Abstract
Purpose
To study vitreous cavity humidity during fluid–air exchange in pars plana vitrectomy.
Methods
Intraocular humidity in the vitreous cavity was recorded for 2 min in six artificial eyes, six enucleated pig eyes, and ten patient eyes, after the eyes had been filled with either humidified air (75% humidity) or dry air (8% humidity).
Results
In artificial eyes the humidity levelled off at a value that was approximately equal to the humidity of the infused air, i.e., a mean of 71.9% when humidified air was used and a mean of 14.4% when dry air was used. In enucleated pig eyes humidity increased slightly with humidified air and remained stable with dry air. In patients intraocular humidity increased to over 90%, regardless of whether humidified or dry air was used.
Conclusion
In the living eye, dry air deprives the retinal tissue of humidity, which is lost into the vitreous cavity. This effect can be reduced by using humidified air.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1995, the first observations on visual field defects following pars plana vitrectomy with fluid–air exchange were reported [14]. Since then, these observations have been confirmed in various other publications [1, 3–13, 15, 17, 18, 20, 22]. Since most of the defects have been located in the inferotemporal quadrant, where the air stream enters the eye, it has been speculated that they are the result of a drying process in the retina [21]. Consequently, air humidification has been recommended [19].
The aim of this study was to measure humidity in the vitreous cavity during fluid–air exchange in both experimental and clinical conditions.
Materials and methods
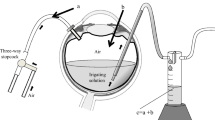
The experiments were carried out on ten human eyes in which macular holes were repaired. To test the validity of the measuring system we carried out control measurements on six MIRA plastic model eyes and six enucleated vitrectomised pig eyes. In all eyes three sclerotomies were performed, an infusion line was anchored in the inferotemporal quadrant, and a multiport illumination system (MIS) was introduced into the upper nasal and upper temporal quadrants. The MIS system was used to provide standardised access to the eye, so that the fluid–air exchange could not be influenced by different sclerotomy sizes and the sclerotomies would not be obstructed by vitreous or other tissue.
Standard pars plana vitrectomy was performed in the pig and human eyes, followed by fluid–air exchange through the infusion line with an automatic air pump attached to the unit (Premiere, Bausch & Lomb, Rochester, N.Y., USA). The model eyes were also perfused with air. Approximately 15 s after completion of the air fill, a fine probe, connected to a hygrometer (Hygrotec, Titisee-Neustadt, Germany) measuring both humidity and temperature, was introduced into the vitreous cavity through one of the sclerotomies and held in the mid-vitreous cavity, while an extrusion needle with its exit held closed was positioned in the other sclerotomy. Intraocular pressure was kept constant with the pump setting at 40 mmHg (pin). The MIS cannulas were very tight, which reduced the force of the jet stream.
The hygrometer consisted of a 20-G probe introduced into the vitreous cavity and an extraocular measuring chamber, with a direct connection between the two. A small opening at the outer surface of the measuring chamber allowed the air to flow through its cavity. The distance between the tip of the probe and the measuring cavity caused a short time lag before the hygrometer displayed the actual humidity readings.
Before entering the eyes the air was passed through Millipore filters. For one-half of each of the three groups of eyes the air came directly from a tank containing compressed air. The humidity of the dry air used straight from the tank was 8% at the point of entry, according to earlier intraluminal measurements at the tip of the infusion system. For the other half of each group the air came from the same tank, but, before being introduced into the eyes, it was humidified by passage through balanced salt solution, as described by Ohji et al. [16]; its humidity was then 75% at the point of entry. The gas temperature was between 22°C and 25°C. Temperature and humidity in the vitreous cavity were measured continuously over 2 min and plotted at intervals of 10 s.
Results
In the plastic model eyes intraocular humidity had dropped to a mean of 14.4% 2 min after perfusion with dry air (Fig. 1a). When humidified air was used the intraocular humidity increased to a mean of 71.9% (Fig. 1b).
In the enucleated pig eyes intravitreal humidity remained at a mean of 68.4% when the eyes were perfused with dry air (Fig. 2a) and climbed to 74.6% when they were perfused with moist air (Fig. 2b).In living human eyes, 2 min after fluid-air exchange, intravitreous humidity increased to a mean of 90.5% when dry air was used (Fig. 3a) and reached a mean of 93.8% when the eyes were perfused with humidified air (Fig. 3b).
Temperature remained constant over the 2-min observation period.
Discussion
The process of drying is defined as the removal or loss of moisture. It depends on the water content and structure of the material exposed, the humidity of the ambient air, the turnover of ambient air, a possible water supply, and the temperature.
In the vitreous cavity of a plastic model eye, with a non-hydrated inner lining, the humidity of the air measured is bound to adapt, becoming closer to the humidity of the air that has been introduced. In our experimental model this was the case after the introduction of both dry and humidified air, underlining the validity of our experimental setting and the hygrometer measurements.
In the enucleated animal eye the situation is different, since the cavity is lined by tissue composed of liquid-containing cells and intercellular spaces. When filled with moist air, the humidity measured in the cavity almost represents the humidity of the air that has been introduced. Since this humidity is already 75% and the air is approximately the same temperature as the surrounding tissue, evaporation is minimal, and humidity in the cavity does not go up within 2 min. Thus, there is almost no drying.
When the eye is filled with dry air, however, evaporation does occur. Consequently, humidity in the vitreous cavity does not decrease as in the model eye, but instead goes up as the surrounding tissue dries. The humidity equilibrates at a much lower level than in the living eye, however, because the inherent moisture on the surface of the retina is not sufficient to saturate the cavity.
In the living human eye the water loss is greater than in the cadaver eye. The lining of the cavity is connected to the circulation and is constantly hydrated. Thus, the increase in intravitreous humidity measured after the eye has been filled with dry air must be the result of fluid extraction from these tissues, together with a small amount of aqueous production [2].
The MIS cannulas were very tight; however, minimal leakage might occur. This, and the fact that a small opening at the outer surface of the measuring chamber allowed minimal air flow, may explain why the intraocular humidity did not reach 100%.
It would be interesting to know the exact amount of water lost from the eye cavity during evaporation. As can be seen in Fig. 3b, the humidity in the living eye changes from approximately 75% to 90% in the case of humidified air, which means an increase from 33 g/m3 to about 40 g/m3. If the volume of the vitreous cavity is considered to be 5 ml, the total amount of water evaporated over 2 min is approximatey 35 μl. As no equilibrium is reached after 2 min it will be even more as time goes on. With regard to the dry air perfusion, and by the same calculation, the amount of water loss is about 150 μl. If it is taken into consideration that the mean aqueous production is regarded to be around 2.75±0.63 μl/min [2], the water evaporated is far more than normal aqueous production. It is conceivable that this procedure is stressful to the retina, and that this “evaporation stress” may produce damage.
Our calculations were based on our surgery setting using MIS cannulas that allow only minimal leakage. In a less tight system with greater leakage the water loss is likely to be more.
Our experimental setting was designed in such a way as to reduce air flow to a minimum to allow us to focus on the “damage owing to evaporation stress” theory and to support this theory with values. Therefore, we did not measure additional stress caused by such air flow parameters as infusion pressure or jet stream. In order to estimate the expected pressure entering the eye we calculated the percentage of the initial air-flow pressure using Hagen–Poiseuille’s law [19]. On the basis of these calculations, where the pressure in a tube is proportional to the length of the infusion line (1.5 m) and r −4 where r (0.9 mm) is the radius of the infusion line, we found that just 5% of the p in reached the eye. We arrived at this percentage by taking into account the fact that there is no turbulent stream or curvature of the hose, which would reduce the entering air pressure even more. If simple geometrical calculations are used and the stream divergence taken into account, the incoming beam cross-section of 2.4 mm2 will blur out, so that the resulting pressure difference is less than the incoming 5% of the p in. This air pressure difference should not cause damage to the retina. Because of this tremendous reduction in air pressure, to less than 5%, the additional pressure-reducing effect of an in-line humidifier is irrelevant.
In conclusion, we have shown that vitreous tamponade with dry air leads to loss of moisture from the surrounding tissues. Water loss is greater in living eyes than in cadaver eyes, implying additional water supply. Quantitative estimation of the water loss in living eyes is greater than the rate of aqueous production. This implies that water is added from the extracellular fluid and, ultimately, via circulation. It may be speculated that this “evaporation stress” has a damaging effect on the surrounding tissues. Until proven otherwise the use of humidified air is therefore advocated. However, this may not be the only precaution that can and should be taken to avoid retinal damage during fluid–air exchange.
References
Boldt HC, Munden PM, Folk JC, Mehaffey MG (1996) Visual field defects after macular hole surgery. Am J Ophthalmol 122:371–381
Brubaker RF (1991) Flow of aqueous humor in humans (The Friedenwald Lecture). Invest Ophthalmol Vis Sci 32:3145–3166
Cullinane AB, Cleary PE (2000) Prevention of visual field defects after macular hole surgery. Br J Ophthalmol 84:372–377
Ezra E, Arden GB, Riordan-Eva P, Aylward GW, Gregor ZJ (1996) Visual field loss following vitrectomy for stage 2 and 3 macular holes. Br J Ophthalmol 80:519–525
Gass CA, Haritoglou C, Messmer EM, Schaumberger M, Kampik A (2001) Peripheral visual field defects after macular hole surgery: a complication with decreasing incidence. Br J Ophthalmol 85:549–551
Hasumura T, Yonemura N, Hirata A, Murata Y, Negi A (2000) Retinal damage by air infusion during vitrectomy in rabbit eyes. Invest Ophthalmol Vis Sci 41:4300–4304
Hirata A, Yonemura N, Hasumura T, Murata Y, Negi A (2000) Effect of infusion air pressure on visual field defects after macular hole surgery. Am J Ophthalmol 130:611–616
Hutton WL, Fuller DG, Snyder WB, Fellman RL, Swanson WH (1996) Visual field defects after macular hole surgery. Ophthalmology 103:2152–2159
Kerrison JB, Haller JA, Elman M, Miller NR (1996) Visual field loss following vitreous surgery. Arch Ophthalmol 114:564–569
Kokame GT (2000) Visual field defects after vitrectomy with fluid–air exchange. Am J Ophthalmol 130:653–654
Kokame GT (2001) Visual field defects after vitrectomy with fluid–air exchange. Br J Ophthalmol 85:121–123
Lee YW, Kwok AKH, Lan DSC (2000) Prevention of visual field defects after macular hole surgery. Br J Ophthalmol 84:1439–1441
Malinowski SM, Pesin SR (1997) Visual field loss caused by retinal vascular occlusion after vitrectomy surgery. Am J Ophthalmol 123:707–708
Melberg NS, Thomas MA (1995) Visual field loss after pars plana vitrectomy with air/fluid exchange. Am J Ophthalmol 120:386–388
Oh KT, Boldt HC, Maturi RK, Folk JC, Kardon RH (2000) Evaluation of patients with visual field defects following macular hole surgery using multifocal electroretinography. Retina 20:238–243
Ohji M, Nao-i N, Saito Y, Hayashi A, Tano Y (1999) Prevention of visual field defect after macular hole surgery by passing air used for fluid–air exchange through water. Am J Ophthalmol 127:62–66
Paques M, Massin P, Santiago PY, Spielmann AC, Gaudric A (1997) Visual field loss after vitrectomy for full thickness macular holes. Am J Ophthalmol 124:88–94
Pendergast SD, McCuen BW II (1996) Visual field loss after macular hole surgery. Ophthalmology 103:1069–1077
Vogel H, Gerthsen C (1997) Gerthsen Physik, vol. 19. Springer, Berlin Heidelberg New York, pp 110–114
Williams JM, Jacobson DM (1997) Visual field loss after vitreous surgery. Arch Ophthalmol 115:434–435
Welch JC (1997) Dehydration injury as a possible cause of visual field defect after pars plana vitrectomy for macular hole. Am J Ophthalmol 124:698–699
Yonemura N, Hirata A, Hasumura T, Negi A (2001) Fundus changes corresponding to visual defects after vitrectomy for macular hole. Ophthalmology 108:1638–1643
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no proprietary interest in any part of the study
Rights and permissions
About this article
Cite this article
Eter, N., Brinken, R., Garbe, S. et al. Intraocular humidity immediately after fluid–air exchange in pars plana vitrectomy. Graefe's Arch Clin Exp Ophthalmo 244, 305–308 (2006). https://doi.org/10.1007/s00417-005-1168-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-005-1168-z