentrations (ICs) were determined by the tube-broth dilution method in Mueller-Hinton broth (Becton Dickinson BBL) and a final inoculum of 105 cfu/ml as described [13] except that the broth was supplemented with 0.01% (instead of 0.1%) BSA. The IC50 was defined as the concentration of staphylolysin that caused a reduction of 50% in absorbance at 600 nm of the bacterial cell suspension after 24-h incubation at 37°C.
Treatment solutions
Staphylolysin was purified from an overproducing P. aeruginosa strain as described [16]. Prior to the experiment, the enzyme was diluted to 1 mg/ml in 0.05 M Hepes 0.15 M NaCl, pH 7.8. For the control, bovine serum albumin (BSA) diluted to 1 mg/ml in the same buffer was used. Both the staphylolysin and BSA solutions were kept at 4°C during the treatment period.
Keratitis study
All of the procedures were performed utilizing a stereoscopic surgical microscope (Wild M690, Wild Heerbrugg, Switzerland). Prior to the induction of keratitis, the central corneal epithelium was marked with a trephine (7.5 mm diameter, 50 µm depth) and then was scraped with a Beever knife in order to avoid permeability issues, thereby ensuring free diffusion of the topically applied protein into the stroma. Then, 100 μl of a freshly prepared S. aureus cell suspension containing 1,000–2,000 viable organisms (cfu) was injected intrastromally at the central part of the cornea with a 1-ml tuberculin syringe and a 30-gauge needle. The same investigator preformed all of the injections.
Each MRSA strain was studied separately. Each experiment included 12 rabbits (n = 6 in both the staphylolysin treatment group and the BSA-treated control group). The MSSA keratitis experiment included 20 rabbits (n = 10 in each, the staphylolysin treatment group and the BSA-treated control group).
Animals were randomly divided into two groups: staphylolysin treatment group and BSA treatment group (control). Topical treatment with either a single drop of staphylolysin (45 μl containing 45 μg of LasA protease) or a drop of BSA as appropriate was applied at each time point, starting 4 h after the induction of infection (to allow development of keratitis) and given every 30 min for 5 h (a total of 11 applications).
One hour after the last drop application, all rabbits with the MRSA-infected eyes and 12 of the MSSA-infected rabbits were killed and their corneas excised for bacterial quantification. The remaining MSSA-infected rabbits were divided into two groups. Four of these rabbits were killed 1 h after the last drop administration and the remaining four rabbits were killed 84 h after administration of the last drop. The eyes were enucleated after killing and submitted for histopathological evaluation.
The observer was not masked due to the need to know which treatment each rabbit should receive, and the need to plan our dilutions appropriately for bacterial counting.
Determination of the number of viable bacteria
For bacterial counting, uniform corneal buttons were removed aseptically from the center of the infected cornea using a 7.5-mm corneal trephine. The corneal buttons were rinsed and homogenized in sterile phosphate-buffered saline (3 ml/cornea) using a Polytron homogenizer. Aliquots of the corneal homogenates were serially diluted in the same buffer, plated in triplicates onto Muller-Hinton agar plates (100 μl per plate) and incubated for 24 h at 37°C to determine the number of colony forming units (cfu) per cornea. The reported cfu numbers (Table 2) were derived from dilutions that yielded 20 to 100 colonies per plate. Homogenates of corneas that produced only a few or no colonies were plated undiluted. The lower limit of detection was one colony per plate, i.e., 30 cfu per cornea.
Toxicity study
The possible toxicity of staphylolysin was evaluated in eight eyes from eight rabbits. Corneal erosion was created in each rabbit as described above in order to maintain the same conditions, but no keratitis was induced. Eyes were treated with either staphylolysin (n = 4) or BSA (n = 4) using the same regimen as in the keratitis study, namely, treatment was initiated 4 h after scraping of the corneal epithelium, and was given every 30 min for 5 h (a total of 11 treatments). One hour after administration of the last drop, all of the rabbits were euthanized and their eyes were enucleated and submitted to histopathological examination.
Histopathological evaluation
The enucleated eyes were fixed in 10% buffered formalin and embedded in paraffin blocks. Specimens included (i) four infected eyes treated with staphylolysin and four infected control eyes (treated with BSA) enucleated 1 h after completion of treatment; (ii) four staphylolysin-treated infected eyes and four infected control eyes (treated with BSA), enucleated 84 h after completion of treatment; (iii) four un-infected staphylolysin-treated eyes and four un-infected control eyes treated with BSA, enucleated 1 h after completion of treatment.
Tissue sections of 4 µm thick of all of these eyes were stained by hematoxylin and eosin (H&E) and examined for corneal erosion, edema, infiltrate, and the presence of bacteria by a masked observer using a light microscope.
Statistical analysis
The individual cfu values were tested for statistical significance by a nonparametric analysis using the Wilcoxon rank-sum test. p values of 0.05 or smaller were considered significant.
Results
Inhibitory concentrations for the various S. aureus strains
The same IC50 value, 5 μg/ml, was obtained for the MSSA strain, the MRSA strain COL and the MRSA clinical isolate 02/48406. The IC50 value obtained for the second MRSA isolate, 02/48103, was 1.8 μg/ml, i.e., about 2.5 fold lower than that of all of the other strains studied (Table 1).
Bacterial number in MSSA and MRSA-infected corneas
Table 2 presents the cfu values obtained for the staphylolysin-treated and control corneas infected with the various S. aureus strains. Staphylolysin completely sterilized all of the six corneas infected with the MSSA strain, whereas the control corneas in this experiment (#1) contained 1.8 × 106 (SD 3.77 × 105) cfu/cornea (p = 0.003). The robust protective effect of staphylolysin was durable, lasting at least 84 h after cessation of treatment (Fig. 2a). Staphylolysin also completely sterilized all of the six corneas infected with strain COL, whereas all of the control corneas (five corneas because one of the control rabbits died before the end of the experiment) contained 4.03 × 105 (SD 4.0 × 105) cfu/cornea (p = 0.004; Table 2, experiment #2). In experiment #3 (corneas infected with the MRSA isolate 02/48406), staphylolysin sterilized five of the six treated corneas, and the sixth staphylolysin-treated cornea contained only 210 cfu/cornea. This is in contrast to the control eyes in which the average cfu value/cornea was 1.43 × 106 (SD 1.38 × 106) (p = 0.004) (Table 2). Staphylolysin reduced the number of bacteria in all of the corneas infected with MRSA isolate 02/48103 (experiment #4) to a mean of 5.0 × 102 cfu/cornea (SD 8.0x102; Median 6.5 × 101) as compared to 4.04 × 106 cfu/cornea (SD 3.6 × 106; Median 4.0 × 106) found in the control group (p = 0.005). However, despite the low IC50 value of this latter strain for staphylolysin (1.8 vs. 5 μg/ml; Table 1), only two of the six staphylolysin-treated corneas were completely sterilized. This apparent discrepancy could result from the relatively high inoculum (2,000 cfu) injected in this particular experiment. Also, consistent with the relatively high cfu value found for the control group in this experiment (mean 4 × 106 as compared to values of 0.4–1.8 × 106 obtained for the other strains; Table 2), perhaps the injected bacteria were at such physiological condition that permitted relatively high intracorneal proliferation rate. Regardless, together, the data clearly reinforce our previous results [13] in a study comprising only one MSSA and one MRSA S. aureus strain, strongly supporting the broad spectrum of staphylolysin action against a variety of MSSA and MRSA clinical isolates.
Histopathological examination
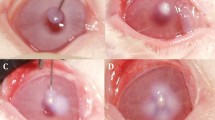
The histopathological analysis of the infected eyes at 1 h after completion of treatment revealed central erosion and stromal edema in all of the corneas regardless of the treatment given. Among the infected corneas that were treated with staphylolysin, three out of four corneas showed no detectable bacteria. The fourth cornea contained a small number of bacteria with no detectable bacterial colonies (Fig. 1a). However, in the control group treated with BSA, bacterial colonies were seen in all of the 4 corneas studied (Fig. 1 b and c).
Histopathology of MSSA-infected corneas 1 h after completion of treatment i.e., at 10 h post-infection. a Staphylolysin-treated eye. The epithelium was denuded, the stroma was edematous and no bacterial colonies were detected. The endothelium was intact. b Control eye treated with BSA. The epithelium was denuded, the stroma was edematous and bacterial colonies were observed (arrows). c Higher magnification of the bacterial colonies seen in b. The sections shown are representative. A similar pattern was observed in the other three specimens evaluated in each of the groups (hematoxylin and eosin; Original magnification of a and b: x100; Original magnification of c: × 400)
All of the infected corneas that were evaluated 84 h following completion of treatment also demonstrated central erosion and stromal edema. In the staphylolysin-treated eyes, dispersed polymorphonuclear leukocytes were seen in the stroma, with intact Descemet's membrane and endothelium (Fig. 2a). However, in the respective control group that was treated with BSA and enucleated 84 h after termination of the treatment, the corneal structure was severely disrupted due to extensive abscess formation. Descemet's membrane remained intact but no endothelium was seen (Fig. 2b).
Histopathological evaluation of MSSA-infected corneas 84 h after completion of treatment. a Staphylolysin-treated eye. The epithelium was denuded and there was stromal edema and infiltration by polymorphonuclear leukocytes, but the stromal lamellae were preserved and the endothelium was intact. b Control eye treated with BSA. The structure of the cornea showed severe destruction due to extensive inflammatory infiltration. Inflammatory infiltrate was also present at the inner corneal surface, and no endothelium was present (hematoxylin and eosin; original magnification: × 100)
Histopathological examination of the staphylolysin-treated un-infected eyes showed central erosion (due to epithelial scraping executed before initiation of the treatment) and only mild stromal edema in all eyes. No inflammatory infiltrate was found, and the Descemet's membrane and the endothelium were intact in all of the specimens, similar to the control group treated with BSA (not shown).
Discussion
The results of the current study indicate that treatment with staphylolysin (also known as LasA protease) from P. aeruginosa can dramatically reduce the number of viable bacteria in S. aureus-infected corneas. This was observed with all of the three MRSA strains studied as well as the MSSA isolate. The number of viable bacteria per cornea in the staphylolysin-treated group was either below the detection level or significantly reduced as compared to the control corneas that contained high numbers of viable bacteria (Table 2). These results are comparable to those obtained in our previous and more limited study [13] in which treatment was also initiated 4 h post-infection, however, it was given for a total of 10 h instead of 5 h as was done here. Thus, the results of the current study both support and expand those of our earlier investigation [13], showing that despite the reduced duration of treatment, full eradication of the MSSA strain and of practically all of the MRSA strains was achieved, demonstrating the strong and immediate effect of staphylolysin.
Dajcs et al. [7] reported similar results for lysostaphin treatment of MRSA-induced keratitis in rabbits. Our current treatment regimen was similar to that used by Dajcs and his colleagues, and the rate of eradication of bacteria achieved in the current study was also comparable to that reported by Dajcs et al. [7]; full sterilization by lysostaphin of the MRSA-infected corneas whereas the untreated control corneas remained heavily infected.
The IC50 values of three of the strains we studied were identical (5 μg/ml). Only one strain was more sensitive to staphylolysin, with an IC50 of 1.8 μg/ml. However, the results of the staphylolysin treatment for all of the four strains studied were practically the same, once again manifesting the strong anti-bacterial power of staphylolysin.
Our histopathological examination also revealed a marked reduction in the level of bacterial infection and inflammation in the staphylolysin-treated corneas as compared to the controls. The beneficial results seen at 84 h after termination of staphylolysin-treatment provided important new information, indicating the long-term effect of staphylolysin treatment. The stromal lamellae and the endothelial layer were both intact in all of the staphylolysin-treated corneas, while in the corneas of the control group, extensive disruption of the stromal structure and complete loss of the endothelium was seen at this point of time (Fig. 2). The rapid eradication of the infecting bacteria by staphylolysin apparently prevented further damage to the inner layers of the cornea in the treatment group. The epithelial erosion performed before the induction of keratitis persisted in all of the animals until killed and was evident even at 84 h after completion of treatment. The un-infected corneas that were examined at 1 h after cessation of treatment had a similar appearance with only mild stromal edema in both the staphylolysin and BSA-treated groups, suggesting that staphylolysin treatment has no (short-term) toxic effect and does not cause a severe structural damage to the cornea.
The possibility that P. aeruginosa endopeptidases can cause corneal damage has been discussed by several investigators [17–19]. There is disagreement, however, concerning the role of staphylolysin in Pseudomonas corneal infection. Cowell et al. [18] found that staphylolysin contributes to Pseudomonas invasion of rabbit corneal epithelial cells. White et al. [20] reported that a staphylolysin-deficient mutant of P. aeruginosa showed significantly reduced virulence in the rabbit eye and was not virulent in mice eyes, although genetic complementation with staphylolysin did not restore virulence in either model of infection. Hobden [19] claimed that alkaline protease, Pseudomonas elastase (LasB) and staphylolysin are not essential for either initiating or maintaining corneal infection. Using a mice model, Preston et al. [21] investigated the damage cause to scarified corneas by topical application of purified staphylolysin. A low dose of 0.5 μg of staphylolysin did not cause ocular damage, while a high dose of 5 μg of staphylolysin produced a toxic reaction leading to eye damage without eliciting cellular inflammation. In the present study, a dose of 45 μg of staphylolysin applied to each eye produced no clinical or histopathological damage. The larger area of the rabbit cornea, as compared to that of the mouse, as well as the difference in sensitivity to staphylolysin between corneas of the two species can partially explain these different results. The minimal toxicity of staphylolysin to scraped corneas found in our study indicates the relative safety of the treatment.
In conclusion, the results of the present study indicate that staphylolysin may serve as a new potential therapeutic tool, eradicating a number of different MRSA strains in a keratitis model in rabbits, with little or no toxic side-effects. These findings give a boost to our previous results [13], demonstrating the strong anti-bacterial activity of staphylolysin in vivo and bringing to light its potency even in a relatively short-term therapeutic regimen. The beneficial effect is attributed to the high capability of staphylolysin as a bacteriolytic enzyme and its immediate action on the bacteria.
References
Alexandrakis GEC, Alfonso E, Miller D (2000) Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology 107:1497–1502
Yilmaz S, Ozturk I, Maden A (2007) Microbial keratitis in West Anatolia, Turkey: a retrospective review. Int Ophthalmol 27:261–268
Solomon R, Donnenfeld ED, Perry HD, Rubinfeld RS, Ehrenhaus M, Wittpenn JR Jr, Solomon KD, Manche EE, Moshirfar M, Matzkin DC, Mozayeni RM, Maloney RK (2007) Methicillin-resistant Staphylococcus aureus infectious keratitis following refractive surgery. Am J Ophthalmol 143:629–634
Bell JM, Turnidge JD (2002) High prevalence of oxacillin-resistant Staphylococcus aureus isolates from hospitalized patients in Asia-Pacific and South Africa: results from SENTRY antimicrobial surveillance program 1998–1999. Antimicrob Agents Chemother 46:879–881
Sharma V, Sharma S, Garg P, Rao GN (2004) Clinical resistance of Staphylococcus keratitis to ciprofloxacin monotherapy. Indian J Ophthalmol 52:287–292
Goldstein MH, Kowalski RP, Gordon YJ (1999) Emerging fluoroquinolone resistance in bacterial keratitis: a 5-year review. Ophthalmology 106:1313–1318
Dajcs JJ, Hume EB, Moreau JM, Caballero AR, Cannon BM, O'Callaghan RJ (2000) Lysostaphin treatment of methicillin-resistant Staphylococcus aureus keratitis in the rabbit. Invest Ophthalmol Vis Sci 41:1432–1479
Kessler E, Ohman DE (2004) Staphylolysin. In: Barrett AJ, Rawlings ND, Woessner JF (eds) Handbook of proteolytic enzymes, 2nd edn. Academic Press, London, United Kingdom, pp 1001–1003
Mansito TB, Falcon MA, Moreno J, Carnicero A, Gutierrez-Navarro AM (1987) Effects of staphylolytic enzymes from Pseudomonas aeruginosa on the growth and ultrastructure of Staphylococcus aureus. Microbios 49:55–64
Perestelo FR, Blanco MT, Gutierre-Navarro AM, Falcon MA (1985) Growth inhibition of Staphylococcus aureus by a staphylolytic enzyme from Pseudomonas aeruginosa. Microbios Lett 30:85–94
Grande KK, Gustin JK, Kessler E, Ohman DE (2007) Identification of critical residues in the propeptide of LasA protease of Pseudomonas aeruginosa involved in the formation of a stable mature protease. J Bacteriol 189:3960–3968
Brito N, Falcón MA, Carnicero A, Gutiérrez-Navarro AM, Mansito TB (1989) Purification and peptidase activity of a bacteriolytic extracellular enzyme from Pseudomonas aeruginosa. Res Microbiol 140:125–137
Barequet I, Ben Simon GJ, Safrin M, Ohman DE, Kessler E (2004) Pseudomonas aeruginosa LasA protease in treatment of experimental Staphylococcal keratitis. Antimicrob Agents Chemother 48:1681–1687
Barequet IS, Habot-Wilner Z, Mann O, Safrin M, Ohman DE, Kessler E, Rosner M (2009) Evaluation of Pseudomonas aeruginosa staphylolysin (LasA protease) in the treatment of methicillin-resistant Staphylococcus aureus endophthalmitis in a rat model. Graefes Arch Clin Exp Ophthalmol 247:913–917
Gill SR, Fouts DE, Archer GL, Mongodin EF, DeBoy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM (2005) Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol 187:2426–2438
Kessler E, Safrin M, Gustin JK, Ohman DE (1998) Elastase and the LasA of Pseudomonas aeruginosa are secreted with their peptides. J Biol Chem 273:30225–30231
Kessler E, Safrin M, Olson JC, Ohman DE (1993) Secreted LasA protease of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem 268:7503–7508
Cowell BA, Twining SS, Hobden JA, Kwong MSF, Fleiszig SMJ (2003) Mutation of LasA and LasB reduces Pseudomonas aeruginosa invasion of epithelial cells. Microbiology 49:2291–2299
Hobden JA (2002) Pseudomonas aeruginosa proteases and corneal virulence. DNA Cell Biol 21:391–396
White CD, Alionte LG, Cannon BM, Caballero AR, O'Callaghan RJ, Hobden J (2001) Corneal virulence of LasA protease-deficient Pseudomonas aeruginosa PAO1. Cornea 20:643–646
Preston MJ, Seed PC, Toder DS, Iglewski BH, Ohman DE, Gustin JK, Goldberg JB, Pier GB (1997) Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect Immun 65:3086–3090
Acknowledgments
This study was supported by the Claire and Amedee Maratier Institute of the Tel-Aviv University Sackler Faculty of Medicine (to I.S.B., M.R., and E.K), the Tel-Aviv University Foundation for Basic Research (to E.K.), Public Health Service grant AI 26187 from the National Institute of Allergy and Infectious Diseases (to D.E.O.), and in part, by Veterans Administration Medical Research Grant I01 BX000477 (to D.E.O.). The authors have full control of all primary data and agree to allow Graefe's Archive for Clinical and Experimental Ophthalmology to review the data upon request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barequet, I.S., Bourla, N., Pessach, Y.N. et al. Staphylolysin is an effective therapeutic agent for Staphylococcus aureus experimental keratitis. Graefes Arch Clin Exp Ophthalmol 250, 223–229 (2012). https://doi.org/10.1007/s00417-011-1822-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1822-6