Abstract
Purpose
To check differences in visual function between Wistar (albino) and Long-Evans (pigmented) rats.
Methods
The animals were born in our facilities and reared under identical light conditions avoiding bright light. Visual electrophysiology was performed at the ages of 1.5, 4, 7 and 10 months (electroretinography, ERG) and at 1.5 and 7 months (visual evoked potentials, VEP).
Results
ERG measurements showed that: 1) The amplitudes of both scotopic and photopic b-waves were markedly larger in Long-Evans rats than in Wistar rats, and also the amplitudes of scotopic oscillatory potentials and photopic 30 Hz Flicker amplitudes, 2) scotopic a-wave amplitudes were larger in Wistar rats at low light intensities, whereas they were smaller in bright light, 3) both a-wave and b-wave latencies were shorter in Wistar rats, 4) the maximum response RmP3 was larger in Long-Evans rats, 5) the sensitivity parameter S was larger in Wistar rats, and 6) the post-receptoral response of cones was smaller in Wistar rats. In the VEP measurements, amplitudes of both photopic and scotopic visual evoked potentials of Long-Evans rats were only slightly larger than those of Wistar rats.
Conclusions
ERG b-wave amplitudes are markedly decreased in Wistar rats, which requires further investigation. As the b/a and OP/a ratios were also decreased in Wistar rats, it can be suggested that post-receptoral processing, in particular, is impaired in albino animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inherited albino defects result in two major visual pathway abnormalities, namely foveal hypoplasia in primates and misrouted optic nerve fibres. Whereas a lot of work has been done on studying the misrouting of the optic nerve fibres by measuring visual evoked potentials and, more recently, through functional magnetic resonance imaging (fMRI) [50], little is known about the influence of pigmentation on retinal function itself. The electroretinographic studies so far performed to compare pigmented with albino animals have not yet given an unequivocal picture. It seems that melanin-related changes in visual physiology vary from species to species and even from strain to strain. Moreover, the experimental conditions often differ between the several studies, which makes comparison of the results difficult.
Taking into account reports about a decreased number of rod photoreceptors in albino animals (see, e.g., [11]), it was of interest to know if such a decrease would be visible in a reduced a-wave amplitude. As a consequence of the reduced number of rods, an impaired post-receptoral response would also be expected. On the other hand, there are several reports that ERG amplitudes are larger in albino humans [44, 51–53]. Therefore, the purpose of this study was to compare the single aspects of retinal function of two closely related strains of pigmented and albino rats by comparing several ERG parameters.
In our study, we used Wistar and Long-Evans rats. These two strains are suitable for comparing albino and pigmented animals, as they have a common origin. Compared to the Long-Evans rat, the Wistar rat has a nucleotide exchange in exon 2 in the Tyr (tyrosinase) gene, causing an amino acid alteration Arg299His [8]. This mutation has already been described in humans affected by type 1 oculocutaneous albinism without any tyrosinase activity and, consequently, complete lack of pigmentation [19].
We performed the ERG measurements on the animals at various ages to check whether possible differences in the parameters did increase or decrease due to ageing. Moreover, visual flash-evoked potentials were measured after Ganzfeld stimulation, in order to check whether a possible alteration of retinal response would have an effect on the VEP, or if cortical response to light stimuli would differ in the case of a similar retinal function.
Materials and methods
Animals
Wistar and Long-Evans rats were used in this study as models for albino and pigmented animals respectively. Pregnant females of both strains were purchased from the Charles River Laboratories, Germany. The animals used in the experiments were born and reared in our facilities under identical light conditions (12 h/12 h cycles of dim light/darkness) and fed ad libitum.
Visual electrophysiology
General procedure
Animals were dark adapted over a period of at least 24 hours. The cornea of the eyes of the anaesthetised animals was de-sensitised with a drop of Novesine (Novartis Ophthalmics). The upper eyelids were retracted slightly by a surgical silk thread. The animals were placed onto a heated platform (37°C) during the measurements to keep their body temperature constant. A gold wire ring electrode was placed into the mouth to serve as a reference electrode. A stainless steel needle electrode was inserted into the tail of the animals for grounding. The pupils were dilated with a drop of Tropicamide (Novartis Ophthalmics). All these manipulations were performed under dim red light, without bringing the animal into ambient light after dark adaptation. The red light was switched off after finishing all the stages of animal preparation. After an additional 5 minutes to allow the pupils to dilate, measurement was started using the commercial RetiPort32 device from Roland Consult Systems (Brandenburg, Germany).
Electroretinography
For the ERG measurements, animals were used from two different litters of both Long-Evans and Wistar rats. The series of measurements was started at the age of 1.5 months with five animals of each strain, and the same animals were used repeatedly for the following measurements at the ages of 4, 7 and 10 months. In total, three animals died as a consequence of anaesthesia accidents during this time. Therefore, five, four and three Long-Evans rats and five, four and four Wistar rats were used at the ages of 4, 7 and 10 months respectively for the ERG measurements.
Animals were anaesthetised by an intraperitoneal injection of a mixture of ketamine and xylazine (120 mg/kg ketamine, 10 mg/kg xylazine). Gold wire ring electrodes placed on the corneas of both eyes served as working electrodes. Standard electroretinographic measurements were performed simultaneously on both eyes, with scotopic flash ERG at five different light intensities (in stages of 10 dB from 0.0003 to 3 cd s/m2), an additional run for scotopic oscillatory potentials, photopic 30 Hz Flicker after 10 minutes of light adaptation, photopic flash ERG, and photopic oscillatory potentials. The light intensity used for the flashes in the photopic ERG measurements was 3 cd s/m2. The time of measurement was 200 ms for scotopic ERG and 160 ms for the other techniques, always 512 data points per measured waveform. The analogue filters of the ERG device were set to the frequency ranges of 0.5 to 200 Hz for both scotopic and photopic flash ERG, 50 to 500 Hz for oscillatory potentials and 10 to 50 Hz for 30 Hz Flicker. In addition, the waveforms of the oscillatory potentials were digitally filtered offline using a DSP filter included in the software of the ERG device (−15 dB for f < 10 Hz). At the age of 10 months, before the animals were sacrificed for histology, additional flash light intensities of up to 100 cd s/m2 were applied in scotopic ERG measurements which, to minimise light damage to the photoreceptors, had not been used beforehand. Amplitudes of a-waves were measured from the baseline to the bottom of the a-wave trough, whereas b-wave amplitudes were measured from the bottom of the a-wave trough to the peak of the b-wave. As a second option, b-wave amplitudes were measured from the baseline to the b-wave peak. Data obtained from both eyes were used and averaged with the data obtained from the other animals of the corresponding group. Parameters of photoreceptor response were computed by using the well-known relationship by Hood & Birch [27], based on the theory by Lamb & Pugh [35]:
with i — stimulus intensity, t — time after flash onset, P3 — sum of individual photoreceptor responses, RmP3 — saturated response, td — delay that allows for biochemical and other recording latencies, S — sensitivity parameter.
Values of a- and b-wave amplitudes were approximated using the equation
introduced (without n) by Naka & Rushton [40], with R — response amplitude, Rmax — maximal response amplitude, i — light intensity, i50 — half-saturating light intensity, n — an exponent describing the slope of the function.
The parameters for the equations (1) and (2) were computed from the data obtained from 10-month-old rats using self-written non-linear least-square routines in Borland Pascal. In order to avoid local minima in the course of the calculations, the fitting procedure was performed with several sets of starting values for the parameters. However, we never obtained different final parameter values as results of the fitting procedure. We performed individual fitting by using the data of each single eye, and averaged the values of the parameters obtained this way. The range of the ERG waveform from the beginning of the a-wave leading edge to the first intrusion of the b-wave was used for the calculation, yielding between 10 and 30 data points. The RmP3 value obtained for each animal at the highest light intensity was used for the calculation of the sensitivity parameters at the lower light intensities [see 9, 27]. The parameter td was left free to obtain the best fit. It did not vary all that much, and was always in the range between 2.7 and 5 seconds. All three parameters of the Naka-Rushton equation (2) were left free during the calculation. Eight data points were used for the fit, according to the eight different light intensities used for stimulation.
Dark adaptation
As a first step, a single-flash (i.e., no averaging) scotopic electroretinogram was recorded in dark-adapted animals at 1 cd s/m2 to obtain base-line values for the parameters. The animals were then exposed to bright light (300 cd/m2) for 10 min including bright flashes of 100 cd s/m2 each 30 seconds in order to bleach the photoreceptors completely. The light was then switched off, and scotopic electroretinograms were recorded during the subsequent dark period, each for 10 min. In order to avoid repeated bleaching of the photoreceptors by the light flashes applied for the measurement, only one flash was used per time point, and the light intensity of all flashes was reset to 1 cd s/m2. The measurements started with seven animals of each strain and were continued until the animals woke up from anaesthesia, in most cases over a period of 70 min (see Results).
Visual evoked potentials
For the VEP measurements, animals were used from two different litters of both Long-Evans and Wistar rats. The first measurements were performed at the age of 1.5 months with five animals of each strain, and the same animals were used at the age of 7 months for the second measurement.
Animals were anaesthetised with an intraperitoneal injection of a solution of chloral hydrate in physiologic saline (0.42 mg chloral hydrate/kg). A stainless-steel screw with a shaft diameter of 1.17 mm (Fine Science Tools, Heidelberg, Germany) served as a measuring electrode and was inserted more than 1 week before the first measurement through the skull into the left visual cortex (2.5 mm laterally to the midline, 2 mm anterior to the lambda), penetrating the cortex to approximately 0.5 mm. For the actual measurement, the skin was opened; the screw was cleaned of connecting tissue and blood, and connected to the amplifier. Both eyes were stimulated for the measurement. Visual evoked potentials were recorded, with scotopic flash VEP at five different light intensities and photopic flash VEP after 10 minutes of light adaptation. The maximum light intensity used for the flashes was 3 cd s/m2. The time of measurement was 160 ms, sample rate 3.2 kHz, and frequency range of 0.5 to 200 Hz. After finishing the measurements, the wound was stitched and antibiotic ointment applied.
The averaged signals of each group were compared with respect to both latency and amplitude.
Statistics
Evaluation of the data was performed by comparing separately the means of each parameter obtained in Long-Evans rats and Wistar rats, using the Student’s t-test.
Results
Electroretinography
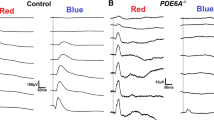
Normal electroretinograms could be recorded from both strains at each age investigated within this study. The typical appearance of the waveforms obtained in both Long-Evans and Wistar rats is shown in Fig. 1.
It is clearly visible that the amplitudes of both scotopic and photopic b-waves, as well as scotopic and photopic oscillatory potentials and photopic 30 Hz Flicker ERG are considerably larger in Long-Evans rats. In contrast, the amplitudes of scotopic a-waves appear to be similar in both long-Evans and Wistar rats, and no great differences in latencies can be seen. In photopic ERG, the so-called “photopic negative response” appears to be missing in Wistar rats.
The parameters of electroretinographic responses were analysed in further detail. A comparison of the amplitudes of scotopic a-wave and b-wave amplitudes at different ages is shown in Fig. 2. The first impression from Fig. 1, that the a-wave amplitudes are quite similar, cannot be confirmed (Fig. 2a). The a-wave amplitudes of Wistar rats are larger at low light intensities, which is even significant at 0.03 cd s/m2. At a light intensity of 0.3 cd s/m2, where cones start to contribute to the ERG response, the a-wave amplitudes of both strains do not show any appreciable difference. The a-wave amplitudes of Long-Evans become significantly larger than those of Wistar rats at light intensities of 3 cd s/m2 or higher.
Comparison of scotopic a-wave amplitudes (a) and scotopic b-wave amplitudes (b, c) between Long-Evans and Wistar rats at different ages of the animals. The shaded areas mark the range of light intensities where cones also contribute to the electroretinographic response. The curves serve as guide to the eye and do not represent a model. In this and all following diagrams, the significance of the differences between the amplitudes obtained in Long-Evans and Wistar rats is given by asterisks as follows: * p ≤ 0.05, ** p ≤ 0.01, and *** p ≤ 0.001
The scotopic b-wave amplitudes are significantly larger in Long-Evans rats than in Wistar rats over the whole range of light intensities (Fig. 2b). In both strains, there is a certain “dent” in the amplitude-light intensity curve in the range of the transition from pure rod response to mixed cone-rod response. The shapes of the dependency of b-wave amplitudes on light intensity change if b-wave amplitudes are calculated from the base line instead of the trough of the a-wave (Fig. 2c). Whereas the calculated b-wave amplitudes of Long-Evans rats increased with increasing light intensity, the b-wave amplitudes of Wistar rats showed no noticeable increase at light intensities above 0.03 cd s/m2.
As expected, the latencies of both a-waves and b-waves decrease with increasing light intensity (Fig. 3). Whereas a-wave latencies decrease continuously with increasing light intensity, b-wave latencies decrease until approximately 0.03 cd s/m2, and remain more or less constant at higher light intensities. Latencies measured in Wistar rats are shorter than those obtained in Long-Evans rats at almost all light intensities and at all ages of the animals.
Comparison of scotopic a-wave latencies (upper row) and scotopic b-wave latencies (lower row) between Long-Evans and Wistar rats at different ages of the animals. The shaded areas mark the range of light intensities where cones also contribute to the electroretinographic response. Asterisks denote statistical significance as described in Fig. 2. The curves serve as a guide to the eye and do not represent a model
The scotopic oscillatory index of amplitudes was calculated by summing up the amplitude values of the first four oscillations. As can be seen in Fig. 1, the a-wave is still present in the waveforms of the oscillatory potentials. However, we did not use more intense filtering of the data, in order to protect the oscillations from being affected by the filtering. Instead, we used the point where the first oscillation became visible to obtain its amplitude. The Wistar rats showed significantly lower amplitudes of oscillatory potentials than the Long-Evans rats (Fig. 4). The larger amplitudes obtained in Long-Evans rats are particularly obvious if both photopic b-wave amplitudes and photopic 30 Hz Flicker amplitudes are compared (Fig. 4).
Comparison of values of the scotopic oscillatory index (top), the photopic b-wave amplitudes (middle) and the photopic 30 Hz Flicker amplitudes (bottom) between Long-Evans and Wistar rats. Asterisks denote statistical significance as described in Fig. 2
Moreover, amplitudes of photopic oscillatory potentials in Long-Evans rats were approximately twice as large as in Wistar rats (not shown).
As a general observation, all amplitudes shown in Figs. 2 and 4 decrease slightly with increasing age of the animals.
The ratio of b-wave and a-wave amplitudes (shortly known as the b/a ratio) is an indication of the relation of the response of post-receptoral systems to the photoreceptor response by which it is elicited. The b/a ratios are shown in Fig. 5 for both strains used in this study. Moreover, the ratio of oscillatory potential amplitudes to a-wave amplitudes, denoted as OP/a, is shown in Fig. 5. The b/a ratio of Long-Evans rats increases with the age of the animals, whereas the b/a ratio of Wistar rats remains relatively stable. At all ages, the b/a ratios are significantly larger in Long-Evans rats than in Wistar rats. The OP/a ratios in Long-Evans rats also show an age-dependent increase, and they are again larger than in Wistar rats.
Comparison of values of the ratio of b-wave and a-wave amplitudes between Long-Evans and Wistar rats. On the right, the values of the amplitude ratio of oscillatory potentials and a-waves are shown. Data were obtained at a light intensity of 100 cd s/m2. Asterisks denote statistical significance as described in Fig. 2
The parameters of a-waves were calculated according to equation (1), and the resulting curves are shown in Fig. 6. The values of maximum response RmP3 were −681 ± 86 µV for Long-Evans rats and −493 ± 24 µV for Wistar rats, which is a significant difference (p = 0.04). As can be seen in Fig. 6, the non-linear fit approximated the waveforms well (R2 was better than 0.99 in most cases).
Measured ERG data points of scotopic a-waves obtained in Long-Evans rats (left) and Wistar rats (right) at light intensities of 100, 30, 10, 3, 1 and 0.3 cd s/m2, and calculated curves using equation (1)
Calculation of the sensitivity parameter S gave different values depending on the light intensity. For light intensities from 100 to 0.03 cd s/m2, S ranged from 0.0017 to 0.0064 cd−1s−1 in Long-Evans rats and from 0.0027 to 0.0170 cd−1s−1 in Wistar rats. Figure 7 shows a double-logarithmic plot of S vs the light intensity of stimulation. It can be seen that log S decreases proportionally with increasing light intensity to the logarithm of the latter (linear fit yields R2 = 0.9028 in Long-Evans rats and R2 = 0.8189 in Wistar rats). In contrast to RmP3 values, sensitivity parameters were smaller in Long-Evans rats than in Wistar rats, with high significance at all light intensities at which the calculations was performed. Moreover, the decrease of S at higher light intensity was more pronounced in Wistar rats. Whereas the difference between two neighbouring data points of S was significant in only two out of five cases in Long-Evans rats, significance was present in four out of five cases in Wistar rats.
Double-logarithmic plot of the sensitivity parameters S of Long-Evans and Wistar rats vs the light intensity of stimulation. Asterisks denote statistical significance as described in Fig. 2
Application of the Naka-Rushton equation (2) on a-wave and b-wave amplitudes at different light intensities resulted in values for the maximal amplitudes Rmax, i50 and n given in Fig. 8. It is obvious that Rmax is significantly larger for Long-Evans rats for both a-waves and b-waves. Whereas there is no significant difference in the i50 values for b-waves, i50 for a-waves is significantly smaller in Wistar rats. The calculated intensity–response functions for both rat strains are shown in Fig. 8.
Diagrams showing averaged a-wave and b-wave amplitudes of Long-Evans and Wistar rats and calculated intensity-response functions according to equation (2). Below, the calculated values of the Naka-Rushton parameters are shown
Using the calculated values for maximum a-wave and b-wave responses Rmax of Long-Evans and Wistar rats, saturation plots were created for both the photoreceptor and post-receptoral response (Fig. 9). The Wistar rats reach their maximum photoreceptor response at lower light intensities than the Long-Evans rats, with a light intensity difference of almost 1 log unit, which is in line with the significant difference observed in i50 values for a-waves. In contrast, the saturation behaviour of the b-wave amplitudes appears to be similar in both strains.
Saturation plots of a-wave and b-wave amplitudes using calculated parameters for maximum responses Ra-max and Rb-max for a-wave and b-wave amplitudes. Asterisks denote statistical significance as described in Fig. 2. The curves serve as a guide to the eye and do not represent a model
Dark adaptation
ERG responses were examined during a short dark adaptation time after exposure to bright light (Fig. 10). As a first step, an ERG was recorded in completely dark-adapted animals at a light stimulus intensity of 1 cd s/m2 to obtain the base-line response. The animals were then exposed to bright light to bleach the photoreceptors completely. Virtually no response could be detected immediately after switching off the bright light (Fig. 10a, waveforms at 0 min). With an increasing time of darkness, retinal function recovered gradually.
Recovery of scotopic ERG after complete bleaching of photoreceptors. a Waveforms obtained in the animals before bleaching and during recovery in darkness at the time points as indicated. b Time course of recovery of a-wave and b-wave amplitudes in pigmented and albino animals, expressed as a percentage of the base line values obtained in dark-adapted animals before bleaching
One Long-Evans rat woke up after 30 min, and the measurements could be completed with the other six animals after 70 min. Out of the Wistar rats, one woke up after 50 min and another one after 60 min, allowing the measurements to be completed for five animals after 70 min.
Numerical evaluation showed that there was a trend of quicker recovery of a-wave amplitudes in Wistar rats, with statistical significance at several time points (Fig. 10B). The advantage of the Wistar rats was more visible in b-wave amplitudes, with a significant difference at 20 min and with a longer duration of dark adaptation.
Visual evoked potentials
Visual evoked potentials were recorded from the animals at the age of 1.5 and 7 months. The resulting averaged waveforms of the scotopic VEPs at different light intensities, and of the photopic VEPs are shown in Fig. 11. At the age of 1.5 months, the waveform looks quite simple, with just one negative deflection. At 7 months, the rising part of the signal is more disturbed by additional waves, in particular in the Long-Evans rat.
The amplitudes obtained in the Wistar rats seem to be slightly smaller than those measured in Long-Evans rats. Numerical evaluation of the parameters confirmed this trend, as shown in Fig. 12. Whereas there is no real difference between the scotopic VEP amplitudes measured in both strains at low light intensities, there is a clear trend towards larger amplitudes at higher light intensities in Long-Evans rats, although the difference does not reach statistical significance (p is in the range from 0.05 to 0.08 in several cases). Photopic VEP amplitudes measured in Long-Evans rats also show a tendency to be larger than in Wistar rats, again without statistical significance.
The N1 latencies were slightly shorter in Wistar rats than in Long-Evans rats at the age of 1.5 months, although not significantly (not shown). At the age of 7 months, the N1 latencies were practically identical in Wistar and Long-Evans rats.
Discussion
We compared electroretinographic and cortical responses towards flash light stimuli of pigmented Long-Evans rats and albino Wistar rats. This is not the first study where properties of pigmented and albino animals have been compared. However, in contrast to many other studies, we used two rat strains that are genetically closely related to each other, except for the one mutation in the tyrosinase gene mentioned in the introduction. In addition, the animals were not purchased directly from a vendor, where the light conditions during breeding and transportation cannot be controlled. Instead, we purchased pregnant females, and the new-born pups were raised in our facilities under identical controlled light conditions. Lastly, the animals were kept under attenuated light in order to avoid light-associated damage to the photoreceptors of albino Wistar rats as far as possible, and, consequently, to extract differences in visual function as independently of the photoreceptor number as possible.
Electroretinography
The most prominent finding in our study was that the amplitudes originating from the activity of post-receptoral systems were remarkably lower in the Wistar rats. This is already obvious from a simple visual comparison of the waveforms measured, as shown in Fig. 1, where the b-waves, oscillatory potentials and 30 Hz Flicker ERGs of Long-Evans rats show larger amplitudes. The following data analysis confirmed that the scotopic b-wave amplitudes (Fig. 2b,c) as well as the photopic b-wave amplitudes, oscillatory potential amplitudes and 30 Hz Flicker amplitudes (Fig. 4) of the Wistar rat were smaller than in the Long-Evans rat, in most of the cases with a clear significance.
Moreover, we checked the so-called b/a ratio, which is calculated as a quotient of b-wave amplitude and a-wave amplitude. The b/a ratios were significantly smaller in Wistar rats, not only at all four ages investigated in this study (Fig. 5), but also at all light intensities (not shown). Additionally, we introduced another ratio here, the quotient of oscillatory potential amplitudes and a-wave amplitudes (OP/a ratio). The OP/a ratios were again smaller in Wistar rats (Fig. 5), although not so drastically as seen in the b/a ratio. These findings also clearly indicate that, in relation to photoreceptor activity, the post-receptoral systems in Wistar rat retinas produce a smaller response.
This clarity cannot be found in the scotopic a-wave amplitudes. A-waves were hardly visible at low intensities of the light stimulus. The a-waves became clearly visible only at light intensities of 0.03 cd s/m2, and their amplitudes were larger in Wistar rats than in Long-Evans rats. Therefore, the finding that amplitudes of b-waves and oscillatory potentials are smaller in Wistar rats cannot be exclusively attributed to a weaker activity of photoreceptors or to a smaller input from the photoreceptors into the post-receptoral circuitries, if at all.
At higher light intensities, however, the a-wave amplitudes measured in Long-Evans rats exceeded those of the Wistar rats. This could indicate that photoreceptors in Wistar rats can generally be exhausted more quickly at high light intensities, and/or that cones in particular show a weaker response in Wistar rats.
We then computed the parameters RmP3 and S for the photoreceptor responses measured in both Long-Evans and Wistar rats. For such calculations, saturated responses are needed in order to obtain reliable values for RmP3. Therefore we only used the ERG data obtained in 10-month-old rats, because we applied flashes of high intensity here. As could be expected from the lower a-wave amplitudes in Wistar rats at high light intensities, the magnitude of RmP3 is smaller in Wistar rats (−493 µV vs −681 µV in Long-Evans rats).
In contrast to the decrease seen in b-wave and oscillatory potential amplitudes as well as RmP3, the sensitivity parameter S of photoreceptor response was found to be larger in Wistar rats (Fig. 7). The higher sensitivity is also visible in the measured waveforms as a steeper decline of the a-waves, and in the larger n value found in Wistar rats (Fig. 8), the latter being a measure for the slope of the sigmoid Naka-Rushton function. Putting these findings in perspective, the shorter latencies in Wistar rats observed in this study can be interpreted.
Hood & Birch [27] reported that sensitivity parameter S did not depend on light intensity in humans. However, they changed the light intensity over just 1.5 orders of magnitude. Breton et al. [9] reported on changes of S when the light intensity was changed over a wide range (6 orders of magnitude). We compared the values of S at light intensities changing over 3.5 orders of magnitude (Fig. 7), which makes changes of S likely, irrespective of species differences between humans and rats. Moreover, changes in S were particularly pronounced in Wistar rats, whereas the humans examined in [27] most probably were not albinos in the majority of cases.
ERG measurements in guinea pigs showed a different picture. In contrast to our results, scotopic a-wave amplitudes were found to be larger in albino animals over the whole range of stimulus intensities, resulting in higher RmP3 values for albinos [10], and post-receptoral responses were also higher in albino guinea pigs [49]. There are obviously large species differences in the influence of pigmentation on the function and structure of the retinas of rats and guinea pigs. On the other hand, the sensitivity parameter S is larger in albino guinea pigs [10] as well as in albino rats (our study), and latencies are also shorter than in the corresponding pigmented animals.
When normalising a-wave amplitudes to Ra-max, there is a clear shift of the curve obtained in Wistar rats to lower light intensities, which is a sign of a certain hypersensitivity of the photoreceptors in albino rats, as suggested by the increased sensitivity parameter S (Fig. 9). On the other hand, there is no significant difference in the normalisation of b-wave amplitudes to Rb-max (Fig. 9), presumably due to the similarity in I50 of both strains.
In zebra fish with a tyrosinase mutation, it was also mainly b-waves and hence post-receptoral neuronal circuits that were affected [42]. However, a sensitivity shift was found in the normalisation of the b-wave, instead of in the a-wave normalisation as shown in our study. Again, interspecies differences could be the reason for this discrepancy.
The same relationship as in RmP3 values was seen when Rmax was calculated using the Naka-Rushton equation (447 µV vs 599 µV). This decrease down to 72.4% (RmP3) or 74.6% (Rmax) compared to Long-Evans rats raises the question as to whether there are considerably less photoreceptors in the retina of Wistar rats, particularly rods, or if, at the least, the total rod outer segment membrane area is smaller in Wistar rats. The assumption of a reduced number of rods is supported by Donatien & Jeffery [11], who found a decrease of rod number in albino mice down to 75% compared to the black C57Bl mice at an age of 4 months. At first sight, this number is an intriguing match with the above-mentioned difference of the RmP3 or Rmax values. Moreover, Jeffery et al. [32] found a smaller number of rod photoreceptors in albino ferrets, and the same group reported that rhodopsin levels were lower in the retina of albino rodents [21]. Gresh et al. [23] checked rods and cones in C57BL/6 and Balb/c mice at different ages, and found similar numbers in juvenile animals. Age-related changes were found in photoreceptor systems of both strains; however, rods appeared to be more susceptible to age and lack of pigmentation. Analogous observations were reported by Weisse et al. [54], who found a similar age-related decrease in the numbers of photoreceptors and retinal neurones in one albino and two pigmented rat strains in general, and a higher death rate of rods compared to cones in albino rats.
We found a decrease of scotopic a-wave and b-wave amplitudes with increasing age in both Wistar and Long-Evans rats at both 0.03 cd s/m2 (rod-driven response) and 3 cd s/m2 (mixed rod and cone response). The degree of this decrease was almost the same in both Wistar and Long-Evans rats and at both stimulus intensities mentioned above (not shown). As the animals were kept under attenuated light, this similarity indicates the absence of other mechanisms of photoreceptor degeneration in Wistar rats, other than light-induced.
We are currently examining histological retina samples from Wistar and Long-Evans rats. Until now, we could not detect any obvious reduction of the photoreceptor nuclei number in the Wistar rat retina compared to the Long-Evans rat retina (in preparation), which is in contrast to the work cited above. Consequently, other explanations for the reduction of a-wave amplitudes at high light intensities have to be found. Reduced b-wave amplitudes in Wistar rats also require an explanation. In the following, we will discuss several factors that could possibly cause the differences in the retinal responses we observed in this study. Our discussion will also include species other than rats, because albinism is a general phenomenon in the animal kingdom, including humans.
As a first point, the passive electrical properties of the eye could differ between pigmented and albino eyes. Smaller b-wave amplitudes were found in patients with higher fundus pigmentation, which led to the hypothesis that this decrease could be related to a higher ocular resistance caused by melanin [52, 53]. Russell-Eggitt et al. [44] recorded ERGs in ocular and oculocutaneous albino children, and found larger a-wave and b-wave amplitudes and shorter latencies than in normally pigmented controls. The authors explained the increase of amplitudes by more light entering the albino eye via the translucent iris, a greater fundal reflectance and light scatter because of lack of light absorption by the RPE. Larger ERG amplitudes in human albinos were also reported by Wack et al. [51], namely at highest flash luminance, and they explained their findings by a higher transscleral illumination of the retina. However, in our pigmented Long-Evans rats, only a-wave amplitudes at low light intensities are smaller, and all other amplitudes were found to be larger in Long-Evans rats than in Wistar rats. Therefore, we doubt that the electrical or optical properties of melanin could influence the results we obtained in our rats in a relevant way.
Secondly, the composition of the extracellular environment could be a different one, in particular with respect to Ca2+ ion concentration during phototransduction, thus leading to different photoreceptor responses. Dräger [13] reported on considerably enhanced binding of Ca2+ ions in pigmented eyes compared to albino eyes. Ca2+ binding occurs in tissues containing melanin, and melanin is known to bind a variety of bivalent and trivalent metal ions. Therefore, the questions arise whether i) melanisation influences the steady-state concentration of Ca2+ ions in the extracellular space within the retina, and ii) Ca2+ binding to melanin influences the Ca2+ flow in photoreceptors and hence the response of photoreceptors towards light stimuli.
To answer these questions, detailed knowledge of Ca2+ concentration in the different compartments of the back of the eye is needed, preferably in both albino and pigmented animals. Unfortunately, there is only a little information in the literature on this subject. Kaila et al. [33] measured Ca2+ concentration in ex vivo carp and frog eye preparations using calcium-sensitive microelectrodes, and found no differences in the Ca2+ concentration of the extracellular fluid within the retina and in the vitreous. In contrast, Lavallee et al. [37] found clear changes in Ca2+ concentration depending on the depth of the electrode in the retina, measured in vivo in both albino and black mice. Moreover, they found significantly higher Ca2+ levels in the retina of albino animals.
A light-evoked decrease of Ca2+ concentration was found in several studies [17, 37, 39]. One possible explanation could be a light-induced expansion of the extracellular space [28] that would lead to a dilution of Ca2+. More importantly, RPE cells were found to take up Ca2+ [45]. Moreover, Ca2+ uptake was found to be dependent on the degree of pigmentation of the RPE [46], and further studies showed Ca2+ uptake and release by melanosomes (see, e.g., [47]).
Unfortunately, there are still not sufficient data available to judge conclusively about the influence of Ca2+ ions and their relationship to melanin on the ERG. Considering the time of 3–10 seconds of Ca2+ concentration increase during illumination [39] before it decreases, and the relatively slow up-take of Ca2+ by melanosomes (Vmax = 62.5 nmoles/mg protein/min [47]), it is unlikely that melanisation of the eye has a direct effect on photoreceptor response by influencing Ca2+ concentration changes, because photoreceptors respond within the first 5–15 ms after the flash.
At least elevated Ca2+ in the retina of albino animals as postulated in [37] could be an explanation for the decrease of b-wave and oscillatory potential amplitudes we found in Wistar rats. Ca2+ influx into retinal neurones could lead to a partially excited state with permanently decreased membrane potential and availability of neurotransmitters, resulting in a decreased post-receptoral activity observed in this study.
The major difference between these two strains on the biochemical level is the diminished activity of tyrosinase and hence the lack of tyrosinase metabolites ranging from l-DOPA to melanin in Wistar rats. This leads to a possible third reason for smaller retinal response in Wistar rats, namely a deficit in neurotransmitter levels and impaired retinal development.
Blaszczyk et al. [6] found slightly reduced levels of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) in the retina of the Wistar rat compared to the Long-Evans rat, and an enhanced glutamate/GABA ratio in the Wistar rat retina, i.e., a higher portion of the excitatory neurotransmitter glutamate. Thomas et al. [48] speculated that the longer rod-driven latencies in pigmented rats could be a manifestation of the inhibitory GABA system because GABA is presumably involved in rod-driven visual signals [34, 38]. Furthermore, enhanced GABA in Long-Evans rat retinas could also contribute to the higher post-receptoral response found in our study.
There have been several reports in the literature that the attenuated activity of tyrosinase in the Wistar rat leads to some disturbances of retinal development, such as a reduced number and activity of rods, a changed projection of ganglion cell axons into the brain, and even physiologic deficiencies in the visual cortex [3, 14, 30, 31]. Consequently, functional deficits have been described in albinos, both in humans and in animals, such as cats and ferrets [1, 16, 18]. Of particular interest here is that pigmented rats have a better visual acuity, spatial contrast sensitivity and spatial learning than albino rats [5, 24, 43]. In addition, motion perception deficits were found in Wistar rats compared to Long-Evans rats [29, 36]. Although motion-sensitive neurones were found in the primary and secondary area of the visual cortex of the rat [20], lesions of the occipital cerebral cortex, including all or the great majority of the visual areas, had no statistically significant effect on opto-kinetic responses of pigmented rats [25].
Instead, retinal deficits could be responsible for the impaired motion perception described. Except for a slightly reduced number of ganglion cells, no obvious anatomical differences with respect to horizontal, amacrine or ganglion cells were found between albino and pigmented rats. Nevertheless, there are less GABA-rich ON-starburst amacrine cells in Wistar rats than in Long-Evans rats, which could be indicate differences in the retinal neuronal circuitry [7]. ON-amacrine cells contribute to the generation of direction selectivity of ganglion cell response innervating the opto-kinetic system. Although the authors discuss that the difference found in GABA-rich ON-starburst amacrine cells may be too subtle to explain the drastic physiological alterations seen in albino rats, at least some of these alterations could be caused by the smaller number of amacrine cells [7]. Donatien et al. [12] reported on a reduced ganglion cell density in albino rabbits. If slight deficits in the numbers of ganglion and amacrine cells were also present in our albino rats, this could help to explain the decreased oscillatory potential amplitudes found in our study, as well as the missing photopic negative response in the albino animals.
Dark adaptation
We also compared the rate of dark adaptation after a complete bleach of the retina in both rat strains. Behn et al. [4] found that albino animals have a slower dark adaptation than pigmented ones. Albino rats had considerably smaller ERG responses after 30 min than after 3 or 12 hours. ERG responses measured in pigmented rats were considerably higher after 30 min than in albino rats, and decreased slightly after 3 or 12 hours of dark-adaptation. In the end, ERG amplitudes were larger in albino animals than in pigmented animals after 12 hours of dark adaptation [4].
Although we followed a different experimental protocol (e.g., we used a higher intensity of bleaching light), it can be stated that these results are in sharp contrast to our findings (Fig. 10). We did not observe that the Long-Evans rats had any advantage over Wistar rats; on the contrary, the Wistar rats even showed a faster recovery of retinal function than Long-Evan rats. After 70 min of dark adaptation, a-wave amplitudes were partly restored to a good 25% of base line values in both strains, and b-wave amplitudes to above 30% in Wistar rats and above 20% in Long-Evans rats. Incidentally, such a slow functional recovery would make the long dark-adaptation period required in the rodent’s ERG protocols understandable.
The reasons for the discrepancies between the observations of Behn et al. [4] and our results are not known. We used Wistar rats instead of Sprague-Dawley rats, which, however, does not explain the different behaviour of the Long-Evans rats that were used in both studies.
Visual evoked potentials
Our measurements of flash-evoked VEP did reveal differences between Long-Evans and Wistar rats. However, these differences did not reach significance, which is surprising in the light of the large differences in post-receptoral processing between both strains.
Dyer & Swartzwelder [15] also compared VEP in albino and hooded rats. They found larger amplitudes and longer latencies in albino rats than in hooded rats, in direct contrast to our findings. Moreover, they reported that changes in the stimulus light intensity produced greater alterations in latencies and N1-P2 amplitudes in hooded rats than in albino rats. Indeed, the amplitudes were enhanced more by increasing light intensity in Long-Evans rats in our study, whereas we did not find any significant changes or differences in latencies.
Green et al. [22] did not find differences in the VEP of albino and black mice. However, they found higher VEP thresholds in albino mice. Balkema & Dräger [2], who, in addition, found similar effects in rabbits, reported the same. No differences were found in the visual threshold in albino and pigmented rats by visual-evoked recordings from cortex and superior colliculus [26]. Moreover, the authors reported that some units in the superior colliculus of the albino rats would respond to very weak light stimuli. This is in accordance with our study, where VEP amplitudes at the lowest light intensity used were slightly higher in Wistar rats.
Thomas et al. [48] performed a different kind of investigation by recording multi-unit visual responses from the surface of the superior colliculus of albino and pigmented rats. In contrast to most of the other work in this field, they found a lower dark-adapted visual threshold in albino rats. The authors stated that several reasons could have contributed to the discrepancies in the outcome of the various studies, such as dark adapting procedures, the kind and length of anaesthesia, variations in surgical procedures, ambient light conditions and form of signal recording. In our opinion, the origin and strain of the animals also have to be taken into account, as it is not possible to control light conditions during breeding by a commercial supplier, and there may be substantial differences inside the various albino and pigmented strains, as seen, for example, in [41].
Moreover, Thomas et al. [48] found shorter latencies in albino rats, in particular at lower light intensities, which is in accordance with our study. They may be a direct consequence of shorter a-wave and b-wave latencies observed in the ERGs.
The waveforms obtained in Long-Evans rats at an age of 7 months show more oscillations after the initial deflection than in Wistar rats. This could indicate a higher complexity of cortical processing in Long-Evans rats. More detailed investigations will be needed to clarify this point further.
To summarise, we found a higher sensitivity and faster response, but a decreased maximum response amplitude of the photoreceptors in Wistar rats. The response of the post-receptoral systems was found to be considerably smaller in Wistar rats than in Long-Evans rats. Although we discussed several possible circumstances that influence retinal function, it is still too early to make final statements about the reasons for the observed differences between Long-Evans and Wistar rats.
References
Abadi RV, Pascal E (1991) Visual resolution limits in human albinism. Vision Res 31:1445–1447 doi:10.1016/0042-6989(91)90063-B
Balkema GW, Dräger UC (1991) Impaired visual thresholds in hypopigmented animals. Vis Neurosci 6:577–585
Barmashenko G, Schmidt M, Hoffmann K-P (2005) Differences between cation-chloride co-transporter functions in the visual cortex of pigmented and albino rats. Eur J Neurosci 21:1189–1195 doi:10.1111/j.1460-9568.2005.03948.x
Behn D, Doke A, Racine J, Casanova C, Chemtob S, Lachapelle P (2003) Dark adaptation is faster in pigmented than albino animals. Doc Ophthalmol 106:153–159 doi:10.1023/A:1022511918823
Birch D, Jacobs GH (1979) Spatial contrast sensitivity in albino and pigmented rats. Vision Res 19:933–937 doi:10.1016/0042-6989(79)90029-4
Blaszczyk WM, Straub H, Distler C (2004a) GABA content in the retina of pigmented and albino rats. Neuroreport 15:1141–1144 doi:10.1097/00001756-200405190-00012
Blaszczyk WM, Telkes I, Distler C (2004b) GABA-immunoreactive starburst amacrine cells in pigmented and albino rats. Eur J Neurosci 20:3195–3198 doi:10.1111/j.1460-9568.2004.03761.x
Blaszczyk WM, Arning L, Hoffmann K-P, Epplen JT (2005) A Tyrosinase missense mutation causes albinism in the Wistar rat. Pigment Cell Res 18:144–145 doi:10.1111/j.1600-0749.2005.00227.x
Breton ME, Schueller AW, Lamb TD, Pugh EN Jr (1994) Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci 35:295–309
Bui BV, Vingrys AJ (1999) Development of receptoral responses in pigmented and albino guinea-pigs (Cavia porcellus). Doc Ophthalmol 99:151–170 doi:10.1023/A:1002721315955
Donatien P, Jeffery G (2002) Correlation between rod photoreceptor numbers and levels of ocular pigmentation. Invest Ophthalmol Vis Sci 43:1198–1203
Donatien P, Aigner B, Jeffery G (2002) Variations in cell density in the ganglion cell layer of the retina as a function of ocular pigmentation. Eur J Neurosci 15:1597–1602 doi:10.1046/j.1460-9568.2002.02022.x
Dräger UC (1985) Calcium binding in pigmented and albino eyes. Proc Natl Acad Sci U S A 82:6716–6720 doi:10.1073/pnas.82.19.6716
Dreher B, Sefton AJ, Ni SY, Nisbett G (1985) The morphology, number, distribution and central projections of Class I retinal ganglion cells in albino and hooded rats. Brain Behav Evol 26:10–48 doi:10.1159/000118764
Dyer RS, Swartzwelder HS (1978) Sex and strain differences in the visual evoked potentials of albino and hooded rats. Pharmacol Biochem Behav 9:301–306 doi:10.1016/0091-3057(78)90289-7
Elekessy EI, Campion JE, Henry GH (1973) Differences between the visual fields of Siamese and common cats. Vision Res 13:2533–2543 doi:10.1016/0042-6989(73)90250-2
Gallemore RP, Li JD, Govardovskii VI, Steinberg RH (1994) Calcium gradients and light-evoked calcium changes outside rods in the intact cat retina. Vis Neurosci 11:753–761
Garipis N, Hoffmann K-P (2003) Visual field defects in albino ferrets (Mustela putorius furo). Vision Res 43:793–800 doi:10.1016/S0042-6989(03)00015-4
Gershoni-Baruch R, Rosenmann A, Droetto S, Holmes S, Tripathi RK, Spritz RA (1994) Mutations of the tyrosinase gene in patients with oculocutaneous albinism from various ethnic groups in Israel. Am J Hum Genet 54:586–594
Girman SV, Sauve Y, Lund RD (1999) Receptive field properties of single neurons in rat primary visual cortex. J Neurophysiol 82:301–311
Grant S, Patel NN, Philp AR, Grey CN, Lucas RD, Foster RG et al (2001) Rod photopigment deficits in albinos are specific to mammals and arise during retinal development. Vis Neurosci 18:245–251 doi:10.1017/S095252380118209X
Green DG, Herreros de Tejada P, Glover MJ (1994) Electrophysiological estimates of visual sensitivity in albino and pigmented mice. Vis Neurosci 11:919–925
Gresh J, Goletz PW, Crouch RK, Rohrer B (2003) Structure-function analysis of rods and cones in juvenile, adult, and aged C57bl/6 and Balb/c mice. Vis Neurosci 20:211–220 doi:10.1017/S0952523803202108
Harker KT, Whishaw IQ (2002) Place and matching-to-place spatial learning affected by rat inbreeding (Dark-Agouti, Fischer 344) and albinism (Wistar, Sprague-Dawley) but not domestication (wild rat vs Long-Evans, Fischer-Norway). Behav Brain Res 134:467–477 doi:10.1016/S0166-4328(02)00083-9
Harvey RJ, De’Sperati C, Strata P (1997) The early phase of horizontal optokinetic responses in the pigmented rat and the effects of lesions of the visual cortex. Vision Res 37:1615–1625 doi:10.1016/S0042-6989(96)00292-1
Herreros de Tejada P, Green DG, Muñoz Tedó C (1992) Visual thresholds in albino and pigmented rats. Vis Neurosci 9:409–414
Hood DC, Birch DG (1994) Rod phototransduction in retinitis pigmentosa: Estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci 35:2948–2961
Huang B, Karwoski CJ (1992) Light-evoked expansion of subretinal space volume in the retina of the frog. J Neurosci 12:4243–4252
Hupfeld D, Hoffmann K-P (2006) Motion perception in rats (Rattus norvegicus sp.): Deficits in albino Wistar rats compared to pigmented Long-Evans rats. Behav Brain Res 170:29–33 doi:10.1016/j.bbr.2006.01.022
Ilia M, Jeffery G (2000) Retinal cell addition and rod production depend on early stages of ocular melanin synthesis. J Comp Neurol 420:437–444 doi:10.1002/(SICI)1096-9861(20000515)420:4<437::AID-CNE3>3.0.CO;2-1
Jeffery G (1997) The albino retina: an abnormality that provides insight into normal retinal development. Trends Neurosci 20:165–169 doi:10.1016/S0166-2236(96)10080-1
Jeffery G, Darling K, Whitmore A (1994) Melanin and the regulation of mammalian photoreceptor topography. Eur J Neurosci 6:657–667 doi:10.1111/j.1460-9568.1994.tb00311.x
Kaila K, Voipio J, Akerman KE (1984) Free extracellular [Ca2+] at photoreceptor level equals that in vitreous in frog and carp eyes. Invest Ophthalmol Vis Sci 25:1395–1401
Kim IB, Lee MY, Oh S, Kim KY, Chun M (1998) Double-labeling techniques demonstrate that rod bipolar cells are under GABAergic control in the inner plexiform layer of the rat retina. Cell Tissue Res 292:17–25 doi:10.1007/s004410051030
Lamb TD, Pugh EN Jr (1992) A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol 499:719–758
Lannou J, Cazin L, Precht W, Toupet M (1982) Optokinetic, vestibular, and optokinetic-vestibular responses in albino and pigmented rats. Pflugers Arch 393:42–44 doi:10.1007/BF00582389
Lavallee CR, Chalifoux JR, Moosally AJ, Balkema GW (2003) Elevated free calcium levels in the subretinal space elevate the absolute dark-adapted threshold in hypopigmented mice. J Neurophysiol 90:3654–3662 doi:10.1152/jn.00736.2003
Li W, Trexler EB, Massey SC (2002) Glutamate receptors at rod bipolar ribbon synapses in the rabbit retina. J Comp Neurol 448:230–248 doi:10.1002/cne.10189
Livsey CT, Huang B, Xu J, Karwoski CJ (1990) Light-evoked changes in extracellular calcium concentration in frog retina. Vision Res 30:853–861 doi:10.1016/0042-6989(90)90054-O
Naka KI, Rushton WAH (1966) S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol 185:536–555
Nusinowitz S, Heckenlively JR (2006) Evaluating retinal function in the mouse retina with the electroretinogram. In: Heckenlively JR, Arden GB (eds) Principles and practice of clinical electrophysiology of vision. 2nd edn. The MIT Press, Cambridge, MA, pp 899–909
Page-McCaw PS, Chung SC, Muto A, Roeser T, Staub W, Finger-Baier KC et al (2004) Retinal adaptation to bright light requires tyrosinase. Nat Neurosci 7:1329–1336 doi:10.1038/nn1344
Prusky GT, Harker KT, Douglas RM, Whishaw IQ (2002) Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behav Brain Res 136:339–348 doi:10.1016/S0166-4328(02)00126-2
Russell-Eggitt I, Kriss A, Taylor DS (1990) Albinism in childhood: a flash VEP and ERG study. Br J Ophthalmol 74:136–140 doi:10.1136/bjo.74.3.136
Salceda R (1989) 45Ca uptake by retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 30:2114–2117
Salceda R, Riesgo-Escovar JR (1990) Characterization of calcium uptake in chick retinal pigment epithelium. Pigment Cell Res 3:141–145 doi:10.1111/j.1600-0749.1990.tb00278.x
Salceda R, Sánchez-Chávez G (2000) Calcium uptake, release and ryanodine binding in melanosomes from retinal pigment epithelium. Cell Calcium 27:223–229 doi:10.1054/ceca.2000.0111
Thomas BB, Aramant RB, Sadda SR, Seiler MJ (2005) Light response differences in the superior colliculus of albino and pigmented rats. Neurosci Lett 385:143–147 doi:10.1016/j.neulet.2005.05.034
Vingrys AJ, Bui BV (2001) Development of postreceptoral function in pigmented and albino guinea pigs. Vis Neurosci 18:605–613 doi:10.1017/S0952523801184105
von dem Hagen EA, Hoffmann MB, Morland AB (2008) Identifying human albinism: A comparison of VEP and fMRI. Invest Ophthalmol Vis Sci 49:238–249 doi:10.1167/iovs.07-0458
Wack MA, Peachey NS, Fishman GA (1989) Electroretinographic findings in human oculocutaneous albinism. Ophthalmology 96:1778–1785
Wali N, Leguire LE (1992) Fundus pigmentation and the dark-adapted electroretinogram. Doc Ophthalmol 80:1–11 doi:10.1007/BF00161226
Wali N, Leguire LE (1993) Fundus pigmentation and the electroretinographic luminance-response function. Doc Ophthalmol 84:61–69 doi:10.1007/BF01203283
Weisse I, Loosen H, Peil H (1990) Age-related retinal changes - a comparison between albino and pigmented rats. Lens Eye Toxic Res 7:717–739
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the 105th congress of the DOG, 20–23 September 2007 in Berlin.
The authors have full control of all primary data and agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review their data upon request.
Rights and permissions
About this article
Cite this article
Heiduschka, P., Schraermeyer, U. Comparison of visual function in pigmented and albino rats by electroretinography and visual evoked potentials. Graefes Arch Clin Exp Ophthalmol 246, 1559–1573 (2008). https://doi.org/10.1007/s00417-008-0895-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0895-3