Abstract
Background
An isolated form of congenital cataract associated with macular hypoplasia and a generally hypopigmented fundus in infancy was observed in a German family. To test the hypothesis that a de-novo mutation had occurred in one of the parental germ lines, a functional candidate gene approach was applied.
Methods
The family was carefully examined by a senior paediatric ophthalmologist according to routine procedures (slit lamp, funduscopy, ERG). Blood was taken from the proband and his parents, genomic DNA was isolated and some candidate genes for cataract (CRYAA, CRYBB2, GJA8) or macular hypoplasia (OA1, P) or both (PAX6) were analyzed.
Results
The proband showed bilateral cataracts at the age of 4 months; the fundus appeared pale, the optic disc grayish, and macular reflexes were absent. After cataract surgery, the nystagmus persisted, and a control ERG at age 9 years showed essentially normal scotopic and photopic wave forms. An infectious aetiology as well as galactosemia were excluded. However, a heterozygous mutation was found in the proband in exon 1 of CRYAA (62 C→T), which leads to an exchange from Arg to Leu at amino acid position 21 (R21L). This sequence alteration was not found in the parents and in 96 randomly selected DNA samples from ophthalmologically normal individuals of the KORA S4 study population. In addition, two heterozygous mutations in P were identified (R419Q and A481T); one of both was present in each of the unaffected parents.
Conclusion
Based upon the unique finding of the mutation and the expression of CRYAA in the lens, this R21L mutation in the CRYAA is considered to be causative for the dominant cataract phenotype. Moreover, the macular hypoplasia has to be considered a concerted interaction with compound heterozygous mutations in the P gene manifesting a mild form of oculocutaneous albinism. Nevertheless, this combination is rare and future studies will focus on identifying similar phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A broad variety of cataract mutations have been characterized in human and mouse within the last 10 years. The analysis of congenital (mainly dominant) cataracts discovered an unexpected high number of mutations in genes coding for γ-crystallins, and to a less extent in those genes coding for α-and β-crystallins. Crystallins are considered to act as structural proteins mainly in the lens; however, some of them have also been detected in other ocular tissues and other organs (for a review see [8]). Even if the crystallins are recognized as highly conserved proteins among the vertebrate species, a high number of polymorphisms exist in the human population [12, 30, 31], and among mouse strains [10]. Further structural proteins in the lens represent membrane proteins. The most prominent ones are the main intrinsic protein (MIP), which belongs to the family of aquaporins, and gap-junction proteins, which belong to the connexin families. Mutations in the corresponding genes MIP/Mip, GJA3/Gja3 and GJA8/Gja8 (encoding the gap junction membrane channel protein α3 and α8, respectively) have been shown to underlie some congenital, dominant cataracts in mouse and human (for review, see [8]). However, none of these mutations has been demonstrated in the past to be associated with retinal disorders.
Macular hypoplasia, frequently associated with nystagmus, is a common feature of various forms of ocular albinism caused mainly by mutations in the OA1 gene (OMIM 300500), the TYR gene (encoding tyrosinase; OMIM 606933) and the P gene (gene symbol for pink-eyed; OMIM 203200; unpublished results, B. Lorenz, M. Preising). However, in these cases no cataract has been observed (as example, see [28]). Moreover, ocular albinism is not only associated with fundus hypopigmentation, but also with iris transillumination, which was not detected in the present case. Macular hypoplasia may also be a feature of aniridia and aniridia phenotypes associated with PAX6 mutations (paired box gene 6; OMIM 607108). A heterozygous PAX6 missense mutation was reported in one patient with isolated foveal hypoplasia [1].
There are few reports dealing with both features, cataract as well as foveal hypoplasia. The first appeared in 1982 and described this new association of autosomal dominant foveal hypoplasia and (presenile) cataract [28] in a four-generation family; however, neither were mapping data given, nor has a subsequent molecular analysis been reported. Moreover, there were a few other reports on such a syndrome demonstrating the involvement of various PAX6 mutations in this particular phenotype [11, 32].
The present paper demonstrates clinical data for a congenital cataract associated with macular hypoplasia in an isolated patient of a small family. Therefore, linkage analysis was not possible. Based upon the functional candidate approach, CRYAA (encoding αA-crystallin), CRYBB2 (encoding βB2-crystallin), GJA8, OA1, P and PAX6 have been tested. Since a novel mutation was identified in the CRYAA gene, which was not detected in 96 controls nor in the parents, it is considered to be causative for the cataract, while the macular hypoplasia was caused by the concerted interaction of two heterozygous mutations in the P gene (compound heterozygosity).
Materials and methods
The study respected the tenets of the Declaration of Helsinki, as the family members were fully informed of their role in the study and the outcome, prior to securing their informed consent, prepared according to standard norms. The study was approved by the Ethics Committee of the University of Regensburg. Clinical details were recorded including the family’s health history. The proband and his parents were examined by a senior paediatric ophthalmologist (B. Lorenz) with a hand-held slit-lamp (Kowa Genesis, Japan) and a Zeiss slit-lamp (Zeiss, Oberkochen, Germany), respectively. As part of a prospective study, the patient was photographed at the time of surgery with a hand-held fundus camera (Kowa SL 14, Japan). Lens material and blood serum were subjected to microbiological analysis to exclude intrauterine infections. Various forms of galactosemia were ruled out by analysis of blood serum according to a previously published protocol [33]. Because of the retinal changes, a Ganzfeld electroretinogram (ERG) was performed on a Nicolet Spirit examination unit (Nicolet, Madison, Wisc., USA) using DTL electrodes according to the ISCEV standard for electroretinography [23].
To test the hypothesis that a de-novo mutation had occurred in one of the parental germ lines, a functional candidate gene approach was applied to identify a causative mutation. Therefore, we tested CRYAA, CRYBB2, GJA8 and PAX6 as candidate genes. To rule out albinism as a cause for the macular hypoplasia, we screened OA1 and P.
Blood samples (5–10 ml) were collected from the family members and additionally, cells of the mucous membrane of the oral cavity from the proband; genomic DNA was isolated according to standard procedures [24]. The functional candidate gene PAX6 was amplified by PCR [7] CRYBB2 and GJA8 were amplified as described previously [16, 30]; the primer and PCR conditions for the amplification of the three exons of CRYAA are listed in Table 1. OA1 and P exons were amplified using primers according to [28] and [18], respectively. Amplimers were screened by Single Strand Conformation Polymorphism Analysis and those showing aberrant banding pattern were sequenced directly; sequencing was done commercially (Sequiserve, Vaterstetten, Germany, or Seqlab, Göttingen, Germany).
The KORA (Cooperative Health Research in the Augsburg Region) Survey 2000 studied a population-based sample of 4261 subjects aged 25–74 years during the years 1999–2001 [13]. Ninety-six randomly chosen ophthalmologically normal individuals of this cohort were analyzed for the CRYAA mutation.
Results
Clinical findings
The proband V.C., born in 1993, was first seen at the Department of Paediatric Ophthalmology, Strabismology and Ophthalmogenetics at the age of 4 months because of poor fixation, nystagmus and bilateral cataract (Fig. 1a). He was born at term to non-consanguineous parents after an unremarkable pregnancy with a birth weight of 3020 g. The parents had noticed poor fixation, nystagmus and strabismus since birth. The family history was unremarkable, as was the clinical eye examination of the parents as to lens opacities. The patient has two siblings, an older and a younger brother, who are both healthy and in particular have no lens opacities or any other eye abnormalities.
Clinical features. a Anterior segments of both eyes prior to pars plana lentectomy at the age of 4 months. There was a dense opacification of the fetal nucleus more pronounced in the right eye, and with a clear periphery of the lens (Genesis hand-held fundus camera; Kowa Company Ltd., Tokyo, Japan). b Fundus photographs of both eyes at age 10 years (Zeiss Digital Fundus Camera; Zeiss, Oberkochen, Germany). Note pronounced hypoplasia of the macula. The temporal part of the optic disc is also hypoplastic. Increased visibility of the larger choroidal vessels most pronounced in the macular area indicative of hypopigmentation of the retinal pigment epithelium (RPE) or thinning of the RPE
At the age of 4 months, V.C. showed bilateral dense central lens opacities that were more pronounced in the posterior part with a clear periphery (Fig. 1a). Biometric measures were normal for age: the ultrasonographic axial length was 18.4 mm RE, and 17.7 mm LE, (standardized A-scan after Ossoinig). The keratometric readings were 6.85/6.85 RE, and 6.84/7.01 LE (Alcon keratometer, USA). The fundus appeared pale, the optic disc grayish, and macular reflexes were absent. No iris translucency was noted. A bilateral pars plana lensectomy (PPL) was performed, and the postoperative aphakia was corrected with contact lenses. To exclude an infectious aetiology, the lens material saved at the time of surgery as well as blood serum were tested for cytomegalovirus, herpes zoster, and toxoplasma, which were all negative. IgG for herpes simplex and rubella were positive, IgM negative, indicative of maternal antibodies still present at the age of 4 months. Uridyltransferase, galactokinase, epimerase and GAL-1-p were measured in the blood, and all were normal, excluding a metabolic defect in the galactose pathway. The ERG measured in the dark-and light-adapted state under general anaesthesia prior to the PPL in the second (left) eye showed normal rod and cone responses. The nystagmus persisted after surgery, visual acuity remained below age-normal values, and an abnormal head posture developed with dampening of the nystagmus with a right head turn. Because of the head turn and an additional esotropia and hypertropia, several squint surgeries were performed in the follow-up. At the last follow-up at age 11 years, best corrected visual acuity at distance was 0.2 in both eyes (with contact lenses). The anterior segments were unremarkable except for the surgical aphakia. On ophthalmoscopy, the temporal part of the optic disc as well as the macula were hypoplastic. The choroidal vessels were clearly visible in particular in the macular area indicative of increased translucency of the retinal pigment epithelium. Choroidal pigmentation had considerably increased, and there were even intensely pigmented patches most likely on the level of the RPE (Fig. 1b). A control ERG at age 9 years showed essentially normal scotopic and photopic wave forms. Only cone flicker amplitudes in the light adapted state were slightly below the age-matched 5th percentile. Multifocal ERG that would test retinal function spatially resolved in the macular area was not feasible due to the pronounced nystagmus.
Molecular genetics
A few polymorphic sites were identified in the proband that were not considered to be causative for the observed clinical feature, as they have been described previously as a polymorphic site or because no alteration of the amino acid sequence could be predicted (Table 2).
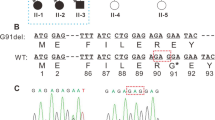
In exon 1 of the CRYAA gene, a heterozygous mutation was found in the proband (62 C→T; Fig. 2a). This mutation was confirmed by an HpaII digest of the PCR-amplified exon 1 of the CRYYA gene (Fig. 2c,d); similarly, the heterozygous mutation was also confirmed in DNA prepared from the mucous membrane of the oral cavity excluding the presence of a mosaic. Moreover, the mutation was seen only in the proband, but not in his healthy parents (Fig. 2b,d). Finally, this HpaII restriction site could not be found in 96 randomly selected human DNA samples of Caucasian origin (data not schown). DNA translation programs predict an exchange of the highly conserved Arg at amino acid position 21 to Leu (R21L); Arg is present at this position in all sequences available (of rat, mouse, bovine, Xenopus, zebrafish, cave-fish and epigean fish).
CRYAA mutation in the family 786. a A short fragment of the first exon of the CRYAA gene is given: at sequence pos 71 (=position 62 in CRYAA exon 1, counting the A of the ATG start codon as #1), the heterozygous situation of the proband (a) is obvious (arrow). The corresponding fragment of the mother (b) is identical with the database entry (AP001748.1); the arrow points to the base altered in the proband. The underlined bases (CCGG) define a HpaII restriction site, which is destroyed by the mutation. The amino acid sequence is given above the colored DNA sequence; the new Leu is underlined and given in bold. c A schematic overview of exon 1 of the CRYAA gene is given indicating the position of the ATG start codon and the two HpaII restriction sites. They lead to three fragments of 48, 90 and 118 bp. The 5’-HpaII site (indicated by an asterisk) is destroyed by the mutation leading to two fragments only (48 bp and 206 bp). d Exon 1 of the CRYAA gene was amplified in family 786 (1, proband; 2, mother; 3, father); the fragment was analyzed on an agarose gel before (−) and after (+) HpaII treatment. The larger fragment of 206 bp can be observed in the proband only. All smaller fragments are present in all family members; however, the 90/118 bp fragments are of lower intensity in the proband, indicating the heterozygous situation; M marker (the weak bands in lane 1 are derived from the marker lane)
A first attempt to understand the underlying molecular mechanisms might come from the computer-assisted analysis of the structure of the mutated R21L αA-crystalline. Using the proteomics program of the Expasy server http://www.expasy.ch), we compared several features among the wild-type and the mutant protein. No major differences were found in the extend of α-helices or β-sheets at this particular position. The exchange of the basic amino acid Arg by an aliphatic side chain as in Leu alters the isoelectric point only slightly (from pH 5.77 to pH 5.60); however, the hydrophobicity of this motif is significantly enhanced in the mutant (Fig. 3).
Enhanced hydrophobicity of the R21L mutant áA-crystallin. The hydrophobicity of the wild-type αA-crystallin (a) is compared with the mutant form (b). The red circle marks the region of the mutation (amino acid 21) and shows the area of enhanced hydrophobicity. In the mutant, it reaches almost the same size as in the unaffected C-terminus, whereas the corresponding region in the wild-type αA-crystallin ranges at about zero levels. Calculation was done using the bioinformatrics tool of the Expasy server http://www.expasy.ch)
To test also candidate genes for the macular hypoplasia, mutations were checked in the OA1 and P gene. Since no mutation could be shown in OA1, screening of P resulted in two mutations in exon 13 [R419Q (c.1256G→A)] and exon 14 [A481T (c1441G→A)], which have been reported previously [15, 17, 25, 29, 34, 35]. While A481T occurred only once in our set of albinism patients, R419Q was quite frequent, with ten cases from 95 tested. Segregation of these mutations was tested by SmaI and Fnu4HI digest showing an independent origin from both parents (data not shown).
Discussion
We describe a young boy suffering from congenital cataracts, hypopigmentation of the retinal pigment epithelium, and macular hypoplasia together with temporally hypoplastic optic discs. His parents as well as his two brothers are healthy. Because of the small size of the family, linkage analysis could not be done. Therefore, we molecularly analyzed functional candidate genes. The combination of cataract, macular hypoplasia, and nystagmus has been described in some cases as a manifestation of PAX6 mutations [11, 32]. However, in our case no mutation was observed in PAX6. Similarly, no mutation was detected in the OA1 gene.
Several genes have been demonstrated to be involved in cataractogenesis in human as well as in model organisms like the mouse coding for structural proteins (e.g. the crystallins) or membrane proteins (e.g. the connexins; for a recent review see [8]). Most of these genes are expressed predominantly in the lens, and a mutation in one of these genes would be a reasonable explanation for the cataract phenotype. Therefore, the observed unique mutation in the CRYAA gene is in line with previous reports on dominant cataracts caused by other CRYAA alleles in humans [20, 21]. Moreover, a complete list of the allelic series of CRYAA mutations also demonstrates their diversity, since one of the CRYAA mutations exhibits a recessive mode of inheritance [27]. This genetic diversity is very similar to the situation in the mouse, where the null mutation shows the cataract phenotype in the homozygous null state only [2]. Two additional mouse cataract mutants have been described: one suffers from a dominant [9], the other from a recessive form [3].
However, the position mutated in the patient reported here lies in the N-terminal domain, which has been demonstrated to be important in the αA-crystallin self-interaction [5]. It is at the very beginning of the highly conserved helical motif SRLFDQFFG, being identical in both αA- and αB-crystallins. It shares a high homology also to other mammalian small heat-shock proteins (RLFDQXFG). Deletion of this highly conserved motif showed in in-vitro studies that the multimeric size of the mutated protein is significantly reduced [26]. Moreover, in a recent paper Ghosh and Clark [6] demonstrated that this motif interacts with αB-crystallin also.
Since this change in hydrophobicity is comparable with others (R116C or V124E) known to be causative for cataracts, it is an additional line of evidence for the pathological nature of the R21L mutation reported here. Future biochemical investigations will add the experimental proof of this suggestion.
In contrast to the involvement of αA-crystallin in cataract formation, its participation in retinal disorders is not yet established. The mouse mutation V124E occurred on the genetic background of the C3H strain; this strain is known to suffer from retinal degeneration caused by a Pde6b nonsense mutation ([4], and references therein). The other mouse mutation affecting Cryaa, lop18, has been investigated histologically only during embryonic development [3]. Also for the human CRYAA mutations, no information on a retinal phenotype is available; either it was not analyzed because of the severity of the cataract, or it was not present. Retinal effects may be expected, since CRYAA is expressed in the retina [14, 19, 22]. However, to fit the features of fundus hypopigmentation and macular hypoplasia, we screened the two most probable genes (OA1 and P) involved in X-linked (OA1) and autosomal recessive ocular albinism (OA3) or in a light form of oculocutaneous albinism (OCA2), respectively. The mutations identified have been reported previously as a polymorphism (R419Q) [25, 34] or involved in darker iris colour (R419Q) [29], as well as the cause of subclinical OCA or mild OA, respectively (A481T) [15, 17, 35]. Both mutations are quite frequent in the Causasian and Japanese population (R419Q: ∼8 %, A481T: ∼12% in Japan, 1/50 carrier in Caucasian) [15, 25, 29, 35]. Therefore, they are not likely to cause the macular hypoplasia by themselves, although A481T is involved in subclinical OCA [15]. Unfortunately, R419Q has never been functionally tested like A481T which shows 70% activity in melanogenesis [35], and therefore, its influence cannot be evaluated completely. Our findings support the notion that this case has to be regarded as the rare incidence of two independent genetic causes which emphasizes the necessity of considering P in cases of macular hypoplasia and light features of albinism overlapping with congenital cataract.
However, such coincidence appears to be rare, and therefore the findings reported here might encourage colleagues in the field to look also for retinal changes at least in some cataract patients as well as in corresponding model systems.
In conclusion, mutations in the αA-crystallin encoding gene lead to diverse phenotypes with respect to the form of cataracts and mode of inheritance. Additional involvement in findings in the retina could not be ruled out but should be considered as a result of mutations identified in P.
References
Azuma N, Nishina S, Yanagisawa H, Okuyama T, Yamada M (1996) PAX6 missense mutation in isolated foveal hypoplasia. Nat Genet 13:141–142
Brady JP, Garland D, Duglas-Tabor Y, Robison WG Jr, Groome A, Wawrousek EF (1997) Targeted disruption of the mouse αA-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein alpha B-crystallin. Proc Natl Acad Sci USA 94:884–889
Chang B, Hawes NL, Roderick TH et al. (1999) Identification of a missense mutation in the αA-crystallin gene of the lop18 mouse. Mol Vis 5:21
Dalke C, Loster J, Fuchs H et al. (2004) Electroretinography as a screening method for mutations causing retinal dysfunction in mice. Invest Ophthalmol Vis Sci 45:601–609
Fu L (2002) Detection of protein-protein interactions among lens crystallins in a mammalian two-hybrid system assay. J Biol Chem 277:4225–4260
Ghosh JG, Clark JI (2005) Insights into the domains required for dimerization and assembly of human αB crystallin. Prot Sci 14:684–695
Glaser T, Walton DS, Maas RL (1992) Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat Genet 2:232–239
Graw J (2003) The genetic and molecular basis of congenital eye defects. Nat Rev Genet 4:876–888
Graw J, Loster J, Soewarto D et al. (2001) Characterization of a new, dominant V124E mutation in the mouse αA-crystallin-encoding gene. Invest Ophthalmol Vis Sci 42:2909–2915
Graw J, Neuhäuser-Klaus A, Klopp N, Selby PB, Löster J, Favor J (2004) Genetic and allelic heterogeneity of Cryg mutations in eight distinct forms of dominant cataract in the mouse. Invest Ophthalmol Vis Sci 45:1202–1213
Gupta SK, De BI, Tremblay F, Guernsey DL, Neumann PE (1998) Genotype/phenotype correlations in aniridia. Am J Ophthalmol 126:203–210
Heon E, Priston M, Schorderet DF et al. (1999) The γ-crystallins and human cataracts: a puzzle made clearer. Am J Hum Genet 65:1261–1267
Illig T, Bongardt F, Schopfer A et al. (2003) The endotoxin receptor TLR4 polymorphism is not associated with diabetes or components of the metabolic syndrome. Diabetes 52:2861–2864
Kapphahn RJ, Ethen CM, Peters EA, Higgins L, Ferrington DA (2003) Modified áA crystallin in the retina: altered expression and truncation with aging. Biochemistry 42:15310–15325
Kawai M, Suzuki T, Ito S, Inagaki K, Suzuki N, Tomita Y (2005) A patient with subclinical oculocutaneous albinism type 2 diagnosed on getting severely sunburned. Dermatology 210:322–323
Klopp N, Heon E, Billingsley G et al. (2003) Further genetic heterogeneity for autosomal dominant human sutural cataracts. Ophthalmic Res 35:71–77
Lee ST, Nicholls RD, Bundey S, Laxova R, Musarella M, Spritz RA (1994) Mutations of the P gene in oculocutaneous albinism, ocular albinism, and Prader-Willi syndrome plus albinism. N Engl J Med 330:529–534
Lee ST, Nicholls RD, Schnur RE et al. (1994) Diverse mutations of the P gene among African-Americans with type II (tyrosinase-positive) oculocutaneous albinism (OCA2). Hum Mol Genet 3:2047–2051
Li D, Sun F, Wang K (2004) Protein profile of aging and its retardation by caloric restriction in neural retina. Biochem Biophys Res Commun 318:253–258
Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG (1998) Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet 7:471–474
Mackay DS, Andley UP, Shiels A (2003) Cell death triggered by a novel mutation in the αA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet 11:784–793
Maeda A, Ohguro H, Maeda T, Nakagawa T, Kuroki Y (1999) Low expression of αA-crystallins and rhodopsin kinase of photoreceptors in retinal dystrophy rat. Invest Ophthalmol Vis Sci 40:2788–2794
Marmor MF, Zrenner E (1995) Standard for clinical electroretinography (1994 update). Doc Ophthalmol 89:199–210
Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucl Acids Res 16:1215
Passmore L, Käsmann-Kellner B, Weber BHF (1999) Novel and recurrent mutations in the tyrosinase gene and the P gene in the German albino population. Hum Genet 105:200–210
Pasta SY, Raman B., Ramakrishna T, Rao CM (2003) Role of the conserved SRLFDQFFG region of α-crystallin, a small heat shock protein. J Biol Chem 278:51159–51166
Pras E, Frydman M, Levy-Nissenbaum E et al. (2000) A nonsense mutation (W9X) in CRYAA causes autosomal recessive cataract in an inbred Jewish Persian family. Invest Ophthalmol Vis Sci 41:3511–3515
Preising M, op de Laak JP, Lorenz B (2001) Deletion in the OA1 gene in a family with congenital X linked nystagmus. Br J Ophthalmol 85:1098–1103
Rebbeck TR, Kanetsky PA, Walker AH et al. (2002) P gene as an inherited biomarker of human eye color. Cancer Epidemiol Biomarkers Prev 11:782–784
Santhiya ST, Manisastry SM, Rawlley D et al. (2004) Mutation analysis of congenital cataracts in Indian families: identification of SNPS and a new causative allele in CRYBB2 gene. Invest Ophthalmol Vis Sci 45:3599–3607
Santhiya ST, Shyam MM, Rawlley D et al. (2002) Novel mutations in the gamma-crystallin genes cause autosomal dominant congenital cataracts. J Med Genet 39:352–358
Schröder HW, Orth U, Meyer-König E, Gal A (2003) Familiare foveahypoplasie-klinische einordnung. Klin Monatsbl Augenheilkd 220:559–562
Shin YS (1990) Galactose metabolites and disorders of galactose. In: Hommes FA (ed) Techniques in diagnostic human biochemical genetics. Wiley-Liss, New York, pp 267–284
Spritz RA, Lee ST, Fukai K et al. (1997) Novel mutations of the P gene in type II oculocutaneous albinism (OCA2). Hum Mutat 10:175–177
Suzuki T, Miyamura Y, Tomita Y (2003) High frequency of the Ala481Thr mutation of the P gene in the Japanese population. Am J Med Genet A 118:402–403
Acknowledgements
Various forms of galactosemia were ruled out biochemically by Professor Y. Shin, University Children´s Hospital Munich, Germany. An infectious etiology was ruled out at the Institute of Medical Microbiology, University of Regensburg (Head: Professor Dr. H. Wolf). We thank Dr. Jack Favor (Neuherberg) for critical comments on the manuscript.
The excellent technical assistance of Birgit Langer and Günther Schuch (Regensburg) and of Erika Bürkle, Michaela Bunge, Sabrina Hauser and Monika Stadler (GSF Neuherberg) is gratefully acknowledged. Oligonucleotides were synthesized by Utz Linzner, GSF-Institute of Experimental Genetics.
The KORA Group consists of H.E. Wichmann (speaker), H. Löwel, C. Meisinger, T. Illig, R. Holle, J. John and their co-workers who are responsible for the design and conduct of the KORA studies.
The KORA research platform (KORA: Cooperative Research in the Region of Augsburg) was initiated and financed by the GSF - National Research Center for Environment and Health, which is funded by the German Federal Ministry of Education and Research and by the State of Bavaria; part of this work was also supported by the National Genome Research Network (NGFN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Graw, J., Klopp, N., Illig, T. et al. Congenital cataract and macular hypoplasia in humans associated with a de novo mutation in CRYAA and compound heterozygous mutations in P. Graefe's Arch Clin Exp Ophthalmo 244, 912–919 (2006). https://doi.org/10.1007/s00417-005-0234-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-005-0234-x