Abstract
Background
We investigated the influence of chronic smoking on ocular vascular reactivity during breathing of 100% oxygen.
Methods
Retinal vascular reactivity was tested during inhalation of 100% oxygen over 10 min. The observer-masked two-cohort study was performed in 24 healthy male volunteers (12 smokers and 12 nonsmokers) using the Zeiss Retinal Vessel Analyzer and laser Doppler velocimetry. From these parameters retinal blood flow was calculated.
Results
Hyperoxia significantly decreased arterial (smokers: p<0.001 vs baseline; nonsmokers: p=0.003 vs baseline) and venous (smokers: p<0.001 vs baseline; nonsmokers: p<0.001 vs baseline) diameters. This decrease was significantly more pronounced in smokers (arterial diameter: p<0.001, venous diameter: p=0.003). Hyperoxia decreased venous blood flow velocity (smokers: p=0.02 vs baseline; nonsmokers: p<0.001 vs baseline) to a comparable degree (p=0.51). The two groups showed a comparable decrease in retinal blood flow during hyperoxia (smokers: p<0.001 vs baseline; nonsmokers: p<0.001 vs baseline; p=0.76 between groups). The decrease of PCO2 during inhalation of 100% oxygen was significantly more pronounced in smokers than in nonsmokers (p=0.038).
Conclusion
The present study indicates an abnormal retinal vascular response to hyperoxia in smokers. Further studies are needed to identify possible neural or humoral factors involved in this shifted vasoconstrictory status in smokers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oxidative properties of tobacco smoking are a risk factor for a variety of ocular vascular disorders, such as hypertensive retinopathy, age-related macular degeneration and anterior ischemic optic neuropathy [25]. Tobacco smoke is composed of more than 4,000 active substances, including nicotine, tars, nitrosamines, polycyclic aromatic hydrocarbons, hydrogen cyanide, formaldehyde and carbon monoxide [4], all known to be involved in smoking-induced disorders.
A number of studies investigated effects of acute cigarette smoking on ocular blood flow parameters. One study, using the laser speckle method, showed a decrease of blood velocity in the optic nerve head and possibly in the choroid in habitual smokers, suggesting a significant increase in vascular resistance [28]. Another study investigated the acute effect of cigarette smoking on macular capillary white blood cell flux, using the blue field simulation technique, and demonstrated an increase in macular leukocyte velocity [24]. In diabetic patients and healthy controls, retinal blood flow at baseline and during breathing of 100% oxygen (O2) was assessed with laser Doppler velocimetry before and after smoking [17]. In both groups, smoking induced a marked decrease in retinal blood flow, more pronounced in diabetics than in healthy controls. Oxygen reactivity was reduced in controls and eliminated in diabetic patients after cigarette smoking.
However, only few data regarding effects of chronic cigarette abuse on ocular vascular reactivity are available. In animal studies, chronic smoking resulted in a significant increase of choroidal vascular resistance, whereas choroidal blood flow was not affected [9]. Ultrasound investigations comparing blood flow velocities in ocular vessels between smokers and nonsmokers revealed conflicting results. Kaiser et al. described significantly higher blood flow velocities in the ophthalmic artery, the central retinal artery and the posterior ciliary arteries in chronic smokers than in healthy controls [11]. Other investigators, however, found that chronic cigarette smoking is associated with lower velocities in the ophthalmic artery [29] and showed a decrease in flow velocity in the central retinal artery and the posterior ciliary arteries in smokers compared with nonsmokers [27].
The present study was undertaken to gain insight into the pathophysiological mechanisms but also possible therapeutic targets of smoking-related ocular disorders. For this purpose we investigated the effects of chronic smoking on retinal vascular reactivity to systemic hyperoxia. Hyperoxia was induced by breathing 100% O2 and the reactivity in retinal vessel diameter and retinal red blood cell velocity were compared between smokers and nonsmokers.
Material and methods
Study population
The present study was performed in adherence to the Declaration of Helsinki and the Good Clinical Practice guidelines. After approval of the study protocol by the Ethics Committee of the Vienna University School of Medicine and after written informed consent had been obtained, 24 healthy male volunteers were enrolled in the study (age range 19–35 years; mean 25±3 SD). Twelve participants had been smoking for at least 2 years and regularly smoked between 15 and 25 cigarettes per day. The other 12 volunteers had no history of smoking. All volunteers were drug-free for at least 3 weeks prior to inclusion and underwent a prestudy screening during the 4 weeks before the first study day that included physical examination and medical history, 12-lead electrocardiogram, complete blood cell count, activated partial thromboplastin time, thrombin time, clinical chemistry, hepatitis A, B, C and HIV serology, urine analysis, random urine drug screening and ophthalmic examination. Subjects were excluded if any clinically relevant abnormality was found as part of the pretreatment screening. In addition, subjects with ametropia of more than 3 diopters, anisometropia of more than 1 diopter or any evidence of eye disease that might interfere with the purpose of the present trial were excluded.
To objectively distinguish between smokers and nonsmokers, the level of cotinine in urine was determined. This investigation was done with a homogenous immunoassay (EMIT technique, Diagnostic Reagents, Sunnyvale, CA, USA) [8]. In addition the subjects were asked to complete the Fagerstrom Tolerance Questionnaire [21].
The results of the Fagerstrom Tolerance Questionnaire were 0±0 points for the nonsmoking group and 4±0.6 points for the smoking group, indicating nicotine dependence among the smokers. Cotinine levels in urine were 45.1±11.5 ng/ml in nonsmokers and 2,025.7±336.1 ng/ml in smokers. This again clearly indicates that the smokers were adequately selected, because the urine cotinine concentration in nonsmokers is normally below 500 ng/ml, as reported by Jarvis et al. [10].
Design
This study followed an observer-masked design in two cohorts. Subjects were asked to refrain from cigarettes, alcohol and caffeine for at least 12 h before trial days. Dilatation of one pupil was obtained with tropicamide (Mydriaticum “Agepha”-Augentropfen, AGEPHA, Vienna, Austria).
Intervention procedures
After a 20-min resting period in a sitting position, baseline vessel diameters with the Zeiss retinal vessel analyzer (RVA) were obtained during breathing of room air. The measurements were continued without cessation during breathing of 100% O2 (AGA, Vienna, Austria; certified for human use) over a period of 10 min. The gas was delivered through a partially expanded reservoir bag at atmospheric pressure using a two-valve system to prevent rebreathing. Thereafter a minimum time of 30 min was scheduled to reestablish baseline conditions. A second 100% O2 breathing period followed. Measurements were performed with laser Doppler velocimetry following the time schedule described above. Blood gas analysis was carried out at baseline and at the end of the breathing periods.
Systemic hemodynamics
Systolic, diastolic and mean arterial blood pressure (SBP, DBP, MAP) were measured on the upper arm using an automated oscillometric device. Pulse rate (PR) was automatically recorded from a finger-pulse oximeter (HP-CMS patient monitor; Hewlett-Packard, Palo Alto, CA). Systemic hemodynamics were measured at 2-min intervals during O2 breathing periods and at 10-min intervals during resting periods. Pulse rate and real-time ECG were monitored continuously.
Zeiss retinal vessel analyzer
Retinal vessel diameters were determined from retinal images recorded with a fundus camera-based system. The Zeiss RVA is a commercially available system which comprises a fundus camera (Zeiss FF 450, Jena, Germany), a video camera, a real-time monitor and a personal computer with analyzing software for the accurate determination of retinal arterial and venous diameters [3]. Every second a maximum of 25 readings of vessel diameter can be obtained. For this purpose the fundus is imaged onto the charge-coupled device chip of the video camera. The consecutive fundus images are digitized using a frame grabber. In addition, the fundus image can be inspected on the real-time monitor and, if necessary, stored on a video recorder. The retinal arterial diameters were analyzed online and the venous diameters offline from the recorded video tapes. Due to the absorbing properties of hemoglobin each blood vessel has a specific transmittance profile. Measurement of retinal vessel diameters is based on adaptive algorithms using these specific transmittance profile. Whenever a vessel profile is recognized in the region of interest, the RVA can follow this vessel as long as it appears within the measurement window. The system is therefore able to correct automatically for alterations in luminance as induced, for instance, by small eye movements. If the requirements for the assessment of retinal vessel diameters are no longer fulfilled, as occurs during blinks, the system automatically stops the measurement. As soon as an adequate fundus image is achieved again, measurement of vessel diameters restarts automatically. Our previous data showed excellent reproducibility with the Zeiss retinal vessel analyzer [20].
Laser Doppler velocimetry
Retinal blood flow velocity was measured with a commercially available laser Doppler velocimeter (Okulix 5000; Arbaz, Switzerland). The principle of blood flow velocity measurement by laser Doppler velocimetry is based on the optical Doppler effect. Laser light, which is scattered by moving particles (e.g., erythrocytes), is shifted in frequency. This frequency shift is proportional to the blood flow velocity in the retinal vessel. The maximum Doppler shift corresponds to the center-line erythrocyte frequency. Using bidirectional laser Doppler velocimetry the absolute velocity in the retinal vessels can be obtained [22]. Measurements were performed in main inferior or superior temporal retinal veins at the same location as diameter measurements [23].
Retinal blood flow
Retinal blood flow was calculated from the results of the measurements of blood flow velocity and retinal vessel diameter as described previously [23].
Blood gas analysis
Blood gas values were determined from capillary blood samples of the earlobe. After applying an ointment (Finalgon ointment; Thomae, Biberach, Germany) locally to the earlobe to induce capillary vasodilatation, a lancet incision was made. The arterialized blood was drawn into a thin glass capillary tube. Arterial O2 tension (PO2), carbon dioxide tension (PCO2) and O2 saturation (SaO2) were determined with an automatic blood gas analysis system (AVL 995 Hb, Graz, Austria). This technique accurately measures arterial blood gas tensions [19].
Data analysis
Statistical analysis was done with Statistica for Windows (1997; Statsoft, Tulsa, USA). All outcome parameters were averaged on periods of 2 min. Effects of 100% O2 breathing on hemodynamic parameters were analyzed by t-test and repeated-measures ANOVA using absolute values. A p value <0.05 was considered to indicate a significant difference. For data description, relative values are given as means±SEM.
Results
No significant differences in ocular and systemic hemodynamic parameters were observed at baseline between smokers and nonsmokers (Table 1).
Table 2 shows the effects of 10 min of breathing 100% O2 on systemic and ocular hemodynamic parameters and significant differences between the two groups. During administration of 100% O2, SBP significantly decreased in smokers (−5±1%, p=0.008 vs baseline; paired t-test), but not in nonsmokers. The difference in decrease between the two groups was, however, not significant (p=0.84, ANOVA). DBP, MAP and PR remained unchanged in both groups and did not differ significantly between groups.
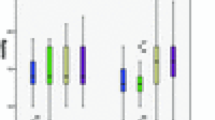
Hyperoxia significantly decreased the diameters of retinal arteries (smokers: −11%±1%, p<0.001 vs baseline; nonsmokers: −6%±2%, p=0.003 vs baseline; Fig. 1a) and veins (smokers: −17%±1%, p<0.001 vs baseline; nonsmokers: −13%±1%, p<0.001 vs baseline; Fig. 1b) in both groups. The decrease in retinal vessel diameters was significantly more pronounced in smokers than in nonsmokers (arterial diameter: p<0.001, venous diameter: p=0.003; ANOVA). Blood flow velocity in retinal veins during O2 breathing (Fig. 1c) decreased less markedly in smokers (−29±11%, p=0.02 vs baseline) than in nonsmokers (−47±7%, p<0.001 vs baseline). However, the difference between the two groups was not significant (p=0.51, ANOVA). Correspondingly, retinal blood flow (Fig. 1d) significantly decreased during O2 breathing in smokers (−52±7%, p<0.001 vs baseline) and nonsmokers (−60±5%, p<0.001 vs baseline). Again, this effect was not significantly different between smokers and nonsmokers (p=0.76, ANOVA).
Retinal arterial diameter (a), retinal venous diameter (b), retinal mean blood velocity (c) and retinal blood flow (d) during breathing of 100% O2. Number sign indicates significant difference vs baseline (t-test, post hoc testing). Asterisk indicates significant difference between smokers and nonsmokers (ANOVA). Data are presented as means±SEM (n=12 in each group)
Data of blood gas analyses are shown in Table 3. At baseline conditions no significant differences in PO2, PCO2 or SaO2 were observed between smokers and nonsmokers. Also the increase of PO2 and SaO2 during O2 breathing was not significantly different between the two groups (p=0.185; ANOVA), but the decrease of PCO2 was significantly more pronounced in smokers than in nonsmokers (p=0.038; ANOVA).
Discussion
Surprisingly, the O2 reactivity of retinal arterial and venous diameters is significantly more pronounced in smokers than in nonsmokers. By contrast, retinal blood velocity and retinal blood flow show a comparable response to O2 breathing in the two groups. Which factors may be responsible for this abnormal retinal vascular response pattern in the retina of smokers?
Our results cannot be explained by differences in blood oxygenation between smokers and nonsmokers, because SaO2 and PO2 were not different between the two groups at baseline or at the end of the breathing period. PCO2 significantly decreased in smokers during hyperoxia, but still remained within the physiological range at the end of the breathing period. We and others have, however, previously shown that the effect of small changes in PCO2 on retinal blood flow is overruled by the strong vasoconstricting effect of O2 [15, 18]. Nevertheless, we can not entirely rule out the possibility that the greater decrease of PCO2 in smokers increased the vasoconstricting effects of O2.
The present data are in contrast to a study assessing retinal and optic nerve head blood flow during a 5-min period of hyperoxia in smokers and nonsmokers with scanning laser Doppler flowmetry (SLDF) [14]. The reduction in retinal and optic nerve head blood flow was significantly more pronounced in the nonsmoking group. Methodological differences may account for the discrepancy between the present data and the previous study. In SLDF measurements the underlying choroid most likely contributes to the received signal, making comparison with the present data difficult. In addition, the acute effects of smoking need to be carefully distinguished from long-term changes in the retinal vasculature induced by chronic smoking. In smokers acute cigarette smoking reduced retinal O2 reactivity [17], whereas in our study chronic smoking was associated with enhanced vasoconstriction in retinal branch veins and arteries.
Permanent alterations to the microcirculation in smokers have also been demonstrated in other vascular tissues. The peripheral resistance of the cutaneous microvasculature is increased, with a corresponding decrease in peripheral skin flow. Also a significantly longer recovery phase is seen in smokers than in nonsmokers, suggesting permanent damage to the microcirculation [16]. In addition, continuous diameter measurements in smokers demonstrated abnormal constriction of the brachial artery during the low-flow period of cuff occlusion [26].
Our observations may in part be explained by the effect of smoking on the production and susceptibility of endothelial-derived vasoactive substances. Animal and human studies found that cigarette smoking is associated with reduced endothelium-dependent vasodilation, nitric oxide (NO) generation, and endothelial NO synthase (eNOS) activity [2, 30]. The expression of eNOS protein is increased in human smokers in the presence of reduced eNOS activity [2]. Moreover, smokers have higher endothelin-1 (ET-1) plasma levels after cigarette smoking [6, 7], whereas basal ET-1 levels are slightly lower in smokers [1]. Short-term smoking enhances ET-1-induced vasoconstriction in the forearm, also indicating the close relation between the endothelin system and smoking habits [12]. This is of interest, because ET-1 plays a major role in hyperoxia-induced vasoconstriction in the human retina [5].
Smoking further alters the expression of a number of endothelial-cell genes encoding for vasoactive and thrombogenetic factors such as eNOS, angiotensin-I converting enzyme, tissue-type plasminogen activator, plasminogen activator inhibitor-I, von Willebrand factor and vascular cell adhesion molecule-I [31]. It may be speculated that the equilibrium between vasoconstricting and vasodilating factors is altered or that the susceptibility to vasoconstricting factors is higher in smokers. This would not affect vessel diameters at basal conditions but might facilitate constriction during exogenous or endogenous stimuli.
At baseline, retinal arterial and venous diameters tended to be greater in smokers than in nonsmokers, although the difference was not significant. Accordingly, retinal arterioles and venules could be in a state of permanent vasodilatation in smokers. This difference in vascular tone could well contribute to the differences in O2 reactivity and is compatible with the more pronounced hyperoxia-induced vasoconstriction in smokers.
A limitation of the present trial is that we do not know the O2 tension in retinal tissue, which is not measurable in humans. Moreover, measurements with the RVA and the laser Doppler velocimeter were not conducted simultaneously, but the effect of O2 on retinal vessels is highly reproducible [13].
In conclusion, the present study showed a difference in the response of retinal vessels to hyperoxia between smokers and nonsmokers. Further studies are needed to identify possible neural or humoral factors involved in this shifted vasoconstriction status in smokers.
References
Ahlborg G, Lundberg JM (2001) Endothelin-1: increased plasma clearance, pulmonary affinity and renal vasoconstriction in young smokers. Clin Physiol 21:693–703
Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG, Saha DC (2001) Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation 104:1905–1910
Blum M, Bachmann K, Wintzer D, Riemer T, Vilser W, Strobel J (1999) Noninvasive measurement of the Bayliss effect in retinal autoregulation. Graefes Arch Clin Exp Ophthalmol 237:296–300
Chiba M, Masironi R (1992) Toxic and trace elements in tobacco and tobacco smoke. Bull World Health Organ 70:270–276
Dallinger S, Dorner GT, Wenzel R, Graselli U, Findl O, Eichler HG, Wolzt M, Schmetterer L (2000) Endothelin-1 contributes to hyperoxia-induced vasoconstriction in the human retina. Invest Ophthalmol Vis Sci 41:864–869
Goerre S, Staehli C, Shaw S, Luscher TF (1995) Effect of cigarette smoking and nicotine on plasma endothelin-1 levels. J Cardiovasc Pharmacol 26:S236–S238
Haak T, Jungmann E, Raab C, Usadel KH (1994) Elevated endothelin-1 levels after cigarette smoking. Metabolism 43:267–269
Haley NJ, Axelrad CM, Tilton KA (1983) Validation of self-reported smoking behavior: biochemical analyses of cotinine and thiocyanate. Am J Public Health 73:1204–1207
Hara K (1991) Effects of cigarette smoking on ocular circulation chronic effect on choroidal circulation. Nippon Ganka Gakkai Zasshi 95:939–943
Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y (1987) Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 77:1435–1438
Kaiser HJ, Schoetzau A, Flammer J (1997) Blood flow velocity in the extraocular vessels in chronic smokers. Br J Ophthalmol 8:133–135
Kiowski W, Linder L, Stoschitzky K, Pfisterer M, Burckhardt D, Burkart F, Buhler FR (1994) Diminished vascular response to inhibition of endothelium-derived nitric oxide and enhanced vasoconstriction to exogenously administered endothelin-1 in clinically healthy smokers. Circulation 90:27–34
Kiss B, Polska E, Dorner G, Polak K, Findl O, Mayrl GF, Eichler HG, Wolzt M, Schmetterer L (2002) Retinal blood flow during hyperoxia in humans revisited: concerted results using different measurement techniques. Microvasc Res 64:75–85
Langhans M, Michelson G, Groh MJ (1997) Effect of breathing 100% oxygen on retinal and optic nerve head capillary blood flow in smokers and non-smokers. Br J Ophthalmol 81:365–369
Luksch A, Garhofer G, Imhof A, Polak K, Polska E, Dorner GT, Anzenhofer S, Wolzt M, Schmetterer L (2002) Effect of inhalation of different mixtures of O2 and CO2 on retinal blood flow. Br J Ophthalmol 86:1143–1147
Monfrecola G, Riccio G, Savarese C, Posteraro G, Procaccini EM (1998) The acute effect of smoking on cutaneous microcirculation blood flow in habitual smokers and nonsmokers. Dermatology 197:115–118
Morgado PB, Chen HC, Patel V, Herbert L, Kohner EM (1994) The acute effect of smoking on retinal blood flow in subjects with and without diabetes. Ophthalmology 101:1220–1226
Pakola SJ, Grunwald JE (1993) Effects of oxygen and carbon dioxide on human retinal circulation. Invest Ophthalmol Vis Sci 34:2866–2870
Pitkin AD, Roberts CM, Wedzicha JA (1994) Arterialised earlobe blood gas analysis: an underused technique. Thorax 49:364–366
Polak K, Dorner G, Kiss B, Polska E, Findl O, Rainer G, Eichler HG, Schmetterer L (2000) Evaluation of the Zeiss retinal vessel analyser. Br J Ophthalmol 84:1285–1290
Pomerleau CS, Carton SM, Lutzke ML, Flessland KA, Pomerleau OF (1994) Reliability of the Fagerstrom tolerance questionnaire and the Fagerstrom test for nicotine dependence. Addict Behav 19:33–39
Riva CE, Grunwald JE, Sinclair SH, O’Keefe K (1981) Fundus camera based retinal LDV. Appl Opt 20:117–120
Riva CE, Grunwald JE, Sinclair SH, Petrig BL (1985) Blood velocity and volumetric flow rate in human retinal vessels. Invest Ophthalmol Vis Sci 26:1124–1232
Robinson F, Petrig BL, Riva CE (1985) The acute effect of cigarette smoking on macular capillary blood flow in humans. Invest Ophthalmol Vis Sci 26:609–613
Solberg Y, Rosner M, Belkin M (1998) The association between cigarette smoking and ocular diseases. Surv Ophthalmol 42:535–547
Stadler RW, Ibrahim SF, Lees RS (1998) Measurement of the time course of peripheral vasoactivity: results in cigarette smokers. Atherosclerosis 138:197–205
Steigerwalt RD Jr, Laurora G, Incandela L, Cesarone MR, Belcaro G, De Sanctis MT (2000) Ocular and orbital blood flow in cigarette smokers. Retina 20:394–397
Tamaki Y, Araie M, Nagahara M, Tomita K, Matsubara M (2000) The acute effects of cigarette smoking on human optic nerve head and posterior fundus circulation in light smokers. Eye 14:67–72
Williamson TH, Lowe GD, Baxter GM (1995) Influence of age, systemic blood pressure, smoking, and blood viscosity on orbital blood velocities. Br J Ophthalmol 79:17–22
Wright JL, Dai J, Zay K, Price K, Gilks CB, Churg A (1999) Effects of cigarette smoke on nitric oxide synthase expression in the rat lung. Lab Invest 79:975–983
Zhang S, Day I, Ye S (2001) Nicotine induced changes in gene expression by human coronary artery endothelial cells. Atherosclerosis 154:277–283
Acknowledgements
Financial support by the Medizinisch-wissenschaftlicher Fonds des Bürgermeisters der Bundeshauptstadt Wien (project 2190) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wimpissinger, B., Resch, H., Berisha, F. et al. Response of retinal blood flow to systemic hyperoxia in smokers and nonsmokers. Graefe's Arch Clin Exp Ophthalmol 243, 646–652 (2005). https://doi.org/10.1007/s00417-004-1083-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-1083-8