Abstract
Background
The purpose of the study was to assess intraobserver and interobserver repeatability of eight ocular components measurement in cataract eyes using the optical low-coherence reflectometer Lenstar LS 900®.
Methods
Five consecutive measurements of ocular components were taken by two examiners using the Lenstar. Components analyzed were: central corneal thickness, lens thickness, anterior chamber depth, axial length, retinal thickness, keratometry, white-to-white distance, and pupillometry. Within-subject standard deviation and the coefficient of variation were calculated for evaluation of intraobserver repeatability. Bland–Altman analysis was used for assessment of interobserver repeatability.
Results
Thirty-two eyes of 22 patients were included. For both observers, the smallest intraobserver coefficient of variation was obtained for axial length, while the largest was found for corneal steepest meridian position. Interobserver repeatability demonstrated less repeatable results for white-to-white distance and corneal steepest meridian position. Considering axial length and anterior chamber depth values, predicted refractive error was 0 ± 0.05 D and 0.02 ± 0.19 D respectively in 95% of observations.
Conclusion
The Lenstar LS 900® evidenced excellent repeatability and observers´ independent results of all components analyzed except white-to-white distance and corneal steepest meridian position measurements. To the best of our knowledge, this is the first study on interobserver repeatability of optical low-coherence reflectometry in cataract eyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most common method for biometric measurement in cataract surgery is the applanation ultrasound A-scan technique [1]. Since the advent of the first commercial ocular biometer in 1999 (IOLMaster, Carl Zeiss Jena GmbH) partial coherence laser interferometry (PCLI) has become widely used method for ocular biometry. A new biometry device Lenstar LS 900® (Haag–Streit AG, Köniz, Switzerland) is now available for measuring ocular optical components: central corneal thickness (CCT), anterior chamber depth (ACD), lens thickness (LT), axial length (AL), retinal thickness (RT), keratometry [corneal dioptric power in the flattest meridian (K1), corneal dioptric power in the steepest meridian (K2) and steepest meridian position (AXIS)], white-to-white distance (WW), and pupillometry (PO).
In clinical measurement, comparison of a new measurement technique with an established one is often needed to see whether the results for the two methods agree sufficiently for the new to replace the old [2]. If the new method agrees sufficiently well with the old, then the two methods may be used interchangeably and the old may be replaced. To assess the agreement of the two methods, it is necessary to know repeatabilities of both techniques, because repeatabilities limit the possible amount of agreement between them [2]. Hence, in this study we assessed the intraobserver and interobserver repeatability of eight ocular components measurements obtained with the new optical low-coherence reflectometer Lenstar LS 900®, so that comparison with established biometric measurement techniques might be performed in the future.
Materials and methods
Patients
A prospective study of 32 eyes (22 patients) undergoing surgery for age-related cataract was conducted at the University Eye Clinic, University Hospital Sveti Duh, Zagreb, Croatia during February and March 2009. Exclusion criteria for the analysis were patients with previous eye surgery, poor expected visual acuity after cataract surgery due to non-cataract-related eye conditions such as macular degeneration, amblyopia, glaucoma, advanced corneal pathology, and those with LOCS III P-scale value greater than 3.5. The study was approved by an institutional review board, and written consent for performing the measurements and analyzing data was obtained from all participants. All study procedures adhered to the recommendations of the Declaration of Helsinki.
Measurement technique
Patients were randomly assigned to have five consecutive measurements of eight ocular components by two examiners with the Lenstar LS 900®. The components analyzed were: CCT, LT, ACD, AL, RT, keratometry (K1, K2, AXIS), WW, and PO. The starting examiner was assigned randomly in each case. A computed average of five serial measurements was automatically registered and displayed on the screen of the Lenstar LS 900® for all the components analyzed except for RT, which was additionally determined by observer positioning the cursor manually on the highest retinal peak.
Biometer characteristics
The Lenstar LS 900® is an ocular biometer that utilizes optical low-coherence reflectometry for measuring CCT, ACD, LT, AL and RT. The principle of measuring axial length with an OLCR system used by the Lenstar LS 900 is similar to that of the partial coherence laser interferometry system used by the Carl Zeiss IOLMaster. Both are based on a specific optical arrangement known as the Michelson interferometer. In theory, the Michelson interferometer arrangement will operate using virtually any type of optical source. The Michelson interferometer portion of the IOLMaster is used to create a pair of coaxial 780 nm infrared light beams with a coherence length of approximately 150 μm. The Lenstar LS 900 uses an 820 nm superluminescent diode as a source. It has wide spectral width properties (20–30 nm), and thus a short coherence length of approximately 30 μm. The result is a high spatial resolution, since it is inversely proportional to the source spectral bandwidth. This quality gives OLCR its main advantage over other reflectometry techniques. The parameters K1, K2 and AXIS are calculated through the position of 32 projected light reflections arranged in two rings with diameters 1.65 mm and 2.30 mm (standard eye R = 7.80 mm). WW is determined using the image of the iris and the eye radii obtained from keratometry. PO is calculated as a diameter of an ideal circle, with the smallest error square to the established pupil border. At the same time, the shift of visual axis towards the centre of the pupil is provided. All of the eight ocular components mentioned are measured in a single shot, with a patient fixating directly on the measurement beam. Loss of fixation and blinking of the patient is registered by the device; hence, only good measurements are analyzed. The measured values may be used instantly to calculate the optimal IOL diopter using integrated IOL power calculator with multivariable IOL power prediction formulas.
Statistical analysis
All numerical computations were performed at the Department of Medical Statistics, Epidemiology and Medical Informatics, Andrija Štampar School of Public Health, Medical School, University of Zagreb, Croatia. Statistical analysis was performed in SPSS ver. 13 (SPSS Inc, Chicago, IL, USA).
Intraobserver repeatability of the five consecutive measurements was assessed using the within-subject standard deviation (Sw), and the coefficient of variation (CV). The common standard deviation of repeated measurement (Sw) estimates the size of the measurement error [3]. The coefficient of variation was calculated as ratio of Sw and mean.
Interobserver repeatability of measurements was evaluated by Bland–Altman analysis [2]. Limits of agreement (LoA) were defined as the mean difference ±1.96 SD of the differences [2]. This standard deviation represents the interobserver range of agreement (1.96 times), with lower values indicating higher repeatability and vice versa. The question of how small something is depends on the clinical context: if a difference between two observers´ measurements (as extreme as that described by the 95% limits of agreement) meaningfully affects the interpretation of the results [4], then the range of agreement is clinically significant, interobserver repeatability not acceptable, and the method analyzed does not provide repeatable measurements. Sample size was defined in a way to provide sufficient statistical power of the study, which was in this case over 90%.
Results
Patients
Measurements were performed on 32 eyes of 22 patients. Mean age of patients was 75 ± 5.58 years (range 64 to 92 years). The Lenstar measured CCT, ACD, LT, AL, RT, K1 and K2 in 32 eyes, while it failed to measure AXIS in four eyes, WW in two eyes and PO in one eye.
Intraobserver repeatability
Table 1 summarizes intraobserver repeatability results for the ocular components analyzed. For both observers, the smallest intraobserver coefficient of variation was obtained for AL, while the largest was found for AXIS.
Interobserver repeatability
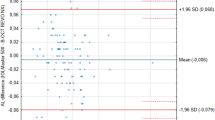
Table 2 summarizes interobserver repeatability results for the ocular components analyzed. A Bland–Altman plot of AL with the smallest range of agreement is shown in Fig. 1. Range and limits of agreement were clinically significant for AXIS and WW, as shown in Figs. 2 and 3 respectively.
Discussion
Repeated measurements of different parameters are often used in clinical research. When two examiners, using the same device for measuring the same parameter on one occasion, gain highly repeatable results, the results are observer-independent and closer to the “true” value of the measured parameter. Hence, the importance of this study is based on the interobserver repeatability of the Lenstar.
The intraobserver repeatability of measurements obtained with the Lenstar in this study was excellent for CCT, ACD, LT, AL, K1 and K2 with the coefficient of variation ≤2%, and was good for WW, PO, RT and AXIS. With regard to interobserver repeatability, limits of agreement were not clinically significant for CCT, ACD, LT, AL, RT, K1, K2, and PO, while WW and AXIS showed less repeatable results, due to a clinically significant span in the 95% limits of agreement of 2.18 mm and 126° respectively.
Published studies reviewed on OLCR analyser for biometric measurements showed excellent repeatability of AL [5–8], CCT [5–8], ACD [5–8], corneal curvature [5–8], and LT [5, 6, 8], with AL being the most repeatable component [5–8]. These results are confirmed with findings in this study. However, to our knowledge only the study by Buckhurst et al. [5] and Cruysberg et al. [6] measured WW, while the study by Rohrer and colleagues [7] is the only one evaluating the AXIS component. Although Lenstar biometric measurement was found to be excellent and highly repeatable with CV ≤2% for WW, when the Lenstar was compared to the IOLMaster the difference in WW measurement demonstrated a span of 1.2 mm [5]. Cruysberg and colleagues reported that the IOLMaster and the Lenstar cannot be used interchangeably for WW measurements, due to large and clinically significant 95% limits of agreement with a span of 2.46 mm [6]. In our study, the interobserver repeatability for WW measured by the Lenstar evidenced less repeatable results because of a clinically significant span of 2.18 mm in the 95% of observations. We believe that the higher intraobserver CV and clinically significant interobserver differences in measurement for WW component obtained in this study might reflect the variation in the measurement process. WW is measured by an imaging technique using corneoscleral contrast zones as a reference mark. However, identification of limbus and hence measurement of WW can be biased by arcus senilis, limbal pannus, and other anatomic variations because of interfering signals reflected on iris.
The coefficients of variation for AL, CCT, ACD, average radius of corneal curvature and AXIS of the flattest radius obtained with the Lenstar by Rohrer et al. were comparable with our results [7]. The most variable component was AXIS of the flattest meridian with CV higher than 9% [7]. When Lenstar was compared with the IOLMaster for measurement of AXIS of the flattest radius, the span of 86° in the 95% limits of agreement was found [7]. In addition, with regard to keratometry parameters, it was shown that the OLCR measured 0.04 D [9], 0.05 D [5], and 0.11 D [10] flatter cornea than the PCI unit. However, it cannot be verified which of the two methods is more correct. A possible explanation for these keratometry variabilities might be related to different measurement methods used by the two devices. With the Lenstar, the corneal curvature value is calculated using two rings of diameter 1.65 mm and 2.30 mm of 16 light spots, each reflected off the air–tear interface. The IOLMaster keratometry values are measured through image analysis of six light points arranged in a 2.3 mm diameter hexagonal pattern. The averaging over 32 instead of six light reflections can improve the accuracy of K1, K2, and AXIS. However, we speculate that even the very least amplitude of eye movements as seen during breathing and provoking small re-fixation saccades has greater influence on AXIS than on K1 and K2 value, to the extent of increasing the variability of AXIS measurements to a clinically significant level. We could find no data on interobserver repeatability of AXIS measured with PCLI technique searching the Medline database to compare it to our results. When compared to the contact ultrasound biometer in cataract eyes, the Lenstar demonstrated higher reproducibility with regard to AL, CCT and ACD, whereas no significant difference in repeat accuracy was found for LT [11].
Precise measurement of WW and AXIS is of extreme importance in cataract surgery and clear lens extraction. Third generation formulas for IOL calculation use WW as a parameter in determining the IOL power [12], while the selection of angle supported and posterior chamber phakic intraocular lenses (pIOL) is based on calculations using the WW [13]. Angle-supported pIOLs have been associated with complications which arise from poor pIOL sizing. The accurate AXIS value measurement is an important preoperative determinant when toric IOL implantation is performed.
In this study, Bland–Altman analysis showed that the results of the two observers measuring AL differed by 0 ± 0.019 mm, corresponding to a refractive error of about 0 ± 0.05 D in 95% of observations [14]. With regard to ACD value, the difference between the two examiners' results in 95% of observations was 0.014 ± 0.124 mm, corresponding to a refractive error of about 0.02 ± 0.19 D [14]. These data are impressive, because achievement of about 95% of eyes within ±0.50 D of the targeted refraction would be an excellent refractive outcome. Reported number of predictions within ±0.5 D, ±1.0 D and ± 2.0 D of the expected outcome was 55.6%–92% [15–18], 87%–95.7% [15, 17–19], and 99.9%–100% [15, 18] of cases with PCLI, compared to 45.5%–69.6% [15, 17, 18], 77.3%–93.5% [15, 17–20], and 98.4%–100% [15, 18, 20] of cases with ultrasound respectively.
As a result of our findings, we conclude that the Lenstar LS 900® is a non-contact, patient, and user-friendly method which offers easy measuring of CCT, ACD, LT, AL, RT, WW, PO, K1, K2, and AXIS in a single setting. Our findings demonstrated excellent repeatability of all parameters analyzed with the Lenstar LS 900®, except for WW and AXIS. Further studies are warranted to determine the method with the highest accuracy for WW and AXIS measurements.
References
Leaming DV (2004) Practice styles and preferences of ASCRS members—2003 survey. J Cataract Refract Surg 30:892–900
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Bland JM, Altman DG (1996) Measurement error. BMJ 313:744
Myles PS, Cui J (2007) Using Bland–Altman method to measure agreement with repeated measures. Br J Anaesth 99:309–311
Buckhurst PJ, Wolffsohn JS, Shah S, Naroo SA, Davies LN, Berrow EJ (2009) A new optical low coherence reflectometry device for ocular biometry in cataract patients. Br J Ophthalmol 93:949–953
Cruysberg LP, Doors M, Verbakel F, Berendschot TTJM, De Brabander J, Nuijts RMMA (2010) Evaluation of the Lenstar LS 900 all-in-one non contact biometry meter. Br J Ophthalmol 94:106–110
Rohrer K, Frueh BE, Wälti R, Clemetson IA, Tappeiner C, Goldblum D (2009) Comparison and evaluation of ocular biometry using a new noncontact optical low-coherence reflectometer. Ophthalmology 116:2087–2092
Liampa Z, Kynigopoulos M, Pallas G, Gerding H (2010) Comparison of two partial coherence interferometry devices for ocular biometry. Klin Monatsbl Augenheilkd 227:285–288
Holzer MP, Mamusa M, Auffarth GU (2009) Accuracy of a new partial coherence interferometry analyser for biometric measurements. Br J Ophthalmol 93:807–810
Hoffer KJ, Shammas HJ, Savini G (2010) Comparison of 2 laser instruments for measuring axial length. J Cataract Refract Surg 36:644–648, Erratum in: J Cataract Refract Surg 36:1066
Tappainer C, Rohrer K, Frueh BE, Waelti R, Goldblum D (2010) Clinical comparison of biometry using the non-contact optical low coherence reflectometer (Lenstar LS 900) and contact ultrasound biometer (Tomey AL-3000) in cataract eyes. Br J Ophthalmol 94:666–667
Baumeister M, Terzi E, Ekici Y, Kohnen T (2004) Comparison of manual and automated methods to determine horizontal corneal diameter. J Cataract Refract Surg 30:374–380
Piñero DP, Plaza Puche AB, Alió JL (2007) Corneal diameter measurements by corneal topography and angle to angle measurements by optical coherence tomography: Evaluation of equivalence. J Cataract Refract Surg 34:126–131
Olsen T (2007) Calculation of intraocular lens power: a review. Acta Ophthalmol Scand 85:472–485
Kiss B, Findl O, Menapace R, Wirtitsch M, Petternel V, Drexler W, Rainer G, Georgopoulos M, Hitzenberger CK, Fercher AF (2002) Refractive outcome of cataract surgery using partial coherence interferometry and ultrasound biometry. Clinical feasibility study of a commercial prototype II. J Cataract Refract Surg 28:230–234
Packer M, Fine IH, Hoffman RS, Coffman PG, Brown LK (2002) Immersion A-scan compared with partial coherence interferometry. Outcomes analysis. J Cataract Refract Surg 28:239–242
Narváez J, Cherwek DH, Stulting RD, Waldron R, Zimmerman GJ, Wessels IF, Waring GO 3rd (2008) Comparing immersion ultrasound with partial coherence interferometry for intraocular lens power calculation. Ophthalmic Surg Lasers Imaging 39:30–34
Olsen T (2007) Improved accuracy of intraocular lens power calculation with the Zeiss IOLMaster. Acta Ophthalmol Scand 85:84–87
Rajan MS, Keilhorn I, Bell JA (2002) Partial coherence laser interferometry vs conventional ultrasound biometry in intraocular lens power calculation. Eye 16:552–556
Haigis W, Lege B, Miller N, Schneider B (2000) Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol 238:765–773
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have full control of all primary data, and they agree to allow Graefe's Archive for Clinical and Experimental Ophthalmology’ to review their data if requested.
None of the authors has a financial or proprietary interest in any material or method mentioned.
Rights and permissions
About this article
Cite this article
Bjeloš Rončević, M., Bušić, M., Čima, I. et al. Intraobserver and interobserver repeatability of ocular components measurement in cataract eyes using a new optical low coherence reflectometer. Graefes Arch Clin Exp Ophthalmol 249, 83–87 (2011). https://doi.org/10.1007/s00417-010-1546-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1546-z