Abstract
Background
This study was conducted to evaluate whether polyunsaturated fatty acids (PUFA) such as γ-linolenic acid (GLA) and eicosapentaenoic acid (EPA), as found in the diet, may affect the lipid composition of conjunctival epithelium and whether these modifications affect prostaglandin (PG) production after inflammatory stimulation.
Methods
Chang and IOBA-NHC conjunctival human cells were treated with GLA and/or EPA at 5, 10, 20, 30, 40, or 50 μg/ml for 72 h and then were stimulated with interferon-gamma (IFN-γ) for 48 h. Changes in the composition of neutral lipids and phospholipids were monitored by gas chromatography. PGE1 and PGE2 levels were measured by enzyme immunoassay.
Results
PUFA supplementations in the culture medium induced incorporation of these fatty acids and of their metabolites in neutral lipids and phospholipids of the conjunctival cells. The fatty acid composition of neutral lipids and phospholipids was not affected by stimulation with IFN-γ. The production of PGE1 and PGE2 was affected by GLA supplementation whereas it was not modified by EPA supplementation. A combined supplementation of EPA and GLA did not change the production of PGE1 but decreased the production of PGE2.
Conclusions
These results suggest that modulation of fatty acid composition and PG production by PUFA supplementation is possible in the conjunctival epithelium, which is an important site of inflammation in dry eye syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
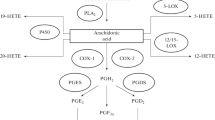
Dietary polyunsaturated fatty acids (PUFA) are of great interest since they can modulate the composition of the cell membrane due to their incorporation into a variety of tissues including the lacrimal gland, the retina, and the brain [1–4]. One benefit of increased dietary n-3 PUFA is that their incorporation into plasma and cell lipids occurs frequently at the expense of arachidonic acid (AA). By inhibiting the delta-5 desaturase, eicosapentaenoic acid (EPA) decreases the conversion of dihomo gamma-linolenic acid (dGLA) to AA [3, 5, 6]. Limiting the AA level is beneficial because AA is the precursor of prostaglandin E2 (PGE2), which has potent pro-inflammatory properties whereas EPA is the precursor of PGE3 known to be less pro-inflammatory than PGE2 [7]. Another strategy aimed at modulating PG production is the supply of gamma-linolenic acid (GLA). Increased dietary intake of GLA results in the accumulation of its elongation product, dGLA, which is the precursor of PGE1, and is less pro-inflammatory than PGE2 [7]. EPA and dGLA also compete with AA at the level of substrate for the cyclo-oxygenase (COX)-dependent production of PG [8]. Hence, PG synthesis is affected by altering the fatty acid composition of the cell membrane and can be modulated by manipulation of PUFA intake. This was already shown in several tissues including the retina [9] and lacrimal glands [4], but also in tears [11]. A detailed diagram of the metabolic pathway of the PUFA from n-6 and n-3 series is shown in Fig. 1.
The benefits of PUFA from the n-6 and n-3 series were documented in inflammatory diseases such as dry eye syndrome, which results in the inflammation of the components of the lacrimal functional unit [10]. Interventional studies have suggested the potential benefit of n-6 or n-3 PUFA alone or a combination of both on the clinical signs of ocular dryness [11–15]. The observational women's health study has shown that a high ratio of dietary intake of n-3 PUFA to n-6 PUFA may be associated with a high prevalence of dry eye syndrome, pointing out the importance of the dietary balance between PUFA [16]. The beneficial effect of GLA has also been shown in other chronic inflammatory conditions such as rheumatoid arthritis [17]. There is limited information concerning the accumulation of dietary PUFA in the lacrimal functional unit. Data in lacrimal glands showed that dietary supplementation in GLA and/or EPA induced an increase in n-6 and/or n-3 PUFA [3, 4]. However, no data is available concerning the accumulation of dietary PUFA in the conjunctiva even if this tissue has the ability to synthesize PGE2 and PGE3 [18]. Hence, the aim of the present study was to investigate whether dietary PUFA such as GLA and EPA may affect the composition of conjunctival epithelium and whether these modifications impact PGE1 and PGE2 levels. This was investigated on cultured conjunctival cells by assessing the effect of GLA and/or EPA supplementation on the fatty acid composition of neutral lipids and phospholipids as well as on the PG production in response to stimulation with interferon-gamma (IFN-γ). We have compared the results from two cell lines: the Wong-Kilbourne derivative of Chang conjunctival cell line, which has been widely used, and the IOBA-NHC cell line, to lessen the impact of the presence of HeLa marker chromosomes in the Chang cells. This in vitro study also allowed the possibility to evaluate the effect of EPA alone given that natural dietary EPA is always accompanied by DHA.
Materials and methods
Cells
Two conjunctival cell lines of human origin were cultured in standard conditions (5% CO2, 37°C, humidity saturated environment). Chang cells (Wong-Kilbourne derivative of Chang conjunctiva, clone 1-5c-4, ATCC CCL-7) were cultured in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) with a high glucose concentration (4.5 g/L) (GibcoTM, Invitrogen, Bioblock, Illkirch, France) supplemented with 10% fetal calf serum (FCS) (Eurobio, Courtaboeuf, France), 25 mM HEPES (GibcoTM), 20 μg/ml gentamicin (GibcoTM). A second human conjunctival cell line spontaneously arising from a primary culture of human conjunctival epithelium (IOBA-NHC) was cultured in DMEM/F-12 (1:1) (GibcoTM) supplemented with 2 ng/ml epidermal growth factor (EGF) (AbCys, Paris, France), 1 μg/ml bovine pancreas insulin (Sigma-Aldrich, Saint Quentin Fallavier, France), 0.1 μg/ml cholera toxin (Sigma-Aldrich), 0.5 μg/ml hydrocortisone (Sigma-Aldrich), 10% FCS, and 20 μg/ml gentamicin. Cells from passages 9 and 10 (following ATCC initial passage 65 or following the provision of IOBA-NHC cells) were used in all experiments. The medium was changed every 2–3 days, and cell growth was assessed daily by phase-contrast microscopy. Cells were seeded at a density of 105 cells/mm².
Cell treatment
Cells were supplemented with 5–50 μg/ml of EPA and/or GLA (Cayman Chemical, Spi-Bio, Montigny le Bretonneux, France) prepared in ethanol [19]. Non-supplemented cells received the same quantity of ethanol (0.4% of total medium volume). Cells were then incubated without hydrocortisone. After 72 h, the cells were stimulated with 300u/ml IFN-γ (Sigma-Aldrich) prepared in phosphate buffer solution (PBS). Non-stimulated cells received PBS alone. After 48-h stimulation, supernatants were collected and 10% protease inhibitor cocktail (Sigma-Aldrich) was added. Samples were stored at −80°C until prostaglandin measurement. Cells were collected, rinsed, immersed in chloroform/methanol (2:1, by vol) and stored at −20°C until lipid analyses.
Measurement of prostaglandin levels in the supernatant
PGE1 and PGE2 were measured using an enzyme immunoassay kit (Assay Designs, Euromedex, Mundolsheim, France and Cayman Chemical, respectively) according to the manufacturers’ instructions. Supernatants were diluted with the kit buffer to 1/50th and 1/500th for PGE1 and PGE2 measurements, respectively. PGE1 and PGE2 levels in the supernatants were expressed in ng/ml.
Lipid analyses
Lipids from cells were extracted according to the method developed by Folch et al. [20]. A total of 1 mg of total lipids was separated into neutral lipids and phospholipids using Sep-Pak silica cartridges (Waters, Guyancourt, France) according to the technique developed by Juaneda and Rocquelin [21]. Neutral lipids and phospholipids were submitted to fatty acid methylation according to Morrison and Smith (1964) as previously described [22]. Fatty acid methyl esters (FAME) were analyzed using gas chromatography on a Hewlett Packard Model 5890 gas chromatograph (Palo Alto, CA, USA) using a CPSIL-88 column (100 m × 0.25 mm internal diameter; film thickness, 0.20 μm; Varian, Les Ulis, France). Hydrogen was used as a carrier gas (inlet pressure, 210 kPa). The oven temperature was held at 60°C for 5 min, increased to 165°C at 15°C/min and held for 1 min, and then to 225°C at 2°C/min and finally held at 225°C for 17 min. The injector and the detector were maintained at 250°C. FAME were identified by comparison with commercial and synthetic standards. The data were computed using the Galaxie software (Varian). The proportion of each fatty acid was expressed as a percentage of total fatty acids.
Statistical analyses
Data were expressed as mean ± standard deviation (SD). The Student–Newman–Keuls test was used to compare the data from the different groups using the SAS software (SAS Institute, Cary, NC, USA). Linear regression correlation coefficients were calculated to determine the relationship between GLA or EPA concentrations and cell fatty acids (Figs. 2 and 3). p values less than 0.05 were considered significant and the tests were two-tailed.
Effect of GLA and/or EPA supplementations on the PUFA precursors of PG in neutral lipids of conjunctival cells stimulated with IFN-γ (300 u/ml) for 48 h. Fatty acid incorporation was defined by a linear regression (ax + b) as a function of the concentration of GLA or EPA added in the culture media. a dGLA, b AA, c EPA
Effect of GLA and/or EPA supplementations on the PUFA precursors of PG in the phospholipids of conjunctival cells stimulated with IFN-γ (300u/ml) for 48 h. Fatty acid incorporation was defined by a linear regression (ax + b) as a function of the concentration of GLA or EPA added in the culture media. a dGLA, b AA, c EPA
Results
Effect of PUFA supplementation on the fatty acid composition of the neutral lipids
Fatty acid composition of the neutral lipids of the conjunctival cells. Effect of IFN-γ
The fatty acid composition of the neutral lipids of the two conjunctival cell lines is shown in Table 1. Without supplementation, neutral lipids of IOBA-NHC cells contained significantly more C16:0 and C18:0 when compared to the neutral lipids of Chang cells (19.1 versus 13.4%, p < 0.0001, and 3.4 versus 2.0%, p < 0.0001, respectively). They contained significantly less C18:1 n-7 (27.5 versus 31.0%, p < 0.0005), less n-6 PUFA (1.4 versus 2.7%, p < 0.0001) and less n-3 PUFA (1.6 versus 3.9%, p < 0.0001). These compositions were not modified by the stimulation with IFN-γ.
Effect of GLA supplementation on the neutral lipid fatty acid composition of the conjunctival stimulated cells
A supplementation with GLA strongly modified the fatty acid composition of the neutral lipids in conjunctival cells stimulated with IFN-γ. The figs. 2a and b show the incorporation of dGLA and AA in the neutral lipids as a function of the GLA concentration. GLA supplementation induced an increase in dGLA level (from 25 μg/ml in Chang cells, p < 0.05 and from 10 μg/ml in IOBA-NHC cells, p < 0.05) and in AA level (from 10 μg/ml in Chang cells, p < 0.0001 and from 5 μg/ml in IOBA-NHC cells, p < 0.0001). Other n-6 PUFA levels were increased: GLA (from 5 μg/ml in Chang and IOBA-NHC cells, p < 0.01 and p < 0.001, respectively) and C22:4 n-6 (from 10 μg/ml in Chang cells, p < 0.0001 and 5 μg/ml in IOBA-NHC cells, p < 0.0001). It is noteworthy that GLA supplementation induced the formation and the incorporation of atypical n-6 PUFA: C16:3 n-6 from 25 μg/ml (0.1% in Chang cells, p < 0.01 and 0.2% in IOBA-NHC cells, p < 0.0001); C22:3 n-6 from 10 μg/ml (0.2% in both cell lines, p < 0.05); C24:4 n-6 from 10 μg/ml in Chang cells (3.3%, p < 0.0001) and from 5 μg/ml in IOBA-NHC cells (0.2%, p < 0.001). The contents of these fatty acids increased with the GLA concentration.
Effect of EPA supplementation on the neutral lipid fatty acid composition of the conjunctival stimulated cells
A supplementation with EPA increased the incorporation of EPA (significantly from 10 μg/ml in Chang cells, p < 0.0001 and from 5 μg/ml in IOBA-NHC cells, p < 0.05) (Fig. 2c), and C22:5 n-3 (from 5 μg/ml in Chang and IOBA-NHC cells, p < 0.01 and p < 0.0001, respectively). In Chang cells, DHA content was significantly increased with EPA supplementation at 10 μg/ml (2.8%, p < 0.0001) and 50 μg/ml (1.2%, p < 0.05) whereas it was increased for all concentrations in IOBA-NHC cells with a maximum with 25 μg/ml of EPA (1.3%, p < 0.0001). EPA supplementation also induced the formation and the incorporation of atypical fatty acids: C16:4 n-3 and C18:4 n-3 only with 50 μg/ml of EPA (0.2% in Chang cells, p < 0.0001 and 0.3% in IOBA-NHC cells, p < 0.0001); C20:4 n-3 from 10 μg/ml in Chang cells (0.2%, p < 0.0001) and 25 μg/ml in IOBA-NHC cells (0.5%, p < 0.0001); C24:5 n-3 from 10 μg/ml in Chang cells (5.9%, p < 0.0001) and 5 μg/ml in IOBA-NHC cells (0.8%, p < 0.0005). The contents of C20:4 n-3 and C24:5 n-3 were improved with increasing EPA concentration.
Effect of GLA + EPA supplementation on the neutral lipid fatty acid composition of the conjunctival stimulated cells
A combined supplementation of GLA and EPA induced an increase in dGLA level (significantly from 10 μg/ml of both fatty acids, p < 0.05 and p < 0.001 in Chang and IOBA-NHC cells, respectively), AA (significantly from 5 μg/ml of both fatty acids, p < 0.0005 and p < 0.0001 in Chang and IOBA-NHC cells, respectively) and EPA (significantly from 5 μg/ml of both fatty acids, p < 0.05 and p < 0.005 in Chang and IOBA-NHC cells, respectively) (Fig. 2a, b and c). Other n-6 and n-3 PUFA levels were increased: GLA (from 10 μg/ml of both fatty acids, p < 0.005 and p < 0.001 in Chang and IOBA-NHC cells, respectively), C22:4 n-6 (from 5 μg/ml of both fatty acids, p < 0.005 and p < 0.0005 in Chang and IOBA-NHC cells, respectively) and C22:5 n-3 (from 5 μg/ml of both fatty acids, p < 0.0001 in Chang and IOBA-NHC cells). DHA was also increased in Chang cells for a supplementation of GLA and EPA of 5 μg/ml (2.9%, p < 0.0001) and 10 μg/ml (2.5%, p < 0.0001) whereas in IOBA-NHC cells it was increased for all the concentrations tested with a maximum of incorporation with 10 μg/ml of both fatty acids (1.2%, p < 0.0001). The combined supplementation also induced the incorporation of the atypical fatty acids: C16:3 n-6 from 10 μg/ml (0.1% in Chang cells, p < 0.0001 and 0.2% in IOBA-NHC cells, p < 0.0001); C22:3 n-6 from 5 μg/ml in Chang cells (0.2%, p < 0.05) and from 10 μg/ml in IOBA-NHC cells (0.3%, p < 0.0005); C24:4 n-6 from 5 μg/ml (1.6% in Chang cells, p < 0.0005 and 0.2% in IOBA-NHC cells, p < 0.005); C16:4 n-3 and C18:4 n-3 from 25 μg/ml (0.1% in both cell lines); C20:4 n-3 from 5 μg/ml in Chang cells (0.1%, p < 0.05) and 10 μg/ml in IOBA-NHC cells (0.1%, p < 0.01); and C24:5 n-3 from 5 μg/ml (3.3% in Chang cells and 0.8% in IOBA-NHC cells, p < 0.0001).
Effect of PUFA supplementation on the levels of fatty acids involved in prostaglandin synthesis in phospholipids
Fatty acid composition of the phospholipids of the conjunctival cells. Effect of IFN-γ
The phospholipids of IOBA-NHC cells contained significantly more C18:0 (7.8 versus 6.1%, p < 0.0001) as compared to those in Chang cells, more C18:1 n-9 (24.7 versus 20.7%, p < 0.0001) and less C16:1 n-9 (0.8 versus 1.0%, p < 0.005), C16:1 n-7 (10.6 versus 12.4%, p < 0.05) and C18:1 n-7 (18.5 versus 21.8%, p < 0.0001) (Table 2). They contained also more dGLA (0.30 versus 0.15%, p < 0.0001), and less C22:4 n-6 (0.4 versus 0.6%, p < 0.0001) and C22:5 n-3 (0.9 versus 1.2%, p < 0.01). The only change observed with the IFN-γ stimulation was a slight increase in dGLA in IOBA-NHC cells (0.30 to 0.32%, p < 0.05).
Effect of GLA supplementation on the levels of fatty acids involved in prostaglandin synthesis in phospholipids of the conjunctival stimulated cells
A supplementation with GLA induced an increase in dGLA, the precursor of PGE1 and AA, the precursor of PGE2, in the phospholipids of Chang and IOBA-NHC cells stimulated with IFN-γ (Fig. 3a and b). dGLA was increased from 25 μg/ml in Chang cells (p < 0.0005) and from 5 μg/ml in IOBA-NHC cells (p < 0.05), AA from 5 μg/ml in Chang and IOBA-NHC cells (p < 0.0005 and p < 0.0001, respectively). The increase in dGLA was superior to the increase in AA because the Δ5 desaturase index (AA/dGLA) was significantly decreased in both cell lines from 5 μg/ml of GLA (Fig. 4a). The Δ5 desaturase index was minimal with 50 μg/ml of GLA (3.9, p < 0.0001 and 1.5, p < 0.0001 in Chang and IOBA-NHC cells, respectively). The ratio of the fatty acids precursors of “anti-inflammatory” PG (dGLA + EPA) to the fatty acid precursor of “pro-inflammatory” PG (AA) was not modified by a GLA supplementation in Chang cells and was significantly increased only with the 50 μg/ml dose in IOBA-NHC cells (p < 0.0001) (Fig. 4b).
Effect of GLA and/or EPA supplementations on the ratio between PUFA precursors of PG in the phospholipids of conjunctival cells stimulated with IFN-γ (300u/ml) for 48 h. a Δ5 desaturase index, b (dGLA + EPA)/AA ratio. ** p < 0.01, # p < 0.005, $$ p < 0.0001 as compared to non-supplemented stimulated cells
Effect of EPA supplementation on the levels of fatty acids involved in prostaglandin synthesis in phospholipids of the conjunctival stimulated cells
A supplementation in EPA induced an increase in EPA, the precursor of the PGE3 in the phospholipids of conjunctival cells (Fig. 3c). This increase was significant from 5 μg/ml of EPA (p < 0.005 in Chang cells and p < 0.0001 in IOBA-NHC cells). EPA supplementation induced a significant decrease of the Δ5 desaturase index from 10 μg/ml (p < 0.0001 in Chang cells and p < 0.01 in IOBA-NHC cells, Fig. 4a). The rise in EPA induced a strong increase in the ratio (dGLA + EPA)/AA (8.0 in Chang cells, p < 0.0001 and 8.2 in IOBA-NHC cells, p < 0.0001 for a supplementation of 50 μg/ml, Fig. 4b).
Effect of GLA + EPA supplementation on the levels of fatty acids involved in prostaglandin synthesis in phospholipids of the conjunctival stimulated cells
A combined supplementation of GLA and EPA induced an increase in the three PUFA precursors of PG, dGLA, AA, and EPA (Fig. 3a, b and c). The increase in dGLA with a supplementation of 25 μg/ml of both fatty acids was higher than that observed with a supplementation of 25 μg/ml of GLA alone, only in Chang cells (4.7 versus 1.7%, p < 0.0001). In opposition, the increase of AA was lower with a combined supplementation of 25 μg/ml of both fatty acids than that observed with 25 μg/ml of GLA alone, only in IOBA-NHC cells (8.4 versus 13.7%, p < 0.0001). The incorporation of EPA was also diminished with the combined supplementation as compared to a supplementation with EPA alone, only in IOBA-NHC cells (5.3 versus 10.7%, p < 0.0001). These modifications favored the anti-inflammatory synthesis of PG since the Δ5 desaturase index decreased and the ratio (dGLA + EPA)/AA increased (Fig. 4a and b). The Δ5 desaturase index was significantly lower with a combined supplementation of 25 μg/ml of EPA and GLA as compared to a supplementation of EPA alone (p < 0.0001). As compared to the GLA supplementation, this decrease was not significant. The ratio (dGLA + EPA)/AA was significantly higher with a combined supplementation of EPA and GLA as compared to a supplementation of GLA alone (p < 0.05 in Chang cells and p < 0.0001 in IOBA-NHC cells) but significantly lower as compared to a supplementation of EPA alone (p < 0.0001 in both cell lines).
Effect of PUFA supplementation on prostaglandin production
Prostaglandin production in the conjunctival cells. Effect of IFN-γ
The production of PGE1 and PGE2 was only slightly stimulated by IFN-γ but this increase was not significant (Table 3). It was significantly higher in IOBA-NHC cells than in Chang cells (p < 0.0001 for PGE1 and p < 0.01 for PGE2). The IOBA-NHC cells produced more PGE2 than PGE1 (×17, p < 0.05 for the control cells and × 20, p < 0.005 for the stimulated cells, respectively).
Effect of GLA and/or EPA supplementation on prostaglandin production in conjunctival stimulated cells
A supplementation with GLA induced a significant increase in the production of PGE1 (with 50 μg/ml in Chang cells, p < 0.05, and with 10 μg/ml, p < 0.0001 and 50 μg/ml, p < 0.005 in IOBA-NHC cells) (Fig. 5). As compared to the non-supplemented cells, PGE1 production was maximal with 50 μg/ml of GLA in Chang cells (×26) and with 10 μg/ml in IOBA-NHC cells (×17). PGE2 production was increased from 5 μg/ml of GLA (p < 0.05 in Chang cells and p < 0.01 in IOBA-NHC cells). As compared to the non-supplemented cells, PGE2 production was maximal with a 50 μg/ml GLA supplementation in Chang cells (×11, p < 0.0001) and with a 25 μg/ml GLA supplementation in IOBA-NHC cells (×5, p < 0.0001). The production of PGE1 and PGE2 was not significantly modified by the EPA supplementation. A combined supplementation of EPA and GLA did not change the production of PGE1 but increased the production of PGE2, only in Chang cells (with 10 μg/ml of GLA and EPA, p < 0.05 and 25 μg/ml, p < 0.01). However, these increases remained lower than those observed with a supplementation with GLA alone (with 10 μg/ml, p < 0.001 and 25 μg/ml, p < 0.0001).
Effect of GLA and/or EPA supplementations on prostaglandin production in the conjunctival cells stimulated with IFN-γ (300u/ml) for 48 h. a PGE1, b PGE2. Normalized [PGE1] or [PGE2] is defined as the ratio between the concentration of PGE1 or PGE2 in the stimulated supplemented cells as compared to the concentration of PGE1 or PGE2 in the stimulated non-supplemented cells. * p < 0.05, ** p < 0.01, # p < 0.005, ## p < 0.001, $$ p < 0.0001 as compared to non-supplemented stimulated cells
Discussion
In this study, we show that different PUFA supplementations in the culture medium induce incorporation of these fatty acids and of their metabolites in the neutral lipids and the phospholipids of the human conjunctival cells. This result demonstrates the possibility to modulate the content of the precursors of PG, dGLA, AA, and EPA in conjunctival epithelial cells. The level of DHA, which exhibits anti-inflammatory properties, was slightly modified with a supplementation in EPA, suggesting that a supplementation in DHA should be recommended to increase the level of this fatty acid. The supplementations with GLA and/or EPA induced modifications in the PUFA metabolism, leading to the formation and incorporation of atypical fatty acids in the neutral lipids. Among the PUFA conversion enzymes, the limiting steps are those that involve the Δ6 and Δ5 desaturases [23, 24]. In particular, PUFA supplementation inhibited the desaturase activity [25]. In this way, we have observed in our study an unusual accumulation of C24:4 n-6 and C24:5 n-3, that are substrates of Δ6 desaturase. We have observed that the supplementation in GLA induced the formation of C22:3 n-6 by the direct elongation of dGLA at the expense of the synthesis of AA that involves the Δ5 desaturase. The fatty acids C18:4 n-3 and C20:4 n-3 are the intermediate metabolites between α-linolenic acid (ALA) and EPA. Their accumulation and the formation of C16:4 n-3 by β-oxidation of the C18:4 n-3 may be due to the retroconversion of the EPA added to the culture medium. Indeed, the C16:4 n-3 and C18:4 n-3 were formed from successive β-oxidation of EPA [26]. This study highlighted the formation of C16:3 n-6 by successive β-oxidation of AA added in the culture medium. In our study, C16:3 n-6 may be formed directly by β-oxidation of the GLA added.
Our results emphasize the fact that a GLA supplementation affects the production of PGE1 and PGE2 in the conjunctival epithelial cells. Hence, the conjunctiva should be sensitive to PUFA supplementation, further modulating the production of the inflammatory mediators such as PG.
The combination of n-6 and n-3 PUFA favored the incorporation of fatty acids precursors of “anti-inflammatory” mediators (dGLA and EPA) at the expense of AA, which is precursor of “pro-inflammatory” mediators as a result of the competing biochemical pathways of PUFA synthesis [27]. By this way, AA/dGLA was lower than when GLA was added alone and (dGLA + EPA)/AA was higher than when GLA and EPA were added alone. A combined supplementation of GLA and EPA decreased the incorporation of AA and the production of PGE2 as compared to a GLA supplementation. Despite the increase in dGLA, the production of PGE1 was not induced when GLA and EPA were added together. This could be due to the inhibitory effect of EPA on COX-2 required for the synthesis of PG as previously reported by others [28]. As PGE2 were produced in higher amounts than PGE1 [29], this inhibitory effect was observable only for PGE1.
These results suggest that the efficacy of PUFA supplementation observed in dry eye syndrome—a disease inducing inflammation in the conjunctiva [4]—should not only be due to the production of PG. Indeed, all dietary supplementations aimed to modify PG production in tissue will be limited by the fact that the PGE2 will always be the major PG synthesized [29]. Indeed the production of PGE2 by COX-2 is favored at the expense of the production of PGE1 and PGE3, due to the preferential incorporation of AA versus dGLA and EPA into, and release from, the total cellular phospholipid pool [30, 31]. The anti-inflammatory effects of PUFA could be independent of the production of PGs and may be due to the synthesis of other mediators such as resolvins and neuroprotectins [32–34]. It has been shown that a topical treatment with resolvin E1 may have beneficial effect in dry eye mouse model (increase in tear production and decrease in COX-2 activation on the cornea [35]). The protective effects of PUFA may also be mediated through transcription factors implicated in the inflammatory response such as the nuclear factor κB (NF-κB) or the peroxisome-proliferator activated receptor (PPAR) [36, 37]. EPA was already shown to inhibit the activation of NF-κB on monocytic cells [37]. Despite the fact that IFN-γ stimulation did not modify PG production, it has already been described in Chang cells and IOBA-NHC cells as an inflammatory inducer with the activation of NF-κB [38].
We have studied the immortalized Chang cells from human origin [39]. Despite some similarities with in situ conjunctival epithelium (presence of tight junctions, microvillosities, mucin secretion) and their widespread use in the study of the expression of inflammation-related markers or apoptosis [40], they may behave differently. To lessen the impact of the use of Chang cell line, we have also included IOBA-NHC cells in this study. These are non-transfected, and spontaneously immortalized epithelial cell line from normal human conjunctiva. These cells keep morphological and functional epithelial characteristics [41]. Chang cells and IOBA-NHC cells exhibit differences in phospholipid and neutral lipid fatty acid compositions and PG levels but we have found similar results in IOBA-NHC cells and in Chang cells when PUFA were added in the culture medium. This confirms that Chang cells and IOBA-NHC cells are comparable for the lipid metabolism study.
In conclusion, we have shown that the conjunctiva, which is a highly inflamed tissue during dry eye syndrome, is able to incorporate and metabolize fatty acids that can be present in the diet and to modulate the production of PGE1 and/or PGE2. Hence, GLA and EPA may be helpful in the treatment of dry eye syndrome in addition to the conventional therapies.
Abbreviations
- AA:
-
Arachidonic acid
- ALA:
-
α-linolenic acid
- COX:
-
Cyclo-oxygenase
- dGLA:
-
Dihomo γ-linolenic acid
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FAME:
-
Fatty acid methyl ester
- GLA:
-
γ-Linolenic acid
- IFN-γ:
-
Interferon-γ
- LA:
-
Linoleic acid
- NF-κB:
-
Nuclear factor κB
- PG:
-
Prostaglandin
- PPAR:
-
Peroxisome proliferator activated receptor
- PUFA:
-
Polyunsaturated fatty acid
References
Bourre JM (2006) Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 2: macronutrients. J Nutr Health Aging 10:386–99
Schnebelen C, Pasquis B, Salinas-Navarro M, Joffre C, Creuzot-Garcher CP, Vidal-Sanz M, Bron AM, Bretillon L, Acar N (2009) A dietary combination of omega-3 and omega-6 polyunsaturated fatty acids is more efficient than single supplementations in the prevention of retinal damage induced by elevation of intraocular pressure in rats. Graefes Arch Clin Exp Ophthalmol
Schnebelen C, Viau S, Gregoire S, Joffre C, Creuzot-Garcher CP, Bron AM, Bretillon L, Acar N (2009) Nutrition for the eye: different susceptibility of the retina and the lacrimal gland to dietary omega-6 and omega-3 polyunsaturated fatty acid incorporation. Ophthalmic Res 41:216–24
Viau S, Maire MA, Pasquis B, Gregoire S, Acar N, Bron AM, Bretillon L, Creuzot-Garcher CP, Joffre C (2009) Efficacy of a 2-month dietary supplementation with polyunsaturated fatty acids in dry eye induced by scopolamine in a rat model. Graefes Arch Clin Exp Ophthalmol 247:1039–50
Barham JB, Edens MB, Fonteh AN, Johnson MM, Easter L, Chilton FH (2000) Addition of eicosapentaenoic acid to gamma-linolenic acid-supplemented diets prevents serum arachidonic acid accumulation in humans. J Nutr 130:1925–31
Miles EA, Banerjee T, Calder PC (2004) The influence of different combinations of gamma-linolenic, stearidonic and eicosapentaenoic acids on the fatty acid composition of blood lipids and mononuclear cells in human volunteers. Prostaglandins, Leukot Essent Fatty Acids 70:529–38
Calder PC (2006) n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83:1505S–1519S
Culp BR, Titus BG, Lands WE (1979) Inhibition of prostaglandin biosynthesis by eicosapentaenoic acid. Prostaglandins Med 3:269–78
Schnebelen C, Gregoire S, Pasquis B, Joffre C, Creuzot-Garcher CP, Bron AM, Bretillon L, Acar N (2009) Dietary n-3 and n-6 PUFA enhance DHA incorporation in retinal phospholipids without affecting PGE(1) and PGE (2) levels. Lipids 44:465–70
Stern ME, Pflugfelder SC (2004) Inflammation in dry eye. Ocul Surf 2:124–30
Aragona P, Bucolo C, Spinella R, Giuffrida S, Ferreri G (2005) Systemic omega-6 essential fatty acid treatment and pge1 tear content in Sjögren's syndrome patients. Invest Ophthalmol Vis Sci 46:4474–9
Barabino S, Rolando M, Camicione P, Ravera G, Zanardi S, Giuffrida S, Calabria G (2003) Systemic linoleic and gamma-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea 22:97–101
Creuzot-Garcher C (2006) Lacrimal film and the ocular surface. J Fr Ophtalmol 29:1053–9
Macri A, Giuffrida S, Amico V, Iester M, Traverso CE (2003) Effect of linoleic acid and gamma-linolenic acid on tear production, tear clearance and on the ocular surface after photorefractive keratectomy. Graefes Arch Clin Exp Ophthalmol 241:561–6
Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP (2011) Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea 30:308–14
Miljanovic B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA (2005) Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am J Clin Nutr 82:887–93
Leventhal LJ, Boyce EG, Zurier RB (1993) Treatment of rheumatoid arthritis with gammalinolenic acid. Ann Intern Med 119:867–73
Kulkarni PS, Srinivasan BD (1989) Cyclooxygenase and lipoxygenase pathways in anterior uvea and conjunctiva. Prog Clin Biol Res 312:39–52
Goustard-Langelier B, Alessandri JM, Raguenez G, Durand G, Courtois Y (2000) Phospholipid incorporation and metabolic conversion of n-3 polyunsaturated fatty acids in the Y79 retinoblastoma cell line. J Neurosci Res 60:678–85
Folch J, Lees M, Sloane-Stanley G (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Juaneda P, Rocquelin G (1985) Rapid and convenient separation of phospholipids and non-phosphorus lipids from rat heart using silica cartridges. Lipids 20:40–41
Joffre C, Souchier M, Gregoire S, Viau S, Bretillon L, Acar N, Bron AM, Creuzot-Garcher C (2008) Differences in meibomian fatty acid composition in patients with meibomian gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol 92:116–9
Wang Y, Botolin D, Xu J, Christian B, Mitchell E, Jayaprakasam B, Nair MG, Peters JM, Busik JV, Olson LK, Jump DB (2006) Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J Lipid Res 47:2028–41
Zheng X, Torstensen BE, Tocher DR, Dick JR, Henderson RJ, Bell JG (2005) Environmental and dietary influences on highly unsaturated fatty acid biosynthesis and expression of fatty acyl desaturase and elongase genes in liver of Atlantic salmon (Salmo salar). Biochim Biophys Acta 1734:13–24
Nakamura MT, Nara TY (2004) Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr 24:345–76
Williard DE, Kaduce TL, Harmon SD, Spector AA (1998) Conversion of eicosapentaenoic acid to chain-shortened omega-3 fatty acid metabolites by peroxisomal oxidation. J Lipid Res 39:978–86
Sprecher H, Luthria DL, Mohammed BS, Baykousheva SP (1995) Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res 36:2471–7
Obata T, Nagakura T, Masaki T, Maekawa K, Yamashita K (1999) Eicosapentaenoic acid inhibits prostaglandin D2 generation by inhibiting cyclo-oxygenase-2 in cultured human mast cells. Clin Exp Allergy 29:1129–35
Herman CA, Hamberg M, Granstrom E (1987) Quantitative determination of prostaglandins E1, E2 and E3 in frog tissue. J Chromatogr 394:353–62
Raisz LG, Alander CB, Simmons HA (1989) Effects of prostaglandin E3 and eicosapentaenoic acid on rat bone in organ culture. Prostaglandins 37:615–25
Rubin D, Laposata M (1991) Regulation of agonist-induced prostaglandin E1 versus prostaglandin E2 production. A mass analysis J Biol Chem 266:23618–23
He J, Bazan HE (2010) Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostaglandins Leukot Essent Fatty Acids 82:319–25
Mukherjee PK, Chawla A, Loayza MS, Bazan NG (2007) Docosanoids are multifunctional regulators of neural cell integrity and fate: significance in aging and disease. Prostaglandins Leukot Essent Fatty Acids 77:233–8
Serhan CN, Chiang N (2008) Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol 153(Suppl 1):S200–15
Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE (2010) Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther 26:431–9
Christman JW, Lancaster LH, Blackwell TS (1998) Nuclear factor kappa B: a pivotal role in the systemic inflammatory response syndrome and new target for therapy. Intensive Care Med 24:1131–8
Zhao Y, Joshi-Barve S, Barve S, Chen LH (2004) Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr 23:71–8
Tsubota K, Fukagawa K, Fujihara T, Shimmura S, Saito I, Saito K, Takeuchi T (1999) Regulation of human leukocyte antigen expression in human conjunctival epithelium. Invest Ophthalmol Vis Sci 40:28–34
Lavappa KS, Macy ML, Shannon JE (1976) Examination of ATCC stocks for HeLa marker chromosomes in human cell lines. Nature 259:211–3
Guenoun JM, Baudouin C, Rat P, Pauly A, Warnet JM, Brignole-Baudouin F (2005) In vitro comparison of cytoprotective and antioxidative effects of latanoprost, travoprost, and bimatoprost on conjunctiva-derived epithelial cells. Invest Ophthalmol Vis Sci 46:4594–9
Diebold Y, Calonge M, Enriquez de Salamanca A, Callejo S, Corrales RM, Saez V, Siemasko KF, Stern ME (2003) Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Invest Ophthalmol Vis Sci 44:4263–74
Acknowledgements
This work was supported by The Nutrition, Chemical Food Safety and Consumer Behaviour Division from INRA (Dijon, France) for S. Viau (BTH01698). The authors would like to thank Yolanda Diebold in Valladolid (Spain) for providing the IOBA-NHC conjunctival human cells.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support
The Nutrition Chemical Food Safety and Consumer Behaviour Division from INRA (Dijon, France) provided financial assistance to S. Viau (BTH01698).
Rights and permissions
About this article
Cite this article
Viau, S., Leclère, L., Buteau, B. et al. Polyunsaturated fatty acids induce modification in the lipid composition and the prostaglandin production of the conjunctival epithelium cells. Graefes Arch Clin Exp Ophthalmol 250, 211–222 (2012). https://doi.org/10.1007/s00417-011-1801-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1801-y