Abstract
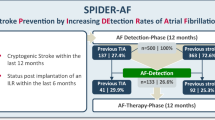
Atrial fibrillation (AF) leads to a high risk of recurrent stroke, and the insertable cardiac monitor (ICM), as a new kind of electrocardiographic monitoring device, has been proven to enhance the recognition rate of AF. The aim of this systematic review was to evaluate the efficacy and safety of the ICM use in AF detection of patients with stroke. We pooled 1233 patients from three randomized controlled trials (RCTs). The detection rate of AF was superior in the ICM group to that in the control group at 6 months (risk ratio [RR], 4.63; P < 0.0001; 95% confidence interval [CI], 2.17–9.90) and 12 months (RR, 5.04; P < 0.00001; 95% CI, 2.93 to 8.68). Patients in the ICM group had a higher rate of oral anticoagulant usage (RR, 2.76; P < 0.00001; 95% CI, 1.89–4.02). However, there was no difference in the time to first detection of AF within 12 months (mean difference, − 8.28; P = 0.82; 95% CI, − 77.84–61.28) or the rate of recurrent ischemic stroke or transient ischemic attack (RR, 0.88; P = 0.51; 95% CI, 0.60–1.28) between the ICM and control groups. In addition, the ICM group experienced more adverse events than the control group within 12 months (RR, 4.42; P = 0.002; 95% CI, 1.69–11.55). To conclude, the sensitivity of ICM is superior to that of conventional external cardiac monitoring. Reducing adverse reactions will be a new development direction of ICM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, stroke remains the second-leading cause of death, as well as the third-leading cause of death and disability combined [1]. According to the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD), there were 12.2 million incident cases of stroke in 2019 and ischemic stroke constituted 62.4% of all new strokes [1]. Due to the high cost of acute treatment and post-care assistance in nursing homes, stroke has resulted in a major burden on the patient’s family and society alike.
Atrial fibrillation (AF) is a well-known cause of ischemic stroke [2]. According to research statistics, across all age groups, non-valvular AF independently increases the risk of stroke by almost five times [3]. Previous studies have reported that ischemic stroke patients with AF have higher recurrence rates [4]. In addition, oral anticoagulants have been confirmed to reduce the risk of recurrent stroke more significantly than antiplatelet therapy among ischemic stroke patients with AF [5]. Therefore, AF detection after ischemic stroke in patients is crucial when making the decision of whether to initiate anticoagulant therapy in clinical practice for secondary prevention [6]. There are several AF monitoring strategies, and these include included insertable cardiac monitors (ICMs) [7, 8] and other conventional external cardiac monitors, such as serial electrocardiography (ECG) [9], monitoring with external loop recorders [10, 11], and Holter monitoring [12]. The detection rates of these strategies range from 0 to 25%.
As a subcutaneous device, an ICM can record the heart rhythm over a period of up to 3 years for patients with paroxysmal AF (PAF) [13]. Unlike traditional operations, due to their small device sizes and simplified procedures, implanting the latest ICMs is considered minimally invasive and can be performed even in an outpatient setting, which vastly contributes to the conservation of medical resources, and reduces costs [14]. Meanwhile, their use has been shown to correlate with fewer surgical complications and higher sensing performance [15]. In addition, with the application of remote monitoring technology to make follow-up more convenient, such has been proven to save hospital resources, require less time, and reduce accidental and emergency visits [16]. However, due to the inconsistencies in the endpoints, eligibility criteria, and monitoring duration of diverse studies, it is difficult to conduct clinical translation and evaluate whether ICM is better than conventional external cardiac monitors.
Currently, there are few systematic analyses of ICMs for AF detection in patients with ischemic stroke. In addition, more evidence of a sufficient quality is needed for clinicians to support clinical decision-making for patients with ischemic stroke. Therefore, we pooled data from previous randomized controlled trials (RCTs) and conducted a systematic review and meta-analysis to investigate the efficacy and safety of ICMs for AF detection in ischemic stroke patients.
Methods
Study protocol
Before the project started, we drafted a research protocol following the Cochrane Collaboration format [17] and the protocol was registered on the INPLASY website (Register number INPLASY2021100108).
Eligibility criteria
We set the inclusion criteria as follows: (1) study type: RCT; (2) language restriction: published in English; (3) participants: adult patients who had received a diagnosis of ischemic stroke or transient ischemic attack (TIA); (4) intervention: ICM and conventional external cardiac monitoring; (5) outcomes: efficacy outcomes including patients detection of AF at 6 months, patients detection of AF at 12 months, time to the detection of AF, recurrent ischemic stroke or TIA and use of oral anticoagulants; safety outcomes including adverse events (AEs). Included RCTs were not requested to supply all the outcomes mentioned above.
We set the exclusion criteria as follows: study type: retrospective studies, cohort studies, case reviews and case reports.
Search strategy
Two independent investigators (XT and XW) systematically searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov to identify relevant studies published until July 31, 2021. The following search strategy was employed: (insertable cardiac monitor [Title/Abstract]) AND (stroke [Title/Abstract]) for MEDLINE; “insertable cardiac monitor”/exp AND “stroke”/exp for EMBASE; “insertable cardiac monitor” in Title Abstract Keyword AND “stroke” in Title Abstract Keyword for CENTRAL; and “insertable cardiac monitor | stroke” for ClinicalTrials.gov. In addition, the reference lists of RCTs, relevant systematic reviews and meta-analyses were also screened independently and manually to ensure a more comprehensive search.
Study selection and data collection
According to the eligibility criteria mentioned above, two authors (XT and ZLW) independently evaluated all records retrieved from the four databases and the reference lists of RCTs and relevant systematic reviews or meta-analyses. Any duplicates and research articles only available as abstracts were excluded. A third author (JZ), who did not participate in the process of data collection, made final decisions concerning disputed data when disagreements emerged among the two authors. The selection process was summarized in Fig. 1. After selection and evaluation, all data from the included RCTs were extracted as follows: basic information and outcome events included for each RCT (Table 1), inclusion and exclusion criteria, and study design; all efficacy and safety outcomes were showed in the online supplementary materials (Table S1).
Risk of bias
The risk of bias plot was evaluated using the Review Manager version 5.3 software program (Cochrane, London, England). The uniform criteria of the Cochrane Collaboration were used to assess the risk of bias for RCTs, including that of: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other potential biases. Each bias criterion was classified as “low”, “high”, or “unclear”.
Summary measures and synthesis of results
The Review Manager version 5.3 software program was used to assess the data. For the dichotomous outcomes, the risk ratio (relative risk [RR]) with the 95% confidence interval (CI) was analyzed and calculated with a random-effects model. The mean difference (MD) was used only for the continuous outcome “time to the detection of AF”. Heterogeneity was estimated via the I2 statistic, where a value of less than 30% suggested “low heterogeneity”; that between 30 and 50% means “moderate heterogeneity”, and that of greater than 50% denotes “substantial heterogeneity”. Sensitivity analysis was used to explore the stability of the consolidated results. For all the analyses, two tailed tests were performed, and a P value of less than 0.05 was considered to be statistically significant.
Results
Search results and study characteristics
MEDLINE, EMBASE, CENTRAL and ClinicalTrials.gov provided 829 titles and abstracts for review. Of these, a total of 710 articles were excluded due to duplication and irrelevance and 119 full articles were further assessed for eligibility; subsequently, 116 articles, including 28 non-randomized clinical trials, seven case reports, five meta-analyses, and 76 reviews. Eventually, three RCTs [18,19,20] containing 1233 patients (613 in the ICM group and 620 placebo group) were selected for qualitative synthesis (Fig. 1). The main characteristics of the included three studies were listed in Table 1.
Efficacy outcome
In this meta-analysis, efficacy outcomes including patients detection of AF at 6 months, patients detection of AF at 12 months, the time to first detection of AF within 12 months, recurrent ischemic stroke or TIA, and use of oral anticoagulants, were investigated. The ICM group showed a significantly higher detection rate of AF than the control group at both 6 months (RR, 4.63; P < 0.0001; 95% CI, 2.17–9.90; Fig. 2A) and 12 months (RR, 5.04; P < 0.00001; 95% CI, 2.93–8.68; Fig. 2B). However, as shown in Fig. 2C, there was no difference in the time to first detection of AF within 12 months between ICM group and control group (MD, − 8.28; P = 0.82; 95% CI, − 77.84–61.28). One RCT was excluded in Fig. 2C due to offering insufficient data of the time to first detection of AF within 12 months [20]. The control group demonstrated a higher rate of recurrent ischemic stroke or TIA than the ICM group over 12 months, though no significant difference was found (RR, 0.88; P = 0. 51; 95% CI, 0.60–1.28; Fig. 2D). A significant difference was also found in the rate of use of oral anticoagulants between the two groups after the detection of AF (RR, 2.76; P < 0.00001; 95% CI, 1.89–4.02; Fig. 2E).
Safety outcome
As for the safety outcome, patients in the ICM group experienced more AEs, such as infection, pain, irritation or inflammation and hemorrhage at the insertion site, than the control group within 12 months (RR, 4.42; P = 0.002; 95% CI, 1.69–11.55; Fig. 3).
Risk of bias in included studies
The details of risk of bias for the three RCTs were exhibited in Fig. 4. All included clinical trials had a low risk of bias in random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data and selective reporting. Blinding of participants and personnel was high in all three included studies as insertion of ICM was an invasive operation. Except for the above-mentioned items, no unclear or high risk of bias in other items was discovered.
Discussion
In general, the results of the present study were based on three RCTs, which included stroke patients with no evidence of AF randomly assigned to ICM implantation or conventional follow-up (control). The results of our meta-analysis presented that, compared to the control group, the ICM group presented significant higher detection rate of AF at both 6 months and 12 months. Furthermore, it indicated that the sensitivity of ICMs was superior to that of conventional external cardiac monitoring. Correspondingly, compared to the control group, a higher rate of oral anticoagulant use after AF was found in the ICM group. However, there was no difference between the ICM and control groups in the time to AF first detection within 12 months. Meanwhile, the ICM group did not exhibit a statistically significant decrease in the rate of recurrent ischemic stroke or TIA relative to the control group during the 12 months.
As a major independent risk factor of stroke, AF is also a leading preventable cause of recurrent stroke [21]. About 20% to 30% of patients with stroke are diagnosed with AF in various periods before and after the event [22]. Previous studies have reported that patients with AF have a 4.8-fold higher risk of stroke onset [23] and experience poorer clinical outcomes than those without AF [4]. Currently, the etiology of 20% to 40% of acute stroke patients is still unclear [24]. As it has been proved, in many patients diagnosed with cryptogenic ischemic stroke (CIS), the cause is attributable to clinically silent AF [22, 25]. In 2018, Katz et al. conducted a cohort study, which indicated that PAF was detectable in 11.8% of non-CIS patients [26]. Furthermore, the choice of anticoagulation therapy is different for stroke patients with or without AF [27]. Therefore, early detection and diagnosis of AF are significant for patients after stroke in clinical practice.
As AF episodes may be symptomatic, asymptomatic or both, and the correlation between AF and symptoms is poor, it is often misdiagnosed and may not be detected by traditional monitoring techniques [28, 29]. Many new monitoring techniques have been developed in recent years. In 1997, ICMs were first introduced to detect cardiac arrhythmias in patients with undocumented palpitations and undetermined syncope [13]. With continuous updates to technology, the size of ICM devices has gradually decreased. Since 2014, a miniaturized device, Reveal LINQ™ (Medtronic, Minneapolis, MN, USA), has drawn attention. This device is inserted into the subcutaneous tissue over the heart using a specially developed insertion tool kit, which leads to fewer surgical complications and higher sensing performance [15]. Although there are a variety of monitoring methods to choose from, the best method for monitoring AF after a stroke is still a controversial issue.
Our analysis presented that there was no difference between the ICM and control groups in the time to first detection of AF within 12 months. This result may be due to the requirements of the automatic detection algorithm duration; in the ICM group, AF was detected when the episode persisted for at least 2 min, rather than 30 s, while in the control group this restriction did not exist, which interfered with the time to first detection of AF. In addition, our analysis also showed that there was no difference between the two groups on the rate of recurrent ischemic stroke or TIA over 12 months. At present, the minimum burden of AF to initiate anticoagulation is not yet clear [30], which may interfere with the timing of anticoagulation initiation and the proportion of anticoagulant treatment coverage in the two groups. Finally, it may affect the comparison of the recurrence rate of stroke or TIA between the two groups. Moreover, previous studies have shown that the risk of stroke is tied to a synergism between the severity of associated comorbidities and AF duration. A subanalysis of data from the ASymptomatic atrial fibrillation and Stroke Evaluation in pacemaker patients and the atrial fibrillation Reduction atrial pacing Trial (ASSERT) indicated that a 24-h duration of subclinical AF may be significant enough to increase the stroke risk [31]. The definition of AF in the RCTs we included ranged from 30 s to 2 min, i.e., they remained short. Therefore, although the ICM group had a higher rate of AF detection, the duration of AF detected by conventional follow-up was mostly longer, which may explain the small difference in the stroke recurrence rate between the ICM and control groups, though no significant difference was detected.
In addition, compared to the control group, the placement of an ICM statistically increases the risk of AEs, such as infection, pain, inflammation, and hemorrhage. In 2017, a relevant clinical trial showed that whether implantation was performed in a traditional electrophysiology laboratory or in an outpatient clinic, the incidence of complications following ICM implantation is very low [32]. The Reveal LINQ™ used in the trial mentioned above is the smallest ICM available at present, and the ICMs involved in our analysis were not all the latest generation equipment. We believe that, with further improvements to the technology, the adverse reactions of ICMs will be even more reduced. To our knowledge, this study is the most comprehensive systematic analysis of the ICM in patients with ischemic stroke to date.
However, several limitations of the present meta-analysis should not be ignored. First, our analysis was performed based on limited data. Despite an extensive search, only three published RCTs were pooled to test the efficacy and safety of ICMs. Second, in the analysis of the first time of AF detection, a high level of heterogeneity (92%) was found. This may be partly because we were unable to extract data from one RCT. However, our sensitivity analysis finally demonstrated that all the statistics were robust (Fig. S1). Certainly, we will continue to focus on large-scale and high-quality studies in the future.
Conclusion
In conclusion, the present study indicated that ICM implantation in patients after ischemic stroke is superior to the conventional follow-up in the detection of AF. The ICM group is prone to experiencing more AEs due to the invasive operation. From a comprehensive point of view, ICMs constitute a promising electrocardiographic monitoring method for patients after ischemic stroke, and further improving the sensitivity and accuracy of ICM and reducing adverse reactions will be a new direction for post-stroke electrocardiographic monitoring. More large-scale, high-quality studies are needed to identify further strategies for monitoring approaches.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
References
Feigin VL et al (2021) Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol 20(10):795–820. https://doi.org/10.1016/s1474-4422(21)00252-0
Freedman B, Potpara TS, Lip GY (2016) Stroke prevention in atrial fibrillation. Lancet 388(10046):806–817. https://doi.org/10.1016/s0140-6736(16)31257-0
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN et al (2020) Heart disease and stroke statistics-2020 update: a report from the american heart association. Circulation 141(9):e139–e596. https://doi.org/10.1161/cir.0000000000000757
Kimura K, Minematsu K, Yamaguchi T (2005) Atrial fibrillation as a predictive factor for severe stroke and early death in 15,831 patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry 76(5):679–683. https://doi.org/10.1136/jnnp.2004.048827
(1993) Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) Study Group. Lancet 342(8882):1255–1262
Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G (2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 33(21):2719–2747. https://doi.org/10.1093/eurheartj/ehs253
Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ (2013) Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 80(17):1546–1550. https://doi.org/10.1212/WNL.0b013e31828f1828
Etgen T, Hochreiter M, Mundel M, Freudenberger T (2013) Insertable cardiac event recorder in detection of atrial fibrillation after cryptogenic stroke: an audit report. Stroke 44(7):2007–2009. https://doi.org/10.1161/STROKEAHA.113.001340
Douen AG, Pageau N, Medic S (2008) Serial electrocardiographic assessments significantly improve detection of atrial fibrillation 2.6-fold in patients with acute stroke. Stroke 39(2):480–482. https://doi.org/10.1161/STROKEAHA.107.492595
Jabaudon D, Sztajzel J, Sievert K, Landis T, Sztajzel R (2004) Usefulness of ambulatory 7-day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke 35(7):1647–1651. https://doi.org/10.1161/01.STR.0000131269.69502.d9
Higgins P, MacFarlane PW, Dawson J, McInnes GT, Langhorne P, Lees KR (2013) Noninvasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke: a randomized, controlled trial. Stroke 44(9):2525–2531. https://doi.org/10.1161/strokeaha.113.001927
Manina G, Agnelli G, Becattini C, Zingarini G, Paciaroni M (2014) 96 hours ECG monitoring for patients with ischemic cryptogenic stroke or transient ischaemic attack. Intern Emerg Med 9(1):65–67. https://doi.org/10.1007/s11739-012-0755-3
Burkowitz J, Merzenich C, Grassme K, Bruggenjurgen B (2016) Insertable cardiac monitors in the diagnosis of syncope and the detection of atrial fibrillation: a systematic review and meta-analysis. Eur J Prev Cardiol 23(12):1261–1272. https://doi.org/10.1177/2047487316632628
Kanters TA, Wolff C, Boyson D, Kouakam C, Dinh T, Hakkaart L, Rutten-Van Mölken MP (2016) Cost comparison of two implantable cardiac monitors in two different settings: Reveal XT in a catheterization laboratory vs. Reveal LINQ in a procedure room. Europace 18(6):919–924. https://doi.org/10.1093/europace/euv217
De Angelis MV, Di Stefano V, Franciotti R, Furia N, Di Girolamo E, Onofrj M, Faustino M (2020) Cryptogenic stroke and atrial fibrillation in a real-world population: the role of insertable cardiac monitors. Sci Rep 10(1):3230. https://doi.org/10.1038/s41598-020-60180-6
Drak-Hernández Y, Toquero-Ramos J, Fernández JM, Pérez-Pereira E, Castro-Urda V, Fernández-Lozano I (2013) Effectiveness and safety of remote monitoring of patients with an implantable loop recorder. Rev Esp Cardiol (English ed) 66(12):943–948. https://doi.org/10.1016/j.rec.2013.06.009
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clin Res ed) 339:b2700. https://doi.org/10.1136/bmj.b2700
Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F et al (2014) Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 370(26):2478–2486. https://doi.org/10.1056/NEJMoa1313600
Bernstein RA, Kamel H, Granger CB, Piccini JP, Sethi PP, Katz JM, Vives CA, Ziegler PD, Franco NC, Schwamm LH et al (2021) Effect of long-term continuous cardiac monitoring vs usual care on detection of atrial fibrillation in patients with stroke attributed to large- or small-vessel disease: the STROKE-AF randomized clinical trial. JAMA 325(21):2169–2177. https://doi.org/10.1001/jama.2021.6470
Buck BH, Hill MD, Quinn FR, Butcher KS, Menon BK, Gulamhusein S, Siddiqui M, Coutts SB, Jeerakathil T, Smith EE et al (2021) Effect of implantable vs prolonged external electrocardiographic monitoring on atrial fibrillation detection in patients with ischemic stroke: the per diem randomized clinical trial. JAMA 325(21):2160–2168. https://doi.org/10.1001/jama.2021.6128
Kulach A, Dewerenda M, Majewski M, Lasek-Bal A, Gasior Z (2021) Supraventricular Runs in 7-day holter monitoring are related to increased incidence of atrial fibrillation in a 3-year follow-up of cryptogenic stroke patients free from arrhythmia in a 24 h-holter. J Cardiovasc Dev Dis. https://doi.org/10.3390/jcdd8070081
Kishore A, Vail A, Majid A, Dawson J, Lees KR, Tyrrell PJ, Smith CJ (2014) Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke 45(2):520–526. https://doi.org/10.1161/strokeaha.113.003433
Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22(8):983–988. https://doi.org/10.1161/01.str.22.8.983
Li L, Yiin GS, Geraghty OC, Schulz UG, Kuker W, Mehta Z, Rothwell PM (2015) Incidence, outcome, risk factors, and long-term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population-based study. Lancet Neurol 14(9):903–913. https://doi.org/10.1016/s1474-4422(15)00132-5
Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, Sacco RL, Connolly SJ (2014) Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 13(4):429–438. https://doi.org/10.1016/s1474-4422(13)70310-7
Katz JM, Eng MS, Carrazco C, Patel AV, Jadonath R, Gribko M, Arora R, Libman RB (2018) Occult paroxysmal atrial fibrillation in non-cryptogenic ischemic stroke. J Neurol 265(10):2237–2242. https://doi.org/10.1007/s00415-018-8982-9
Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Georg Haeusler K, Oldgren J, Reinecke H, Roldan-Schilling V et al (2018) The 2018 european heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace 20(8):1231–1242. https://doi.org/10.1093/europace/euy054
Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C et al (2012) Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 366(2):120–129. https://doi.org/10.1056/NEJMoa1105575
Seet RC, Friedman PA, Rabinstein AA (2011) Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation 124(4):477–486. https://doi.org/10.1161/circulationaha.111.029801
January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL et al (2019) 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol 74(1):104–132. https://doi.org/10.1016/j.jacc.2019.01.011
Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, de Graaf JJ, Freericks M, Verma A, Wang J, Leong D et al (2017) Subclinical atrial fibrillation in older patients. Circulation 136(14):1276–1283. https://doi.org/10.1161/CIRCULATIONAHA.117.028845
Diederichsen SZ, Haugan KJ, Højberg S, Holst AG, Køber L, Pedersen KB, Graff C, Krieger D, Brandes A, Svendsen JH (2017) Complications after implantation of a new-generation insertable cardiac monitor: results from the LOOP study. Int J Cardiol 241:229–234. https://doi.org/10.1016/j.ijcard.2017.03.144
Funding
This work was supported by the Suzhou Health Talents Training Project (GSWS2019002).
Author information
Authors and Affiliations
Contributions
XT and ZLW was the principal investigator. XT and XW designed the study and developed the analysis plan. XW, XT and JZ analyzed the data and performed meta-analysis. TX and ZLW contributed in writing of the article. ZMS and YJQ revised the manuscript and polish the language. ZQC, ZW and GC supervised the project. All authors read and approved the final submitted paper.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethics approval
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tan, X., Wang, Z., Wu, X. et al. The efficacy and safety of insertable cardiac monitor on atrial fibrillation detection in patients with ischemic stroke: a systematic review and meta-analysis. J Neurol 269, 2338–2345 (2022). https://doi.org/10.1007/s00415-021-10903-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10903-0