Abstract
Background
Malignant gliomas (MG) are aggressive brain tumours in adults. The standard of care is concurrent radiation plus temozolomide (TMZ) [chemo-radiotherapy (CRT)] followed by TMZ maintenance up to 6 months. TMZ is considered to have a low toxicity profile, but several studies reported occurrence of severe myelosuppression, especially during the concomitant phase. Toxicity may be prolonged, thus treatment should be discontinued.
Purpose
To evaluate the risk of recurrente myelotoxicity during adjuvant chemotherapy (CT) in patients who recovered from severe myelotoxicity during CRT.
Methods
We retrospectively collected data on patients with MG who developed and recovered from severe myelotoxicity during CRT from eight Italian neuro-oncology centers.
Results
We included 87 patients. Histology was Glioblastoma (GBM) in 78 patients (89.7%); 60% of patients were female. After myelotoxicity recovery, 54 (62%) received treatment. The majority of them (82%, n = 44) received adjuvant TMZ and 18% (n = 10) others treatments. Out of 44 patients who received adjuvant TMZ, 34% experienced the re-occurrence of grade 3–4 myelotoxicity which required permanent CT discontinuation in 6 (13%) cases. Patients who received TMZ or other treatments had longer overall (OS) (adjusted HR 0.46, p = 0.008) and progression free survival (PFS) (adjusted HR 0.57, p = 0.034) than those who remained untreated.
Conclusion
Our study suggests that after severe myelotoxicity the majority of patients received treatment, particularly with TMZ. Only a fraction of patients experienced toxicity recurrence, suggesting that TMZ is well tolerated and had an impact on PFS and OS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant gliomas (MG) are the most common and aggressive forms of primary brain tumours in adults.
Today, the standard of care is surgery, followed by concomitant Temozolomide (TMZ) with radiation therapy (CRT), and adjuvant TMZ [1]. Stupp et al. [1] reported that the treatment was reasonably well tolerated as 85% of participants completed the full course of CRT as planned. However, only 47% of patients completed the planned six cycles of adjuvant TMZ, and 8% discontinued due to myelotoxicity. TMZ is considered to be a safe drug; however, several case reports and small series studies have reported severe myelotoxicity occurring during CRT or adjuvant TMZ [2].
In Stupp trial, the main toxicity was grade 3–4 thrombocytopenia, and occurred in 3% of patients during the CRT and 11% of patients during adjuvant chemotherapy (CT), respectively [1].
Gerber et al. [3] in a single-centre study reported severe myelosuppression in a minority of patients: 19% of patients experienced grade 3–4 thrombocytopenia (80% of these occurring during the CRT and 20% during the adjuvant CT), with a median duration of 32 days. Two patients (4%) presented prolonged thrombocytopenia and one died from this complication. A systematic review of the literature [2] showed that severe myelotoxicity mainly occurred during CRT. The myelotoxicity ranged from few weeks till more than 1 year [4, 5] and patients did not receive adjuvant TMZ, because they were considered at risk of new severe toxicity.
Risk factors related to haematological toxicity include clinical parameter as gender, Body Surface Area (BSA), Body Mass Index (BMI), taking steroids, pretreatment platelet count, methylation status of the (O 6-methylguanine–DNA methyl-transferase) MGMT promoter on peripheral mononuclear cells, some specific MGMT polymorphisms and other epigenetic mechanism as single CpG methylation of HSBP2s [5,6,7,8,9,10,11,12,13,14,15,16].
Despite this approach, glioblastoma (GBM) is associated with a poor median overall survival of less than 24 months and relapse is inevitable [1, 17].
However, little data are available about the haematological toxicity recurrence during adjuvant CT in glioma patients who experienced severe haematological toxicity during CRT.
The aim of our retrospective multicentric AINO (Italian Association for Neuro-Oncology) study was to analyse the incidence of second haematological toxicity during adjuvant CT in patients with MG who recovered after severe haematological toxicity during CRT.
Methods
We retrospectively collected data of patients with MG who developed grade 3 or 4 (as the National Cancer Institute (NCI) Common Toxicity criteria (NCICT-CAE) version 3.0 [18]) myelotoxicity during CRT and recovered, from January 2007 to December 2019. The AINO multicentre study included eight Italian neuro-oncology centers. We excluded patients who did not resolve toxicity, who died. Following institutional review board approval, we reviewed demographics, clinical–radiological data [including Karnofsky Performance Scale (KPS) and surgical exeresis extension], histopathological diagnosis and therapy details of all patients. Data on the reason of treatment discontinuation were also collected.
In cases with an adequate amount of tissue, IDH1 was detected [immunochemistry using anti-IDH1 (R132H) or Next Generation sequencing analyses] and MGMT promoter methylation status analyzed as common current practice [Methylation-Specific PCR (PCR) or pyrosequencing].
Myelotoxicity data included: type, onset time and duration, eventually use of growth factors as Granulocyte Colony-Stimulating Factor (G-CSF) and blood/platelet transfusions. For patients treated with adjuvant treatment, we considered type, severity of toxicity, time to progression and reasons for discontinuation. Outcome, including progression-free survival (PFS) and overall survival (OS) were available for all patients.
Statistical analysis
Data are expressed as count (proportion) and as a mean (standard deviation) or median (range), where appropriate. Differences were tested by the Fisher Exact test for categorical variables and by the Mann–Whitney U test for continuous variables.
Survival analyses with Kaplan–Meier curves and proportional hazard Cox regression models explored OS and PFS in patients who started adjuvant chemotherapy versus those who did not. Demographic and clinical variables were also entered in the multivariable Cox models to explore whether there were factors other than adjuvant chemotherapy associated with better or worse prognosis.
Two-sided p values less than 0.05 were considered significant.
Results
We identified 87 patients (34 men, 53 women) with a mean age of 58.4 (10.8) years who were diagnosed with GBM (n = 78) or any other MG (n = 9). Patients’ baseline characteristics are shown in Table 1.
Forty-one patients developed grade 3 (47.1%) or 46 grade 4 (52.9%) myelotoxicity after a median time of 4 weeks (range 2–10) since starting CRT. The resolution of myelotoxicity was observed within a median time of 6 weeks (range 1–80).
Thirty-five patients (40.2%) had 1 blood-cell lineages deficiency; 29 (33.3%) had 2 blood-cell lineages, and 23 (26.5%) had more than 2 blood-cell lineages deficit (see Table 2). Regarding one blood-cell lineages deficiency, the majority of patients presented isolated thrombocytopenia (n = 23, 65.7%), followed by lymphopenia (n = 7, 20%) and neutropenia (n = 5, 14.3%). We found no association of grade or type of myelotoxicity with age, KPS, histology, radiotherapy dose, MGMT promoter methylation and IDH-1 status (all p values > 0.12). The onset of myelotoxicity was significantly shorter in patients who developed grade 4 myelotoxicity respect to those who developed grade 3 toxicity (4.4 ± 1.5 versus 5.4 ± 1.5 weeks, p = 0.001), but there was no difference in terms of duration of toxicity (p = 0.65). One blood-cell-type deficiency occurred later and lasted shorter than two or more blood-cell lineages reductions, as shown in Fig. 1. Administration of G-CSF and/or blood or platelet transfusion were required in 32 (36.7%) and 49 (56%) patients, respectively. No major complications related to myelotoxicity as infection or haemorrhage were observed.
Myelotoxicity after starting adjuvant chemotherapy
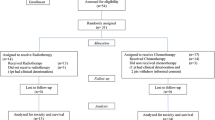
After myelotoxicity resolution, 33 (38%) patients did not receive treatments, whereas 54 (62.1%) patients received therapy (Fig. 2): TMZ 150–200 mg/m2/day (n = 44), metronomic TMZ schedules (n = 4), lomustine (n = 3), bevacizumab (n = 2), tamoxifene (n = 1).
There were no demographic or clinical differences between the two groups (treatments vs. no treatment), including the type and grade of myelotoxicity, except for better KPS at diagnosis (p = 0.046) and after CRT (p = 0.003) in those who did further treatment as compared with those who did not (see also Table 3).
Out of 54 patients who received another therapy after CRT, 21 (38.9%) experienced recurrent grade 3 or 4 myelotoxicity, including isolated thrombocytopenia or neutropenia or lymphopenia (n = 16) and co-occurring thrombocytopenia or neutropenia or lymphopenia (n = 5). Of them, 15/44 (34%) were treated with adjuvant TMZ and 6/10 (60%) treated with other treatments (p = 0.16).
We observed no demographic and clinical differences between patients with (n = 21) and without (n = 33) recurrent myelotoxicity, including type, grade, time of onset and time to resolution of the post-CRT toxicity. These data did not change even after limiting the analysis only to the 44 patients treated with adjuvant TMZ.
Fewer patients treated with adjuvant TMZ (n = 6/44) discontinued the treatment due to toxicity as compared with other therapies (n = 5/10) (p = 0.021). None of them needed G-CSF or blood/platelet transfusion for any blood type deficiency. No major complication related to myelotoxicity was observed.
Outcomes (OS and PFS) after starting adjuvant chemotherapy
Patients who received adjuvant TMZ or other treatments (n = 54) had longer OS (adjusted HR 0.46, p = 0.008) and PFS (adjusted HR 0.57, p = 0.034) than those who remained untreated (n = 33) following resolution of CRT-related myelotoxicity (see also Fig. 3a, b). These data did not change substantially even after limiting the analysis only to adjuvant TMZ 5/28 (n = 44) vs. no treatment (n = 33): adjusted HR for OS = 0.52 (p = 0.025) and adjusted HR for PFS = 0.62 (p = 0.065).
Moreover, patients diagnosed with any MG different from GBM had a reduced risk of death (adjusted HR 0.17, p = 0.016) and of disease progression (adjusted HR 0.27, p = 0.007) than those with GBM (see also Tables 4, 5).
The MGMT promoter methylation status and IDH1 mutation status did not affect both the OS and PFS, possibly because of the reduced sample size (data on MGMT and on IDH1 status were available only for 67 and 56 patients, respectively).
Predictor factors for iatrogenic myelotoxicity
We performed an additional retrospectively analysis to identify the potential predictors for iatrogenic myelotoxicity. For this reason we identified a control group in the Neuro-Oncology Unit's database of Regina Elena, collecting 1500 primary brain tumors. From these 1500 patients, we only selected those glioma patients, from Janauray 2007 to December 2019, who did not experience myelotoxicity during CRT. We considered 456 MG patients. We then compared the clinical and histo-molecular variables between the study cohort (n = 87) to control cohort (n = 456). We observed that female sex (p < 0.001), age (p = 0.01), histology (p = 0.001) and IDH-1(p = 0.041) are predictors factors at baseline for myelotoxicity (see Table 6). Lastly, we estimated no difference between patients who developed myelotoxicity and those who did not in terms of PFS and OS (data not shown).
Discussion
Our study suggest that starting adjuvant CT or another therapy after resolution of CRT-related myelotoxicity results in longer OS and PFS and in a relatively small risk of recurrent myelotoxicity, especially with adjuvant TMZ.
TMZ treatment has been considered to have a low toxicity profile, as reported by Stupp et al. and other different studies reported an overall incidence of 5–8% for grade 3 or 4 myelotoxicity [1, 7, 8]. Niewald et al. [19] reported high rates of myelotoxicity with discontinued treatment in 50% of GBM treated with CRT.
Subsequent studies showed that severe myelotoxicity could be even higher during CRT compared with adjuvant. Today, there are no guidelines about how to treat patients with glioma after remission of severe myelotoxicity during CRT. However, given the lack of clear evidence, in clinical practice, patients may or not receive adjuvant CT after remission of their myelotoxicity on the basis of single-centre case by case evaluation of the risk.
In this multicentre retrospective study, we observed that the majority of patients that recovered from CRT-induced severe myelotoxicity are largely treated with adjuvant CT (61.6%), and only 38.4% patients did not receive any treatment.
There were not significant clinical differences between the two groups. However, considerable caution is required in interpreting such data due to the design of the present study. Among the treated patients, the most received standard adjuvant TMZ where 34% experienced myelotoxicity re-occurrence, but only 13% discontinued adjuvant TMZ for this reason.
In previous data, patients that develop prolonged severe myelosuppression during CRT usually did not receive CT for high risk of recurrence of severe toxicity [2]. Lombardi et al. [5] observed that all patients discontinuing TMZ during the concomitant phase for severe haematological toxicity did not receive adjuvant treatment. Gerber et al. [3] concluded that around 20% of patients with newly diagnosed MG receiving TMZ are at risk of severe thrombocytopenia, and half of them with risk of sustained, prolonged and potentially irreversible toxicity.
Lin et al. [20] analysed the impact of concurrent versus adjuvant CT on the severity of lymphopenia in gliomas and showed that CRT appears to be the dominant contributor to the severity of acute lymphopenia.
An analysis on 3400 patients concluded that the hematologic toxicity due to TMZ represent a significant concern and the mechanisms involved could be different from the other alkylants [14].
During the CRT, our patients with myelotoxicity initially reported one blood-cell lineages, and later deficiencies involving more than one blood-cell lineages as thrombocytopenia and neutropenia or thrombocytopenia, neutropenia and anaemia. During adjuvant CT phase, we observed only cases of isolated thrombocytopenia or neutropenia or lymphopenia.
Our data are in line with the literature, suggesting that thrombocytopenia is the first sign of severe myelosuppression [3,4,5].
In our study, the median time of myelotoxicity occurrence during CRT was 4 weeks (range 1–10) with a median time of 6 (range 1–80) weeks for resolution. Gerber et al. [3] reported that the median onset was on day 52 of therapy.
In addition, some of the above-mentioned risk factors of myelotoxicity as clinical factors and epigenetic profile, also the use of antiepileptic drugs (AED) may have an impact [13, 15, 16]. Given the absence of a control group of patients who did not develop myelotoxicity, we cannot confirm any reported associations between toxicity and the use of AED or MGMT methylation status.
The main goal of our study was to assess the risk of myelotoxicity induced by adjuvant CT in patients recovered by previous severe myelotoxicity during CRT of Stupp regimen. The results of this study show that the majority of patients received treatment, in particular TMZ, and may be safely concluded in the majority of them.
Moreover, patients treated with adjuvant CT showed a positive outcome trend: nevertheless, such data need to be confirmed in larger prospective studies. To our knowledge, survival advantage in patients that did not experienced pancytopenia have been reported in few small studies [21,22,23,24]. The reasons for the positive correlation between leukopenia/thrombocytopenia and positive outcome have also been hypothesized, and need to be proved. Firstly, it remains unclear whether patients who do not show a notable decrease in blood counts in response to TMZ are treated below therapeutic range. Second, leukopenia could be a positive prognostic factor in relation to the knowledge that neutrophil exhibit a pro-tumorigenic activity [25]. Besides, induced-bone marrow failure can include also reduction of bone marrow-derived cells (BMDC), endothelial Precursor Cell (EPC) and myeloid-derived suppressor cells (MDSC), all cell populations essential for MG development and progression [26, 27].
The present study is not exempt from limitations, mainly due to its retrospective-real life design and small sample size. Even if database plan was discussed and defined by reaching an agreement on the minimum dataset to be analysed by a devoted network, we still cannot exclude a certain degree of inter-centre variability. Despite these limitations, we are confident that our study provides new insights into the management of patients who presented severe myelotoxicity during CRT, an area that has never been investigated before in patients with MG.
Conclusion
We concluded that the majority of patients who received adjuvant TMZ after resolution from myelotoxicity occurred during CRT, where only a fraction of them experienced of toxicity. In addition, the adjuvant CT can positively impact on PFS and OS.
Data availability
The data that support the findings of this study are available on request from the corresponding author, [V.V.]. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Dixit S, Baker L, Walmsley V, Hingorani M (2012) Temozolomide-related idiosyncratic and other uncommon toxicities: a systematic review. Anticancer Drugs 23(10):1099–1106. https://doi.org/10.1097/CAD.0b013e328356f5b0
Gerber DE, Grossman SA, Zeltzman M, Parisi MA, Kleinberg L (2007) The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas. Neuro Oncol 9(1):47–52. https://doi.org/10.1215/15228517-2006-024 (Epub 2006 Nov 15)
Lombardi G, Caccese M, Bellu L, Pambuku A, Bergo E, Berti F, Gardiman MP, Della Puppa A, Denaro L, Dal Pos S, Zagonel V (2018) Good tolerability of maintenance temozolomide in glioblastoma patients after severe hematological toxicity during concomitant radiotherapy and temozolomide treatment: report of two cases. Anticancer Drugs 29(9):924–928. https://doi.org/10.1097/CAD.0000000000000678 (PMID: 30080691)
Lombardi G, Rumiato E, Bertorelle R, Saggioro D, Farina P, Della Puppa A, Zustovich F, Berti F, Sacchetto V, Marcato R, Amadori A, Zagonel V (2015) Clinical and genetic factors associated with severe hematological toxicity in glioblastoma patients during radiation plus temozolomide treatment: a prospective study. Am J Clin Oncol 38(5):514–519. https://doi.org/10.1097/COC.0b013e3182a790ea
Robins HI, Eickhoff J, Gilbert MR, Armstrong TS, Shi W, De Groot JF, Schultz CJ, Hunter GK, Valeinis E, Roach M, Youssef EF, Souhami L, Howard SP, Lieberman FS, Herman JG, Zhang P, Mehta MP (2019) The association between BMI and BSA-temozolomide-induced myelosuppression toxicities: a correlative analysis of NRG oncology RTOG 0525. Neurooncol Pract. 6(6):473–478. https://doi.org/10.1093/nop/npz006
Arulananda S, Lynam J, Sem Liew M, Wada M, Cher L, Gan HK (2018) Clinical correlates of severe thrombocytopenia from temozolomide in glioblastoma patients. Intern Med J 48(10):1206–1214. https://doi.org/10.1111/imj.14000
Armstrong TS, Cao Y, Scheurer ME et al (2009) Risk analysis of severe myelotoxicity with temozolomide: the effects of clinical and genetic factors. Neurooncology 11:825–832
Yin AA, He YL, Etcheverry A et al (2019) Novel predictive epigenetic signature for temozolomide in non-G-CIMP glioblastomas. Clin Epigenetic 11(1):76. https://doi.org/10.1186/s13148-019-0670-9
Altinoz MA, Elmaci I, Bolukbasi FH, Ekmekci CG, Yenmis G, Sari R, Sav A (2017) A MGMT gene variants, temozolomide myelotoxicity and glioma risk. A concise literature survey including an illustrative case. J Chemother 29(4):238–244. https://doi.org/10.1080/1120009X.2017.1312752 (PMID: 28436299)
Becker-Schiebe ME, Wetzel M, Wetzel F, Christansen H, Hoffmann W (2015) Hematologic toxicity of temozolomide and radiation in glioblastoma patients—correlation with clinicopathological factors. Clin Med J1:63–69
Gupta T, Mohanty S, Moiyadi A, Jalali R (2013) Factors predicting temozolomide induced clinically significant acute hematologic toxicity in patients with high-grade gliomas: a clinical audit. Clin Neurol Neurosurg 115(9):1814–1819
Sabharwal A, Waters R, Danson S, Clamp A, Lorigan P, Thatcher N, Margison GP, Middleton MR (2011) Predicting the myelotoxicity of chemotherapy: the use of pretreatment O6-methylguanine-DNA methyltransferase determination in peripheral blood mononuclear cells. Melanoma Res 21(6):502–508. https://doi.org/10.1097/CMR.0b013e32832ccd58
Villano JL, Letarte N, Yu JM, Abdur S, Bressler LR (2012) Hematologic adverse events associated with temozolomide. Cancer Chemother Pharmacol 69(1):107–113
Tinchon A, Oberndorfer S, Marosi C, Gleiss A, Geroldinger A, Sax C, Sherif C, Moser W, Grisold W (2015) Haematological toxicity of Valproic acid compared to Levetiracetam in patients with glioblastoma multiforme undergoing concomitant radio-chemotherapy: a retrospective cohort study. J Neurol 262(1):179–186. https://doi.org/10.1007/s00415-014-7552-z (PMID: 25359262)
Handoko KB, Souverein PC, van Staa TP et al (2006) Risk of aplastic anemia in patients using antiepileptic drugs. Epilepsia 47:1232–1236
Ronning PA, Helseth E, Meling TR, Johannesen TB (2012) A population-based study on the effect of temozolomide in the treatment of glioblastoma multiforme. Neuro Oncol 14:1178–1184
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P (2003) CTCAE v30: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13(3):176–181
Niewald M, Berdel C, Fleckenstein J, Licht N, Ketter R, Rube C (2011) Toxicity after radiochemotherapy for glioblastoma using temozolomide—a retrospective evaluation. Radiat Oncol 6:141
Lin AJ, Campian JL, Hui C, Rudra S, Rao YJ, Thotala D, Hallahan D, Huang J (2018) Impact of concurrent versus adjuvant chemotherapy on the severity and duration of lymphopenia in glioma patients treated with radiation therapy. J Neurooncol 136(2):403–411. https://doi.org/10.1007/s11060-017-2668-5 (PMID: 29143923)
Williams M, Liu ZW, Woolf D, Hargreaves S, Michalarea V, Menashy R, Kooner I, Wilson E (2012) Change in platelet levels during radiotherapy with concurrent and adjuvant temozolomide for the treatment of glioblastoma: a novel prognostic factor for survival. J Cancer Res Clin Oncol 138(10):1683–1688
Vaios EJ, Nahed BV, Muzikansky A, Fathi AT, Dietrich J (2016) Bone marrow response as a potential biomarker of outcomes in glioblastoma patients. J Neurosurg 14:1–7. https://doi.org/10.3171/2016.7.JNS16609
Ho KG, Uhlmann EN, Wong ET, Uhlmann EJ (2020) Leukopenia is a biomarker for effective temozolomide dosing and predicts overall survival of patients with glioblastoma Mol. Clin Oncol 13(6):80. https://doi.org/10.3892/mco.2020.2150
Fontanilles M, Marguet F, Alexandru C, Langlois O, Veresezan O, Gilard V, David M, Laquerriere A, Hanzen C, Tennevet I, Di Fiore F, Clatot F (2019) Early platelet variation during concomitant chemo-radiotherapy predicts adjuvant temozolomide-induced thrombocytopenia in newly diagnosed glioblastoma patients. Support Care Cancer 27(2):477–484. https://doi.org/10.1007/s00520-018-4336-5 (PMID: 29978325)
Coffelt SB, Wellenstein MD, de Visser KE (2016) Neutrophils in cancer: neutral no more. Nat Rev Cancer 16(7):431–446. https://doi.org/10.1038/nrc.2016.52
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322
Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, Dhara S, Simpson K, Gardner EE, Iacobuzio-Donahue CA, Brennan CW, Tabar V, Gutin PH, Joyce JA (2016) Macrophage Ontogeny Underlies Differences in Tumor-Specific Education in Brain Malignancies. Cell Rep. 17(9):2445–2459. https://doi.org/10.1016/j.celrep.2016.10.052
Funding
None.
Author information
Authors and Affiliations
Contributions
Conception and design of study: VV, AP. Acquisition data: AP, AF, GL, RR, PG, SR, LG, CS, EP, GS, GT. Data analysis: LP. Interpretation: AE, VV, AP. Drafting: VV, LP. Revising: AE, AP. Final approval: VV, AP. All authors read and approved submission.
Corresponding author
Ethics declarations
Conflicts of interest
The author declares that they have no conflict of interest.
Consent to participate
Informed consent, in line with the principles of the Declaration of Helsinki, was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Villani, V., Anghileri, E., Prosperini, L. et al. Adjuvant chemotherapy after severe myelotoxicity during chemoradiation phase in malignant gliomas. Is it feasibile? Results from AINO study (Italian Association for Neuro-Oncology). J Neurol 268, 2866–2875 (2021). https://doi.org/10.1007/s00415-021-10438-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10438-4