Abstract
Background and purpose
There has been scant research on the consequences of discontinuing second-line disease-modifying treatment (DMT) in middle-aged patients with multiple sclerosis (MS). The objective was therefore to examine the occurrence of focal inflammatory activity after the discontinuation of second versus first-line DMT in patients over 45 years.
Methods
Patients who had been treated for at least 6 months with second (natalizumab, fingolimod, anti CD20) or first-line DMT and who stopped their DMT were retrospectively included. Kaplan–Meier survival curves were used to study the occurrence of relapse and MRI activity according to the type of DMT stopped. Proportional hazard Cox models were calculated to identify factors associated with focal inflammatory activity. The annualized relapse rate was calculated under treatment and for every 3 months after DMT discontinuation.
Results
We included 232 patients (median age: 52.8 years), 49 of whom stopped second-line DMT. The probability of having a relapse within the year following discontinuation was 6% for first-line DMT, 9% for fingolimod and 43% for natalizumab. In multivariate analysis, the probability of relapse after DMT discontinuation was significantly increased with natalizumab compared to first-line DMT (HR = 3.24; 95% CI [1.52; 6.90]). A peak of relapse was observed at 0–3 months after stopping natalizumab or fingolimod.
Conclusion
Our study suggests that the risk of inflammatory activity is greater after discontinuation of natalizumab compared to other DMT even in middle-aged patients. As for younger patients, natalizumab discontinuation should only be considered if there is an adequate substitution of a different therapy.
.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical expression of multiple sclerosis (MS) is dynamic and changes according to patients’ age and disease duration. Natural history studies have consistently described a spontaneous reduction in relapses and focal MRI activity with age and disease duration in patients with relapsing-onset MS [1, 2]. Consequently, the benefits of disease-modifying treatments (DMT), which mainly act on this focal inflammatory component of MS, also decrease with patients’ age and disease duration [3]. Furthermore, treatment may cause serious side effects, especially after lengthy exposure and in older patients, such as infections [4, 5]-including progressive multifocal leukoencephalopathy with natalizumab [6]-, and cancer with fingolimod [7].
Although this is a common enough situation in clinical practice, there is still a lack of clear recommendations regarding DMT discontinuation [8]. For first-line DMT, retrospective studies consistently suggest that the risk of relapse after discontinuation decreases with patients’ age, particularly after 45 years, and DMTs can probably be safely stopped in patients over 55 or 60 years whose disease has remained stable for at least 5 years [9, 10]. For second-line DMTs, initial available data are reassuring concerning the risk of rebound in young patients after stopping anti-CD20 treatment [11]. By contrast, the discontinuation of natalizumab or fingolimod without adequate substitution of a different highly effective therapy is generally not recommended, owing to the possibility of a rebound effect, or at the very least the return of pre-existing focal inflammatory activity. Rebound after stopping natalizumab is well documented [12, 13], and it has also been described after the discontinuation of fingolimod [14]. For these second-line DMTs, however, we lack data concerning discontinuation after age 45 years (i.e. in a population potentially benefiting from the spontaneous reduction in MS inflammatory activity with age). Many questions therefore remain unanswered in daily clinical practice concerning the possibility of stopping second-line DMTs in older patients: is it possible to simply stop the immunosuppressive treatment without recourse to an alternative treatment and without risk for the patient? Should it be followed by another highly effective treatment, as in younger patients?
The objectives of the present study were therefore to (i) compare the occurrence of focal inflammatory activity (clinical relapse and/or MRI activity) and disability progression after first-line versus second-line DMT discontinuation in patients with MS aged over 45 years, (ii) compare the occurrence of focal inflammatory activity according to the subtype of second-line DMT stopped, (iii) compare the annualized relapse rate (ARR) under treatment and for every 3 months after DMT discontinuation.
Materials and methods
Study design
We carried out a retrospective study, based on data prospectively acquired at two centers in France (Rennes and Nantes University Hospitals) and recorded in the European Database for MS (EDMUS; [15]). All patients signed an informed consent form allowing their medical data to be included in EDMUS and used for research after anonymization. Data confidentiality and security were consistent with the recommendations of the French Data Protection Agency (CNIL).
Study population
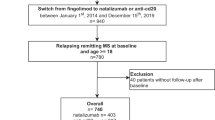
We included all patients who were registered at two EDMUS participating centers (Rennes and Nantes) in November 2021 and who met the following inclusion criteria: (1) relapsing–remitting, primary or secondary progressive MS according to 2017 McDonald criteria [16]; (2) treatment with the same first-line (interferon beta-1a IM, interferon beta-1b, interferon beta-1a, glatiramer acetate, dimethyl fumarate, or teriflunomide) or second-line (fingolimod, natalizumab, ocrelizumab, or rituximab) DMT for at least 6 months before treatment discontinuation; (3) DMT discontinuation with no initial intention of switching to another treatment; and (4) regular (at least yearly) follow-up at Rennes or Nantes MS centers during treatment and for at least 6 months after treatment discontinuation. Only patients seen at least once in the last 5 years were included. Patients treated with mitoxantrone, alemtuzumab or cladribine were not included.
Data collection
The following data were extracted from EDMUS in November 2021: demographic data, clinical data (disease phenotype, relapse, and disability), DMT data (history of treatments and reasons for discontinuation and resumption), radiological data (MRI date and MRI activity, for each MRI scan between 3 years prior discontinuation until the end of follow up). In addition, patients’ medical records were systematically checked to ensure that DMT had been discontinued without any intention of switching to another treatment (to exclude switches with extended intervals). Relapses and MRI activity that occurred after DMT was stopped were also systematically checked in medical records.
End-point definition
A relapse was defined as the appearance, recurrence or aggravation of neurological symptoms for a period of at least 24 h, without fever. We only counted relapses validated by the treating neurologist. Focal inflammatory activity was defined as clinical relapse or MRI activity (contrast enhancement after injection of gadolinium and/or new T2 lesion), whichever occurred first. Disability was assessed as the Expanded Disability Status Scale (EDSS) score. Disability progression was defined as an increase of at least 1 point if the baseline EDSS was < 5.5, or of 0.5 if the baseline EDSS was ≥ 5.5. This increase had to persist until the end of the patient follow-up.
Statistical analysis
First, we conducted a descriptive analysis of our patients’ characteristics for the whole sample and according to the type of DMT (first-line vs. second-line). Quantitative data were expressed as means and standard deviations (SDs) or medians and interquartile ranges (IQRs), and qualitative data as numbers and percentages. We performed comparisons between groups (first-line vs. second-line DMT) using Student t (parametric) or Mann–Whitney–Wilcoxon (nonparametric) tests for the quantitative data, and χ2 or Fisher tests for qualitative data. Variables with censored data (relapse, focal inflammatory activity, and EDSS score) were presented as Kaplan–Meier survival curves and compared with log-rank tests. The annualized relapse rate (ARR) was calculated according to the DMT used, under treatment during the year prior to treatment discontinuation, and at 3-month intervals after treatment discontinuation, expressed with a 95% confidence interval.
We then ran a univariate analysis to test the association between each of the potential explanatory variables and the variables to be explained after treatment discontinuation (relapse, focal inflammatory activity, and EDSS increase), using a univariate Cox’s model. Multivariate proportional hazard Cox models were used to test the potential impact of the DMT used (either first-line or one of three second-line DMTs: rituximab, fingolimod, or natalizumab) on each outcome (relapse, focal inflammatory activity, and EDSS increase). The potential confusing factors tested were sex, age at treatment discontinuation (45–50 years, 50–55 years, > 55 years), disease duration at treatment discontinuation (< 15 years, ≥ 15 years), treatment duration at treatment discontinuation (< 3 years, 3–5 years, > 5 years), relapse during the 3 years prior to treatment discontinuation, EDSS score at treatment discontinuation (< 3, 3–6, > 6), disease phase at treatment discontinuation (relapsing or progressive), MRI activity in the 3 years prior to treatment discontinuation. Explanatory variables with a p value of < 0.20 in the univariate analysis were introduced in the model. Results are presented as hazard ratios associated with a 95% confidence interval. The hypothesis of proportional risks was verified for each variable. The statistical significance rate was set at p < 0.05. All analyses were performed on the whole population, and after excluding primary progressive MS patients. All analyses were performed using SAS 9.4 statistical software (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics at treatment discontinuation
We included 232 patients in the study. Their characteristics at DMT discontinuation are summarized in Table 1. At discontinuation, their median age was 52.8 years (IQR: 49.2–58.7), and their median DMT duration was 2.6 years (IQR: 1.2–5.4). Of these, 183 were treated with a first-line DMT, and 49 with a second-line DMT (17 natalizumab, 23 fingolimod and 9 rituximab). Twenty-four patients (10.3%) had primary progressive MS (17 treated with first-line DMT, 6 with fingolimod and 1 with rituximab). Treatment duration before discontinuation was significantly shorter for second-line DMTs than for first-line DMTs: 3.0 years (IQR: 1.4–6.3) versus 1.6 years (IQR: 1.2–3.2); p = 0.0003. Main reasons for discontinuation, as entered in the EDMUS database, were a scheduled stop for 63 patients (i.e. with the agreement of the neurologist), convenience for 41 patients (i.e. without the agreement of the neurologist), inefficacy (mainly disability progression without relapse) for 39 patients, adverse events for 85 patients, and other reasons for four patients.
Relapses after treatment discontinuation
In our sample, median follow-up duration was 6.4 years (IQR: 2.9–9.6) after first-line DMT discontinuation and 4.2 years (IQR: 1.7–6.7) after second-line DMT discontinuation.
Probability of relapse after DMT discontinuation
Time from treatment discontinuation to first relapse according to type of discontinued DMT is shown in Fig. 1A and B. The probability of having a relapse at 1 year was 6% for patients who had discontinued first-line therapy, 9% for patients who had discontinued fingolimod, and 43% for patients who had discontinued natalizumab. After excluding primary progressive MS patients, the probability of having a relapse at 1 year remained the same for patients who discontinued first-line therapy (6%) and natalizumab (43%), but increased slightly to 12% for patients who had discontinued fingolimod. None of the nine patients who had discontinued rituximab experienced a relapse following discontinuation, but the mean follow-up duration for this subgroup was short (1.5 ± 0.8 years) and they were mainly patients with progressive MS (1 primary progressive MS, 6 secondary progressive MS and 3 relapsing remitting MS patients). The probability of having a relapse at 5 year was 21% for patients who had discontinued first-line therapy, 19% for patients who had discontinued fingolimod, and 59% for patients who had discontinued natalizumab.
Annualized relapse rate before and after DMT discontinuation
The ARR was not significantly higher during the year following first-line DMT discontinuation than under treatment (0.06 [0.03, 0.12] vs. 0.05 [0.03, 0.07]; p = 0.30). By contrast, it rose to 0.64 [0.32, 1.28] during the year after natalizumab discontinuation, compared with 0.14 [0.05, 0.38] under treatment (p = 0.02). Details of changes in ARR under treatment and after treatment discontinuation, measured every 3 months, are provided in Fig. 2. The increase in ARR over the year after natalizumab discontinuation was not constant over time, as we observed a peak 0–3 months after treatment discontinuation. A similar peak, albeit of smaller magnitude, was observed after stopping fingolimod.
Focal inflammatory activity (MRI and/or relapse) after treatment discontinuation
A total of 228 patients (98%) underwent at least one MRI scan after DMT discontinuation, including 160 (69%) within the first 12 months. The probability of having focal inflammatory activity at 1 year was 17% in patients who had discontinued first-line therapy, 23% in patients who had discontinued fingolimod, and 49% in patients who had discontinued natalizumab. Detailed results are provided in Fig. A1.
In the whole sample, 118 patients (50.9%) experienced focal inflammatory activity after treatment discontinuation during follow-up. In addition to the 52 patients who experienced clinical relapse during follow-up after DMT discontinuation, 66 patients had MRI activity without a clinical relapse.
Disability progression after treatment discontinuation
The median time from treatment discontinuation to disability progression was significantly shorter after stopping second-line DMT than after stopping first-line DMT (5.3 vs. 6.1 years; p = 0.03). Detailed results according to the DMT stopped are provided in Fig. A2.
Treatment resumption
The probability of resuming a treatment within the first year was 10% after first-line DMT discontinuation, 14% after fingolimod discontinuation and 37.5% after natalizumab discontinuation. Median time to treatment resumption was significantly shorter after stopping second-line DMT (12.5 months) than after stopping first-line DMT (27 months) (p = 0.03). The main reason for resuming treatment was clinical relapse (45%, including 9% with no clear MRI activity). The other reasons were MRI activity without clinical relapse (43%), disability progression (6%), and patient’s convenience (5%). Of the whole sample, 97 patients (41.8%) had resumed a DMT for the whole follow-up period.
DMT discontinuation beyond 55 years
Eighty-one patients out of 232 (35%) discontinued DMT after age 55 years, and 16 of them had a relapse (20%). Patients who stopped DMT before age 55 did not differ significantly from those who stopped after 55 years on time to first relapse (Fig. 3A). However, time to first MRI activity was significantly shorter before 55 years than after 55 years (Fig. 3B). For second-line DMT, of the nine patients who discontinued fingolimod after age 55 years, three experienced a relapse and one had MRI activity without a relapse, while two of the four patients who discontinued natalizumab experienced a relapse, and the patient who discontinued rituximab remained relapse-free (details in Table 2).
Factors associated with occurrence of clinical relapse after treatment discontinuation
Multivariate analysis revealed significant associations between the occurrence of relapse after treatment discontinuation and the occurrence of relapse during the 3 years prior to treatment discontinuation (p < 0.0001), treatment duration before discontinuation (p = 0.01), and type of treatment stopped, particularly natalizumab (HR = 3.24; 95% CI (1.52; 6,90) (details in Table 3). The results were similar after excluding primary progressive MS patients from the analysis.
Factors associated with focal inflammatory activity (clinical and/or MRI activity)
Multivariate analysis revealed significant associations between occurrence of focal inflammatory activity after DMT discontinuation and occurrence of relapse in the 3 years prior to treatment discontinuation (p < 0.0001), occurrence of MRI activity in the 3 years prior to treatment discontinuation (p = 0.03), phenotype (relapsing–remitting) of MS (p = 0.01) and treatment duration before discontinuation (p = 0.01). The probability of focal inflammatory activity after DMT discontinuation was not significantly increased with natalizumab compared to first-line DMT, but a trend was observed (details in Table A1).
Factors associated with significant EDSS increase
Multivariate analysis revealed significant associations between EDSS increase after DMT discontinuation and presence of MRI activity in the 3 years prior to treatment discontinuation (p = 0.03), phenotype (progressive) of MS (p = 0.0042), and type of treatment stopped (particularly fingolimod) (p = 0.0026) (details in Table A2).
Discussion
In this retrospective, two-center study of middle-aged patients with MS, the probability of having a relapse within 1 year of stopping DMT was 9%. However, this probability varied greatly depending on the type of DMT stopped, ranging from 6% for first-line DMTs to 43% for natalizumab, suggesting that stopping a continuous immunosuppressant second-line DMT is a different issue than stopping a first-line one, and the type of second-line DMT stopped is crucial, even in older patients.
First-line DMT discontinuation
In the present study, the probability of relapse 1 year after stopping first-line DMT was low (6%), but increased to 21% at 5 years. The probability of MRI activity and/or clinical activity was 17% at 1 year and 55% at 5 years. This probability is in line with that reported in the literature [9, 17,18,19,20], taking into account the median age (53 years) of our population at treatment discontinuation. For example, in a cohort of patients drawn from the MSBase registry who had a lower mean age (43 years), but had been relapse-free for 5 years or more, 36.4% of those who stopped first-line DMT experienced a relapse during the 5-year follow-up [9]. In a cohort of older patients (N = 178) who stopped their DMT after age 60 years, only one had a subsequent relapse [17]. Age at DMT discontinuation and inflammatory activity within the 3–5 years before discontinuation are the factors most often associated with relapse recurrence in the literature [9, 19,20,21]. In our study, having at least one relapse in the 3 years before DMT discontinuation was indeed associated with the occurrence of a relapse after discontinuation, but we did not find any significant association with age. This may have something to do with the size of our cohort and our inclusion criterion of > 45 years of age, as this limited the age range of our population and excluded younger patients for whom the effect of age might have been more pronounced.
Second-line DMT discontinuation
Overall, in our sample, the probability of relapse at 1 year was significantly higher after second-line DMT discontinuation (20%) than after first-line DMT discontinuation (6%). However, this probability varied greatly, depending on which second-line DMT was stopped, ranging from 9% for fingolimod to 43% for natalizumab. The risk of inflammatory disease reactivation after stopping natalizumab is well established in the literature [22], and a rebound effect (i.e. increase in new inflammatory disease activity after discontinuation compared with inflammation prior to DMT start) has also been documented [12, 23, 24]. A meta-analysis revealed that younger age, higher numbers of relapses and gadolinium-enhanced lesions before treatment start, and fewer infusions are associated with increased risk for post-natalizumab disease reactivation [12]. However, the mean age at discontinuation of natalizumab in these studies was 30–40 years, and few studies have been conducted exclusively among older patients. In a small prospective study, treatment was suspended in 15 patients with MS (mean age = 50 years) who had been treated with natalizumab for > 5 years and had no clinical or radiological signs of inflammatory disease activity [25]. Ten patients (66%) experienced a relapse after discontinuation, and five (33%) a rebound (four of these patients were aged over 50 years). These results are therefore in line with ours, and suggest that natalizumab cannot be stopped without a major risk for patients, even after age 50 or 55 years. This high probability of relapse after stopping natalizumab compared to first-line DMT in patients theoretically at lower risk of focal inflammatory activity (61.2% of patients who stopped their second-line DMT had progressive MS vs 46.5% in first-line DMT group) suggests not only a resumption of focal inflammatory activity normal for age, disease duration and MS phenotype but a very significant increase in probability related to stopping natalizumab. It is interesting to note that in the multivariate analysis, the occurrence of relapse after stopping DMT was not significantly related to the phenotype of the disease. Similarly, after exclusion of patients with primary progressive MS, the results regarding the probability of relapse remained unchanged. These results suggest that even patients with progressive MS are at risk for resumption of inflammatory disease activity after stopping natalizumab. Alternative therapy should probably be considered regardless of the MS phenotype.
In our study, the probability of relapse at 1 year after discontinuation of fingolimod was low (9%), but the probability of recurrence of inflammatory activity (relapse and/or MRI activity) was 23%. These probabilities are somewhat lower than those reported in the literature for younger patients (around 30% for disease activity and 10% for rebound) [26, 27]. As previously reported, we observed the increase in ARR within 3 months of discontinuation. As with natalizumab, studies including only older patients are rare in the literature. In a recent retrospective study among patients who had been stable on fingolimod and who had discontinued their treatment, the incidence of disease activity was not significantly lower in older patients (> 50 years) than in younger patients (19% vs. 36.5%, p = 0.17) although a trend was observed. Beyond 60 years, none of the 11 patients concerned exhibited disease activity after stopping fingolimod [27]. In our study, however, two patients who had stopped fingolimod experienced inflammatory activity beyond the age of 60 years. This result, albeit based on a small number of patients, should make us cautious about stopping fingolimod without giving an alternative treatment, even in older patients.
Only nine patients stopped rituximab after age 45 years, and none stopped ocrelizumab in our study. The use of these treatments is more recent in our centers. Consequently, the mean duration of follow-up after stopping rituximab was also short (e.g. 1.6 years vs. 7 years for natalizumab). Moreover, rituximab has a persistent effect (at least 6 months after the infusion). This effect must be taken into account in the interpretation of our results because we considered the date of the last rituximab infusion as the date of treatment discontinuation. In addition, the patients who discontinued rituximab in our study were mainly patients with progressive MS (1 primary progressive MS, 6 secondary progressive MS and 3 relapsing remitting MS patients). None of these patients experienced a relapse after stopping rituximab. These results are in agreement with a recent study in younger relapsing–remitting MS patients [11]. Among the 92 patients (median age around 40 years) treated with rituximab and who had stopped treatment for different reasons, only three of these patients had a relapse, and just four had new MRI lesions during the 30 months of follow up. These encouraging results suggest that rituximab can be discontinued without the need for an alternative treatment in older patients, but will have to be confirmed.
Limitations
This retrospective study had several limitations. First, our sample size was small, especially for second-line DMTs (n = 49), so our preliminary results will need to be confirmed in larger studies dedicated to each type of second-line DMT. Second, our inclusion criterion for age was at least 45 years at treatment discontinuation, which is quite young compared to what is known about the natural history of focal inflammation with age and immunosenescence [2]. However, discontinuation of second-line treatment is still uncommon in older patients. Thus, only 14 patients stopped a second-line DMT after age 55 years in our sample, probably because neurologists and patients are more inclined to stop second-line DMTs earlier than first-line ones, as the risks of these treatments increase with age. Our study was thus a reflection of clinical practice, at least in our two centers. Moreover, given the known risk of recurrence of focal inflammatory activity after discontinuation in younger patients, de-escalation may be performed, rather than discontinuation. Although we focused on discontinuation without a treatment switch, this option should also be investigated in detail. Third, it is important to note that we included patients with both remitting and progressive (secondary and primary) MS in our study. Indeed, progressive patients represented 49.5% of our sample. As there is known to be less focal inflammatory activity in progressive forms of the disease [1], our results cannot be extrapolated to a purely relapsing–remitting MS population, in which the risk of focal inflammatory activity is probably greater. However, the main results of our study were unchanged after excluding primary progressive MS patients. Fourth, as in many retrospective studies, a lack of standardized clinical and radiological follow-up made it difficult to accurately assess MRI activity in patients before and after stopping treatment. Only prospective studies with a predefined MRI schedule can provide good quality MRI data. Finally, it is important to note that both stable (i.e. without relapses and MRI activity) and unstable patients were included in our study, thus increasing the risk of inflammatory activity after stopping DMT. In clinical practice, scheduled discontinuation of DMT is generally envisaged for patients who have been stable for several years.
Perspectives
With the increased use of DMTs and the aging of patients with MS under DMT, the question of possible discontinuation has become central in clinical practice. Several prospective, randomized studies on this subject are currently underway (DISCOMS, STOP-I-SEP, DOT-MS). However, patients on second-line treatments are in the minority in these studies, either because it is an exclusion criterion, or because the neurologist has not included them because of the potential risk of disease reactivation. Large retrospective, multicenter studies are therefore needed on this subject to guide the decisions of neurologists and patients.
Conclusion
Our study suggests that the risk of recurrence of focal inflammatory activity is greater after discontinuation of second-line versus first-line DMTs, even in patients over 45 years of age (and even after 55 years). As for younger patients, natalizumab discontinuation should only be considered if an alternative therapy is available.
References
Ahrweiller K, Rousseau C, Le Page E, Bajeux E, Leray E, Michel L et al (2020) Decreasing impact of late relapses on disability worsening in secondary progressive multiple sclerosis. Mult Scler 26(8):924–935. https://doi.org/10.1177/1352458519848090
Tremlett H, Zhao Y, Joseph J, Devonshire V, UBCMS Clinic Neurologists (2008) Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 79(12):1368–1374. https://doi.org/10.1136/jnnp.2008.145805
Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B (2017) Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol 8:577. https://doi.org/10.3389/fneur.2017.00577
Grebenciucova E, Pruitt A (2017) Infections in patients receiving multiple sclerosis disease-modifying therapies. Curr Neurol Neurosci Rep 17(11):88. https://doi.org/10.1007/s11910-017-0800-8
Luna G, Alping P, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M et al (2020) Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 77(2):184–191. https://doi.org/10.1001/jamaneurol.2019.3365
Sørensen PS, Bertolotto A, Edan G, Giovannoni G, Gold R, Havrdova E et al (2012) Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler 18(2):143–152. https://doi.org/10.1177/1352458511435105
Alping P, Askling J, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M et al (2020) Cancer risk for fingolimod, natalizumab, and rituximab in multiple sclerosis patients. Ann Neurol 87(5):688–699. https://doi.org/10.1002/ana.24651
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D et al (2018) ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 24(2):96–120
Kister I, Spelman T, Alroughani R, Lechner-Scott J, Duquette P, Grand’Maison F et al (2016) Discontinuing disease-modifying therapy in MS after a prolonged relapse-free period: a propensity score-matched study. J Neurol Neurosurg Psychiatry 87(10):1133–1137. https://doi.org/10.1136/jnnp-2016-313760
Kaminsky A-L, Omorou AY, Soudant M, Pittion-Vouyovitch S, Michaud M, Anxionnat R et al (2020) Discontinuation of disease-modifying treatments for multiple sclerosis in patients aged over 50 with disease Inactivity. J Neurol 267(12):3518–3527. https://doi.org/10.1007/s00415-020-10029-9
Juto A, Fink K, Al Nimer F, Piehl F (2020) Interrupting rituximab treatment in relapsing-remitting multiple sclerosis; no evidence of rebound disease activity. Mult Scler Relat Disord 37:101468. https://doi.org/10.1016/j.msard.2019.101468
Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S (2019) Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord 12:1756286419837809. https://doi.org/10.1177/1756286419837809
Kerbrat A, Le Page E, Leray E, Anani T, Coustans M, Desormeaux C et al (2011) Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing remitting multiple sclerosis patients. J Neurol Sci 308(1–2):98–102. https://doi.org/10.1016/j.jns.2011.05.043
Hatcher SE, Waubant E, Nourbakhsh B, Crabtree-Hartman E, Graves JS (2016) Rebound Syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol 73(7):790–794. https://doi.org/10.1001/jamaneurol.2016.0826
Confavreux C, Compston DA, Hommes OR, McDonald WI, Thompson AJ (1992) EDMUS, a European database for multiple sclerosis. J Neurol Neurosurg Psychiatry 55(8):671–676. https://doi.org/10.1016/j.jns.2011.05.043
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Hua LH, Fan TH, Conway D, Thompson N, Kinzy TG (2019) Discontinuation of disease-modifying therapy in patients with multiple sclerosis over age 60. Mult Scler 25(5):699–708. https://doi.org/10.1177/1352458518765656
Bonenfant J, Bajeux E, Deburghgraeve V, Le Page E, Edan G, Kerbrat A (2017) Can we stop immunomodulatory treatments in secondary progressive multiple sclerosis? Eur J Neurol 24(2):237–244. https://doi.org/10.1111/ene.13181
Yano H, Gonzalez C, Healy BC, Glanz BI, Weiner HL, Chitnis T (2019) Discontinuation of disease-modifying therapy for patients with relapsing-remitting multiple sclerosis: Effect on clinical and MRI outcomes. Mult Scler Relat Disord 35:119–127. https://doi.org/10.1016/j.msard.2019.07.021
Bsteh G, Feige J, Ehling R, Auer M, Hegen H, Di Pauli F et al (2017) Discontinuation of disease-modifying therapies in multiple sclerosis—clinical outcome and prognostic factors. Mult Scler 23(9):1241–1248. https://doi.org/10.1177/1352458516675751
Kister I, Spelman T, Patti F, Duquette P, Trojano M, Izquierdo G et al (2018) Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J Neurol Sci 391:72–76. https://doi.org/10.1016/j.jns.2018.06.001
Fox et al. (2014) MS disease activity in RESTORE A randomized 24-we.pdf. https://doi.org/10.1212/WNL.0000000000000355
Killestein J, Vennegoor A, Strijbis EM, Seewann A, van Oosten BW, Uitdehaag BMJ et al (2010) Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol 68(3):392–395. https://doi.org/10.1002/ana.22074
O’Connor PW, Goodman A, Kappos L, Lublin FD, Miller DH, Polman C et al (2011) Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 76(22):1858–1865. https://doi.org/10.1212/WNL.0b013e31821e7c8a
Fagius J, Feresiadou A, Larsson E-M, Burman J (2017) Discontinuation of disease modifying treatments in middle aged multiple sclerosis patients. First line drugs vs natalizumab. Mult Scler Relat Disord 12:82–87. https://doi.org/10.1016/j.msard.2017.01.009
Frau J, Sormani MP, Signori A, Realmuto S, Baroncini D, Annovazzi P et al (2018) Clinical activity after fingolimod cessation: disease reactivation or rebound? Eur J Neurol 25(10):1270–1275. https://doi.org/10.1111/ene.13694
Pantazou V, Pot C, Du Pasquier R, Le Goff G, Théaudin M (2021) Recurrence of disease activity after fingolimod discontinuation in older patients previously stable on treatment. Mult Scler Relat Disord 51:102918. https://doi.org/10.1016/j.msard.2021.102918
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors did not receive support from any organization for the submitted work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chappuis, M., Rousseau, C., Bajeux, E. et al. Discontinuation of second- versus first-line disease-modifying treatment in middle-aged patients with multiple sclerosis. J Neurol 270, 413–422 (2023). https://doi.org/10.1007/s00415-022-11341-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11341-2