Abstract
Purpose
To assess the efficacy and safety of indocyanine green (ICG) dye-enhanced subthreshold diode-laser micropulse (SDM) photocoagulation in patients with chronic central serous chorioretinopathy (CCSC) with no spontaneous resolution 6 months after the onset of the disease.
Study design
Interventional prospective non-comparative case series of seven patients presenting with CCSC with well-defined active leaking sites (ALS) suitable for laser treatment and with serous neuroepithelial detachment persisting for 6 or more months.
Methods
SDM treatment was performed 15 minutes after the injection of 25 mg of ICG in 2 cc of 5% glucose solution. ALS were treated with a series of 50 500-ms exposures separated by 500-ms pauses. Each 500-ms exposure delivered a train of 250 micropulses at 10% duty cycle and 500 mW power. ICG angiographic images were taken after the treatment without new ICG injection, to check for the presence of laser-induced spots of background hypofluorescence at the treated leakage sites.
Results
Within 7-14 days after treatment, all the patients showed improved visual acuity and reduction of serous neuroepithelial detachment on OCT. No signs of laser lesions were visible at fundus examination and on fluorescein angiography. In a period ranging from 4 to 8 weeks, the neuroepithelial detachment was completely resolved in five patients and reduced in two patients. At the 12-month follow-up visits, no recurrence had occurred in the patients, with resolution of the serous neuro-epithelial detachment, and no worsening of the serous detachment or of VA was noted in the patients with incomplete recovery.
Conclusions
These preliminary results suggest that ICG dye-enhanced SMD photocoagulation appears to be an effective treatment, and can represent a viable approach for the management of CSCC with persistent serous neuroepithelial detachment. Post-treatment ICG angiography, without new ICG dye injection, can be used to verify the placement of the SDM laser applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Central serous chorioretinopathy (CSC) is characterized by idiopathic serous detachment of the neurosensory retina secondary to focal defects of the retinal pigment epithelium (RPE), such as multiple small serous detachments and/or localized areas of atrophy [1]. The prevalence of CSC peaks around 45 years of age and the incidence is about six times higher in men than in women, with a mean annual age-adjusted incidence per 100,000 of 9.9 for men and 1.7 for women. There are no significant risk factors identified for CSC [2]. Extra-ocular conditions associated with CSC include type A personalities [3], organ transplantation [4–6], systemic and intranasal use of steroids [7, 8], systemic lupus erythematosus [9, 10], Cushing’s disease [11], and other systemic factors [12]. Usually, CSC regresses spontaneously within several months, but recurrence is observed in 33% to 50% of cases [13, 14]. Direct focal treatment of the RPE active leakage site with conventional laser photocoagulation can accelerate the resolution of the serous detachment, but the final vision acuity (VA) is often similar to that produced by spontaneous healing [15–17]. For this reason, conventional laser photocoagulation is not normally indicated for the treatment of CSC, and it is considered only in specific cases such as those associated with persistent (4–6 months) or progressive detachment (with or without inferior guttering), permanent CSC changes in the fellow eye, multiple recurrences, or the professional need for rapid vision recovery. Complications of conventional laser treatment can include central scotoma, contrast sensitivity loss, accidental foveal damage, retinal distortion and choroidal neovascularization (CNV) [18–20]. CNV develops spontaneously in 15% of patients with CSC [21, 22], an incidence second only to age-related macular degeneration (AMD).
Studies on the treatment of various retinal disorders, including CSC [23–26] support the hypothesis that subthreshold diode-laser micropulse (SMD) treatment of the RPE can produce therapeutic benefits comparable to those of conventional photocoagulation.
Clinical resolution is often reported with no detectable signs of laser-induced iatrogenic damage. This is very encouraging, but the absence of visible endpoint represents a challenge for the surgeon. To overcome this problem, we decided to perform the SDM treatment over ICG-stained RPE cells with the triple goal of: (a) enhancing the selectivity of the treatment for the active leaking sites (ASL), (b) sparing the neurosensory retina, and (c) documenting the placement of SDM applications seen as dark spots in the ICG background fluorescence [27].
Methods
Seven consecutive patients presenting with idiopathic chronic CSC between 2002 and 2007 were enrolled and treated. The patients were male with mean age of 39 ± 8.31. They had persistent serous neuro-epithelial detachment (average 6.57 ± 0.79 months), metamorphopsia and decreased VA. Five patients had refractive hyperopic shift. One or more RPE active leakage sites eligible for focal laser photocoagulation were identified at FA in all patients.
When informed about the risk of complications, all patients declined the conventional 532 nm laser treatment. When requested to choose between the ICG-enhanced SDM treatment or observation, all opted for the treatment and signed the informed consent, despite the specific warning that the absence of intraoperative visible endpoint could lead to an insufficient treatment. Ethics Committee decided approval was not required for this study. Research adhered to the tenets of the Declaration of Helsinki.
For the treatment we used an 810 nm infrared diode laser in the MicroPulse emission mode (IRIS Medical OcuLight SLx, IRIDEX Corporation, Mountain View, CA, USA).
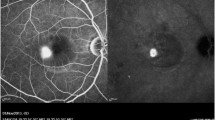
The treatment was started 15-20 minutes after the injection of 25 mg of ICG in 2 cc of 5% glucose solution (Infracyanine SERB), when (a) the hypofluorescent washout of the retinal and choroidal vessels, and (b) the ICG staining of the RPE–Bruch’s membrane complex were visible at ICG angiography (ICGA) [28]. Prior to proceeding with the treatment of the central active leakage site, a test burn was performed either in the nasal mid periphery (Fig. 1a) or on a peripheral active leakage site when present (Fig. 1b) to ensure that no visible or latent retinal burn would result. Each active leakage site was treated with fifty sequential trains of micropulses of 500-ms duration (500 mW power, 10% duty cycle, 0.2 ms “On” time” + 1.8 ms interpulse “Off” time = 2.0 ms), separated by 500-ms pauses, for a total treatment time of 50 seconds.
a ICG angiography (ICGA) 20 minutes after injection, showing the background hyperfluorescence due to the RPE staining and the retinal-choroidal vessels’ hypofluorescence due to the dye wash out. The dark spot under the white arrow is the result of the test run performed on the nasal side. b Late-phase ICGA showing the hyperfluorescent juxtafoveal active leakage site (ALS, red arrow) surrounded by pathologic stained RPE and the test run performed on eccentric ALS (white arrow). The fovea’s position is indicated by the white circle (overlay performed with Topcon ImageNET)
We used a laser spot size of 75 μm diameter in air that, with the Volk PDT laser lens (1.5× spot magnification), produced a spot of 112.5 μm on the retina. With this spot and the laser power set at 500 mW, each micropulse had 5033 W/cm2 irradiance and 1.01 J/cm2 fluence. Although no ophthalmoscopically and angiographically visible effects were produced, each application with these laser parameters totaled 121 times the maximum permissible exposure (MPE) established by the ANSI Z136.1 MPE standard issued by the American Standards Institute and adopted for decades as benchmark for clinical treatment parameters [29]. The 121× MPE level is above the safety margin given for 100 μs laser exposures (100× MPE) [30] and proved adequate to induce cellular effects in the target tissue.
When close to the fovea due to an higher pigment density, as a further precaution, the number of sequential exposures was halved from 50 to 25 (25 seconds treatment time).
Follow-up visits with biomicroscopy, fundus color photography, OCT, UCVA and BCVA evaluations were performed at 1, 2, 4, 6 and 8 weeks, 6 and 12 months in all seven patients. Post-treatment fluorescein angiography (FA) was performed in six patients in a period ranging from 6 to 12 months. One patient accepted all tests, but declined further angiographic evaluations.
Results
The clinical data of the seven patients are summarized in Table 1.
OCT scan revealed complete resolution of the serous neuro-epithelial detachment in five patients and a marked reduction in two patients. Serous detachment recovery times ranged from 4 to 8 weeks (median 6 weeks) in the five patients that shoved a complete resolution. The patients with incomplete recovery reached the maximum improvement in 8 weeks. Visual acuity (VA) improved in six patients and remained stable in one patient. The mean VA improvement at the 12-month follow-up visit was of 0.19 LogMAR (p < 0.05, Friedman test). All patients improved their metamorphopsia-related symptoms.
At the 6- and 12-month follow-up visits, no recurrence occurred in the five patients with complete resolution of the serous neuro-epithelial detachment, and no worsening of the serous neuro-epithelial detachment or of VA was noted in the two patients with incomplete recovery (Fig. 2).
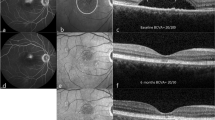
No sign of laser-induced lesion was detectable either ophthalmoscopically or at angiography at any follow-up time in all seven patients, as shown in the post-treatment color retinographies and early (2-week) angiographies (FA and ICGA) of the first patient (Fig. 3). Furthermore, no trace of the treatment was evident at fundus autofluorecence in patients 4 and 7 imaged by mean of HRA2 2 weeks after the treatment (Fig. 4). Six-month angiographies of patients 4 and 6 revealed the lack of any scar due to laser photothermal damage (Fig. 5).
Patient 1: Colour fundus photography (a) and FA showing the active leakage sites before the treatment (b). ICGA without (further dye injection) after the laser treatment: note the two dark spots at the site of the laser treatments (c). Colour fundus photograph (d), fluorangiography (e) and ICG angiography (f) 1 week after the treatment: note the reduction of the retinal serous detachment and the lack of any sign of the laser-induced scar
Patients 4 and 7: fundus autofluorescence at HRA2 before (a,f) and 2 weeks after the treatment (e,j). Active leaking sites at FA (b,g) and at late-phase ICGA: note the hyperfluorescence due to higher dye uptake by pathologic RPE (c,h). Hypofluorescent areas at late phase ICGA (without further dye injection) at the treated leakage sites (d,i)
Discussion
Conventional thermal laser treatment of the ALS has shown to promote the resolution of the exudative manifestations in CSC [15–17]. However, this treatment is controversial, because comparable visual outcomes are normally achieved over time with no intervention and no risks of adverse effects [31, 32]. Recent studies in eyes with chronic CSC have demonstrated a spatial correlation between the ALS at fluorescein angiography and RPE alterations on optical coherence tomography (OCT) [33–35]. This observation suggests that persistent leak and sub-retinal fluid can lead to permanent RPE changes, which cause irreversible visual loss [36]. This provides the rationale for earlier interventions for quicker resolution of leakage and fluid. To this end, alternative laser techniques and protocols have been devised to minimize the iatrogenic damage, improve the benefit/risk ratio and justify earlier treatments prior to the onset of irreversible functional losses. Among them:
-
Selective RPE laser treatment (SRT)
-
ICG–mediated photo-thrombosis (IMP)
-
Subthreshold diode-laser micropulse (SDM) photocoagulation
-
SDM photocoagulation of ICG-stained RPE cells at the active leakage site
Selective RPE laser treatment (SRT) is performed with short pulses (0.8-1.7 μs) from an experimental 527 nm frequency-doubled Nd:YLF laser. It has shown favorable clinical outcomes with RPE-related retinal disorders including active CSC [37–39]. However, the quasi-adiabatic heating interaction causes bubble formation or micro-explosions, disrupting the outer blood-retinal barrier. Fluorescein angiography immediately after the treatment of proper intensity shows the presence of windows defect at the site of the laser treatment. Since there are no publications showing the fluorescein angiography of such treatments after 12 or 18 months from the treatment with SRT, the late development of RPE atrophy cannot be excluded.
It has been proposed that ICG–mediated photo-thrombosis (IMP) selectively occludes choroidal vessels, with minimal damage to the overlying neurosensory retina. In a case series, IMP produced favourable results in patients with persistent CSC, but adverse events, such as mild and transient retinal whitening and retinal capillaries occlusion, occurred in three out of the eleven patients [40]. The severe retinal damage reported in the case of persistent CSC treated with IMP prompted the warning for potential severe adverse events [41].
Subthreshold diode-laser micropulse (SDM) is a photocoagulation technique in which the laser emission is “chopped” in a train of short laser pulses whose “ON” time and inter-pulse “OFF” time are adjustable by the surgeon to control both the intensity and the spread of the heat induced at the absorbing chromophores. This fine control of laser photothermal effects may be of limited value for treatments seeking a visible burn endpoint, but it is very important to safely and consistently perform effective retina-sparing treatments with no intraoperative visible endpoint and, in most cases, with no ophthalmoscopic or angiographic signs of laser lesions at any time [42]. SDM photocoagulation in the treatment of CSC was reported with encouraging results and lack of complications by Bandello in 2003 (Micropulse diode laser treatment of idiopatic central serous chorioretinopathy. A pilot study. Invest Ophthal Vis Sci 44:ARVO E-Abstract 4858) and by Lanzetta at the Macula Society 2004 annual meeting. Both authors indicated that the lack of visible endpoint is somehow challenging for the surgeon.
SDM photocoagulation of ICG-stained RPE cells at the active leakage site is a protocol for the treatment of CSC devised for the purpose of enhancing RPE-selectivity, neurosensory retina sparing and of documenting the otherwise invisible laser applications. The following technical considerations and theoretical advantages guided the design of our protocol:
-
ICG bound to plasma proteins has an absorption peak at 805 to 810 nm, which perfectly matches the 810 nm diode laser emission and enhances its selective absorption by ICG stained RPE cells [43].
-
ICG dye accumulates at higher concentration at the ALS (Fig. 4c,h) and enter more readily RPE cells with damaged membrane as shown by a marked hyperfluorescence in in vitro experiments [44].
-
The 810 nm laser is mostly absorbed by the melanin and by the ICG dye in the RPE cells, and negligibly by hemoglobin and other retinal chromophores. This enhances the selectivity of induced photothermal effects at the RPE, and minimizes the risk of thermal injury to the overlying neurosensory retina and blood vessels [45].
-
The use of repetitive micropulses at low-duty cycle and repetition rate minimizes the axial thermal spread from the RPE toward the adjacent structures [46]. The relatively long laser applications (50 s) with fifty sequential trains of micropulses with 500-ms duration and 500-ms pause, is specifically intended to induce a stronger thermo-tolerance and thermo-resistance to heat damage of the neurosensory retina and to allow more consistent and predictable sub-threshold treatments [47].
The rationale for applying a high number of repetitive pulses is based on the N-1/4 law: “A train with N repetitive pulses of duration t has the same effect as a single pulse with the same duration t if each of the laser pulses has a peak power that is only N -1/4 times the power of the single pulse”. Thus, the higher is the number of pulses N, the lower is the power (and thus the irradiance) that can be used to reach the same effect. A train of 50 pulses has the same effect as one single pulse with only 40% of the irradiance per pulse. With 50 trains of 250 pulses, each application site receives 12,500 pulses, and this permits the use of only 11% of the irradiance that would be needed if the same effect were to be reached with a single pulse.
-
Last, but not least, the dark spots resulting from the quenching of ICG fluorescence, which are detected at the post-treatment ICG angiography [27], provides the angiographic documentation of the placement of the subthreshold laser applications (Fig. 4f,i).
In this case series, both resolution and improvement of leakage and fluid occurred after the subthreshold treatment, with no intraoperative retina blanching and no signs of laser-induced RPE alterations detectable on color fundus photography or on fluorescein angiography at any time postoperatively. The dark hypo-fluorescent spots on postoperative IR-angiography without new ICG injection provided the evidence of the laser placements. The absence of retinal blanching confirms the ability of this treatment to spare the neurosensory retina by axially confining the laser photothermal effects at the RPE, as reported in experimental works: (Chong LP, Kohen L, Kelsoe W, Donovan M, Buzawa D (1992) Selective RPE damage by micro-pulse diode laser photocoagulation. Invest Ophthalmol Vis Sci 33(suppl):722. [Abstract 150]; Chong LP, Kohen L (1993) A retinal laser which damages only the RPE: ultrastructural study. Invest Ophthalmol Vis Sci 34(suppl):960. [Abstract 1270]).
The clinical resolution in the absence of detectable signs of laser-induced lesions suggests that retinal damage is not a prerequisite to the successful treatment of CSC. The mechanism of action of IGG-enhanced SDM photocoagulation in chronic CSC is not clearly understood. Theoretically, the clinical resolution could be the result of a cellular cascade initiated by laser-induced sub-lethal cytotoxic effects to the RPE cells. Photo-thermal and photo-dynamic oxidative stress to the RPE cells could be the laser-induced triggering factors [46]. With thermal lasers, the effect in endogenous absorbing chromophores is normally photothermal in nature. In this case, however, since the melanin-laden RPE cells are also stained with ICG dye (which is an absorbing chromophore, a fluorophore and a photosensitizing agent) the micropulse 810 nm laser applications can produce concomitant ICG-enhanced photothermal effects and photodynamic oxidative stress.
The potential of ICG as a photodynamic photosensitizer has been investigated in different studies [48–52]. Therefore, it is not unreasonable to postulate that the exposure to micropulse 810 nm laser radiation of pathologic ICG-stained RPE cells around the ALS produces an RPE-selective combined photo-oxidative stress that activates a cellular cascade, leading to the beneficial therapeutic effect of the treatment.
This hypothesis is supported by a recent work demonstrating that ICG light-induced decomposition significantly reduced the viability of cultured RPE cells [53].
The pathogenesis of CSC and the mechanism of action of laser photocoagulation are both poorly understood. Two models have been proposed to explain the neuroepithelial detachment in CSC:
-
a primary defect of the RPE cells, resulting from immune, neuro-endocrine, or infective processes, which causes an inversion of transport polarity at the active leakage site and the neuro-epithelial detachment [54, 55].
-
a secondary defect of the RPE cells [56, 57] due to localized choroidal vascular changes which alter the natural permeability of the choriocapillaries, leading to leakage and pooling of fluid beneath the RPE cell layer with stretching of the overlying RPE cells. This would compromise the tight junctions, creating a focal defect of the outer blood retinal barrier (BRB), which constitutes the active leakage site that allows the fluid to enter the subretinal space.
The mechanism of action of SDM photocoagulation over ICG-laden RPE cells could be theoretically explained as a combined thermal and chemical photo-oxidative stress, selective to the targeted RPE cells, which would trigger in response a cellular cascade capable of inducing renovation of pathologic cells in the RPE without causing damage to the neighboring retinal layers. New RPE cells with healthy tight junctions would close the leakage site, restore the integrity of the outer BRB [58], and prompt the resorption of fluid and the resolution of the neuro-epithelial detachment.
Conclusions
These preliminary results suggest that ICG-enhanced subthreshold diode-laser micropulse (SMD) photocoagulation can be a safe and reasonable approach for the management of chronic CSC with persistent serous neuroepithelial detachment.
Despite the several limitations of this report, the absence of complications suggests that ICG-enhanced SDM photocoagulation could be considered for the earlier treatment of RPE-related macular disorders that normally respond to conventional thermal laser photocoagulation, and that could benefit from a less damaging and more RPE-selective subthreshold treatment protocol [59–60]. ICG angiography taken after the treatment without new dye injection allows checking and documenting the actual placement of the sub-visible-threshold laser applications.
References
Gass JDM (1997) Stereoscopic atlas of macular diseases: diagnosis and treatment, 4th edn. Mosby, St. Louis, pp 52–70
Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke J (2008) The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology 115(1):169–173
Yannuzzi LA (1987) Type-A behaviour and central serous chorioretinopathy. Retina 7:111–131, doi:10.1097/00006982-198700720-00009
Friberg TR, EIler AW (1990) Serous retinal detachment resembling central serous chorioretinopathy following organ transplantation. Graefes Arch Clin Exp Ophthalmol 228:305–309, doi:10.1007/BF00920052
Gass JDM, Slamovits TL, Fuller DW, Gieser RG, Lean JS (1992) Posterior chorioretinopathy and retinal detachment after organ transplantation. Arch Ophthalmol 110:1717–1722
Cheng LL, Kwok AK, Wat NM, Neoh EL, Jon HC, Lam DS (2002) Graft-vs-host-disease-associated conjunctival chemosis and central serous chorioretinopathy after bone marrow transplant. Am J Ophthalmol 134(2):293–295, doi:10.1016/S0002-9394(02)01464-2
Gass JDM, Little H (1995) Bilateral bullous exudative retinal detachment complicating idiopathic central serous chorioretinopathy during systemic corticosteroid therapy. Ophthalmology 102:737–747
Haimovici R, Gragoudas ES, Duker JS et al (1997) Central serous chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology 104:1653–1660
Eckstein MB, Spalton DJ, Holder G (1993) Visual loss from central serous retinopathy in systemic lupus erythematosus. Br J Ophthalmol 77:607–609
Cunningham ET Jr, Alfred PR, Irvine AR (1996) Central serous chorioretinopathy in patients with systemic lupus erythematosus. Ophthalmology 103:2081–2090
Bouzas EA, Scott MH, Mastorakos G et al (1993) Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol 111:1229–1233
Tittl MK, Spaide RF, Wong D, Pilotto E et al (1999) Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol 128:63–68, doi:10.1016/S0002-9394(99)00075-6
Gass JDM (1967) Pathogenesis of disciform detachment of the neuroepithelium. I. General concepts and classification. Am J Ophthalmol 63:573–585
Gass JDM (1967) Pathogenesis of disciform detachment of the neuroepithelium. II. Idiopathic central serous choroidopathy. Am J Ophthalmol 63:587–615
Robertson DM, Ilstrup D (1983) Direct, indirect and sham laser photocoagulation in the management of central serous chorioretinopathy. Am J Ophthalmol 95:457–466
Ficker L, Vafidis G, While A, Leaver P (1988) Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol 72:829–834, doi:10.1136/bjo.72.11.829
Watzke RC, Burton TC, Woolson RF (1979) Direct and indirect laser photocoagulation of central serous choroidopathy. Am J Ophthalmol 88:914–918
Khosla PK, Rana SS, Tewari HK, Azad RU, Talwar D (1997) Evaluation of visual function following argon laser photocoagulation in central serous retinopathy. Ophthalmic Surg Lasers 28:693–697
Robertson DM (1986) Argon laser photocoagulation treatment in central serous chorioretinopathy. Ophthalmology 93:972–974
Shatz H, Yannuzzi LA, Fitter KA (1977) Subretinal neovascularization following argon laser photocoagulation treatment for central serous chorioretinopathy: complication or misdiagnosis? Trans Am Acad Ophthalmol Otolaryngol 83:893–906
Folk JC, Pulido JS (1997) Ophthalmology Monographs N. 11 - Laser photocoagulation of the retina and the choroid. Chapter 7: Miscellaneous Chorioretinal Disorders. American Academy of Ophthalmology, San Francisco, pp 239-240
Wang M, Munch IC, Hasler PW, Prünte C, Larsen M Central serous chorioretinopathy. Acta Ophthalmol Scand. OnlineEarly Articles. doi:10.1111/j.1600-0420.2007.00889.x
Friberg TR, Karatza EC (1997) The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology 104:2030–2038
Moorman CM, Hamilton AM (1999) Clinical applications of the MicroPulse diode laser. Eye 13:145–150
Stanga PE, Reck AC, Hamilton AM (1999) Micropulse laser in the treatment of diabetic macular edema. Semin Ophthalmol 14:210–213, doi:10.3109/08820539909069539
Olk RJ, Friberg TR, Stickney KL, Akduman L, Wong KL, Chen MC, Levy MH, Garcia CA, Morse LS (1999) Therapeutic benefits of infrared (810-nm) diode laser macular grid photocoagulation in prophylactic treatment of nonexudative age-related macular degeneration: two-year results of a randomized pilot study. Ophthalmology 106(11):2082–2090, doi:10.1016/S0161-6420(99)90487-6
Ricci F, Missiroli F, Cerulli L (2004) Indocyanine green dye-enhanced micropulsed diode laser: a novel approach to subthreshold RPE treatment in a case of central serous chorioretinopathy. Eur J Ophthalmol 14(1):74–82
Chang AA, Morse LS, Handa JT, Morales RB et al (1998) Histologic localization of ICG dye in aging primate and human ocular tissues with clinical angiographic correlation. Ophthalmology 105:1060–1068, doi:10.1016/S0161-6420(98)96008-0
Sliney DH, Mellerio J, Gabel VP et al (2002) What is the meaning of threshold in laser injury experiments? Implications for human exposure limits. Health Phys 82:335–347, doi:10.1097/00004032-200203000-00006
Mainster MA, Turner PL (2004) Retinal injuries from light: mechanisms, hazards, and preventions. In: Ryan SJ, Ogden TE, Hinton DR, Schachat AP (eds) Retina. Elsevier Publishers, London, p 2
Klein ML, Van Buskirk EM, Friedman E, Gragoudas E, Chandra S (1974) Experience with nontreatment of central serous choroidopathy. Arch Ophthalmol 91:247–250
Brancato R, Scialdone A, Pece A, Coscas G, Binaghi M (1987) Eight-year follow-up of central serous chorioretinopathy with and without laser treatment. Graefes Arch Clin Exp Ophthalmol 225:166–168, doi:10.1007/BF02175443
Montero JA, Ruiz-Moreno JM (2005) Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br J Ophthalmol 89:562–564, doi:10.1136/bjo.2004.049403
Hirami Y, Tsujikawa A, Sasahara M et al (2007) Alterations of retinal pigment epithelium in central serous chorioretinopathy. Clin Exp Ophthalmol 35:225–230, doi:10.1111/j.1442-9071.2006.01447.x
Mitarai K, Gomi F, Tano Y (2006) Three-dimensional optical coherence tomographic findings in central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 244:1415–1420, doi:10.1007/s00417-006-0277-7
Loo RH, Scott IU, Flynn HW Jr, Gass JD, Murray TG, Lewis ML, Rosenfeld PJ, Smiddy WE (2002) Factors associated with reduced visual acuity during long-term follow-up of patients with idiopathic central serous chorioretinopathy. Retina 22:19–24, doi:10.1097/00006982-200202000-00004
Framme C, Brinkmann R, Birngruber R, Roider J (2002) Autofluorescence imaging after selective RPE laser treatment in macular diseases and clinical outcome: a pilot study. Br J Ophthalmol 86:1099–1106, doi:10.1136/bjo.86.10.1099
Roider J, Brinkman R, Wirbelauer C, Laqua H et al (1999) Retinal sparing by selective retinal pigment epithelial photocoagulation. Arch Ophthalmol 117:1028–1034
Elsner H, Pörksen E, Klatt C, Bunse A, Theisen-Kunde D, Brinkmann R, Birngruber R, Laqua H, Roider J (2006) Selective retina therapy in patients with central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 244(12):1638–1645
Costa RA, Scapucin L, Moraes NS, Calucci D et al (2002) Indocyanine green-mediated photothrombosis as a new technique of treatment for persistent central serous chorioretinopathy. Curr Eye Res 25:287–297, doi:10.1076/ceyr.25.5.287.13496
Penha FM, Aggio FB, Bonomo PP (2007) Severe retinal thermal injury after indocyanine green-mediated photothrombosis for central serous chorioretinopathy. Am J Ophthalmol 143(5):887–889, doi:10.1016/j.ajo.2006.12.008
Siviprasad S, Sandhu R, Tandon A, Sayed-Ahmed K, McHugh DA (2007) Subthreshold micropulse diode laser photocoagulation for clinically significant diabetic macular oedema: a three-year follow-up. Clin Experiment Ophthalmol 35(7):640–644, doi:10.1111/j.1442-9071.2007.01566.x
Desmetre T, Deivoselle JM, Mordon S (2000) Fluorescence properties and metabolic features of ICG as related to angiography. Surv Ophthalmol 45:15–27, doi:10.1016/S0039-6257(00)00123-5
Chang AA, Zhu M, Billson F (2005) The interaction of indocyanine green with human retinal pigment epithelium. Invest Ophthalmol Vis Sci 46(4):1463–1467, doi:10.1167/iovs.04-0825
Mainster MA (1986) Wavelenght selection in macular photocoagulation. Ophthalmology 93:952–958
Berger JW (1997) Thermal modelling of micropulsed diode laser retinal photocoagulation. Lasers Surg Med 20:409–415, doi:10.1002/(SICI)1096-9101(1997)20:4<409::AID-LSM6>3.0.CO;2-U
Mainster MA, Reichel E (2000) Transpupillary thermotherapy for age-related macular degeneration: long-pulse photocoagulation, apoptosis, and heat shock proteins. Ophthalmic Surg Lasers 31:359–373
Ricci F, Pucci S, Sesti F, Missiroli F et al (2007) Modulation of Ku70/80, clusterin/ApoJ isoforms and bax expression in indocyanine-green-mediated photo-oxidative cell damage. Ophthalmic Res 39:164–173, doi:10.1159/000103236
Abels C, Fickweiler S, Weiderer P, Baumler W et al (2000) Indocyanine green (ICG) and laser irradiation induce photoo xidation. Arch Dermatol Res 292:404–411, doi:10.1007/s004030000147
Baumler W, Abels C, Karrer S et al (1999) Photo-oxidative killing of human colonic cancer cells using indocyanine green and infrared light. Br J Cancer 80:360–363, doi:10.1038/sj.bjc.6690363
Costa RA, Farah ME, Cardillo JA, Belfort R Jr (2001) Photodynamic therapy with indocyanine green for occult subfoveal choroidal neovascularization caused by age-related macular degeneration. Curr Eye Res 23:271–275, doi:10.1076/ceyr.23.4.271.5449
Farah ME, Cardillo JA, Luzardo AC, Calucci D, Williams GA, Costa RA (2004) Indocyanine green mediated photothrombosis for the management of predominantly classic choroidal neovascularisation caused by age related macular degeneration. Br J Ophthalmol 88(8):1055–1059, doi:10.1136/bjo.2003.035808
Engel E, Schraml R, Maisch T, Kobuch K et al (2008) Light-induced decomposition of indocyanine green. Invest Ophthalmol Vis Sci 49(5):1777–1783, doi:10.1167/iovs.07-0911
Spitznas M (1986) Pathogenesis of central serous retinopathy: a new working hypothesis. Graefes Arch Clin Exp Ophthalmol 224:321–324, doi:10.1007/BF02150023
Spitznas M (1989) Central serous retinopathy. In: Ryan SJ (ed) Retina. vol. 2. CV Mosby, St. Louis, pp 217–227
Marmor MF (1988) New hypotheses on the pathogenesis and treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol 226:548–552, doi:10.1007/BF02169203
Prunte C, Flammer J (1996) Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol 121:26–34
Negi A, Marmor MF (1984) Healing of photocoagulation lesions affects the rate of subretinal fluid resorption. Ophthalmology 91:1678–1683
Luttrull JK, Musch DC, Mainster MA (2005) Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol 89:74–80, doi:10.1136/bjo.2004.051540
Laursen ML, Moeller F, Sander B, Sjoelie AK (2004) Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol 88:1173–1179, doi:10.1136/bjo.2003.040949
Financial Support
None.
Conflict of Interest
G. Dorin is the Director of Clinical Applications Development at Iridex Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ricci, F., Missiroli, F., Regine, F. et al. Indocyanine green enhanced subthreshold diode-laser micropulse photocoagulation treatment of chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 247, 597–607 (2009). https://doi.org/10.1007/s00417-008-1014-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-1014-1