Abstract
Prion real-time quaking-induced conversion (RT-QuIC) is emerging as the most potent assay for the in vivo diagnosis of Creutzfeldt–Jakob disease (CJD), but its full application, especially as a screening test, is limited by suboptimal substrate availability, reagent costs, and incomplete assay standardization. Therefore, the search for the most informative cerebrospinal fluid (CSF) surrogate biomarker is still of primary importance. We compared the diagnostic accuracy of CSF protein 14-3-3, measured with both western blot (WB) and enzyme-linked immunosorbent assay (ELISA), total (t)-tau and neurofilament light chain protein (NfL) alone or in combination with RT-QuIC in 212 subjects with rapidly progressive dementia in which we reached a highly probable clinical diagnosis at follow-up or a definite neuropathological diagnosis. T-tau performed best as surrogate CSF biomarker for the diagnosis of CJD (91.3% sensitivity and 78.9% specificity). The 14-3-3 ELISA assay demonstrated a slightly higher diagnostic value compared to the WB analysis (76.9% vs. 72.2%), but both methods performed worse than the t-tau assay. NfL was the most sensitive biomarker for all sCJD subtypes (> 95%), including those with low values of t-tau or 14-3-3, but showed the lowest specificity (43.1%). When ELISA-based biomarkers were adopted as screening tests followed by RT-QuIC, t-tau correctly excluded a higher number of non-CJD cases compared to NfL and 14-3-3 ELISA. Our study showed that among the CSF surrogate biomarkers of potential application for the clinical diagnosis of CJD, t-tau performs best either alone or as screening test followed by RT-QuIC as a second-level confirmatory test.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prion diseases encompass four major phenotypic entities, namely, Creutzfeldt–Jakob disease (CJD), Gerstmann–Sträussler–Scheinker disease (GSS), fatal insomnia, and variably protease-sensitive prionopathy (VPSPr) [1]. CJD, by far the most common, includes six major clinicopathological subtypes that are primarily determined and classified by the genotype at the methionine (M)/valine (V) polymorphic codon 129 of the PRNP gene and the type (1 or 2) of disease-associated prion protein (PrPSc) accumulating in the brain [2,3,4].
The clinical diagnosis of prion disease is often very challenging given the variety of clinical presentations (especially at onset), which largely overlap with those of other rapidly progressive dementias (RPDs), and the variable rate of disease progression, ranging from acute/subacute to slowly progressive [5].
Over the past two decades, cerebrospinal fluid (CSF) surrogate markers for neuronal damage, such as proteins 14-3-3 and total-tau (t-tau), and magnetic resonance imaging (MRI) with higher resolution, have significantly contributed to improving CJD clinical recognition. Nevertheless, the diagnostic accuracy of these investigations remains suboptimal [6,7,8,9,10].
More recently, CSF prion real-time quaking-induced conversion (RT-QuIC) demonstrated a higher diagnostic value given the virtually full specificity (99–100%) combined with a good to optimal (80–96%) sensitivity [9,10,11,12,13,14]. However, the strict dependence on both availability and batch to batch performance of the recombinant prion protein used in the test, along with its relatively high cost, still represents a limit to the use of RT-QuIC as a routine diagnostic test in many laboratories as well as its application as a screening test to a broader patient population.
As a consequence, the search for the best performing surrogate biomarkers to be used as a screening test is still of importance for reference laboratories worldwide. In this regard, recent studies tested the value of an enzyme-linked immunosorbent assay (ELISA)-based detection of the gamma isoform of protein 14-3-3 in comparison to western blot (WB) [15, 16] and investigated the neurofilament light chain protein (NfL) as a novel marker [17,18,19,20] providing promising results. However, neither NfL nor 14-3-3 ELISA has yet been studied and compared to other CSF assays in a cohort of patients with RPDs in which a highly probable or definite diagnosis was reached at follow-up or post-mortem, respectively.
Here, we compared for the first time the diagnostic value of CSF 14-3-3 WB, 14-3-3 ELISA, t-tau and NfL when tested alone or in combination with RT-QuIC in a large patient series with RPD. Furthermore, we explored the accuracy of CSF assays in the diagnosis of prion disease according to molecular subtypes and different clinical scenarios.
Methods
Inclusion criteria
We retrospectively studied patients affected by a rapidly progressive neurological syndrome which prompted the inclusion of CJD in the differential diagnosis at the time of lumbar puncture. All cases were submitted to the Neuropathology Laboratory at the Institute of Neurological Sciences of Bologna (Italy). Only cases with a highly probable clinical diagnosis of non-CJD at follow-up or with a definite neuropathological diagnosis (either CJD or non-CJD), and with a CSF sample of sufficient volume to complete the ELISA-based assays were selected. The screening of our database yielded a total of 212 cases, including 103 CJD and 109 non-CJD subjects, well representative of the three main definite etiologies of RPD reported in the literature, namely CJD, inflammatory and non-prion neurodegenerative disorders [21,22,23]. To exclude any potential selection bias due to CSF unavailability in the older samples, we also analyzed the results in a group of 127 consecutive and unselected cases with a neuropathological or clinical diagnosis (either CJD or non-CJD), which were submitted to our laboratory between January 2011 and December 2018 (65 CJD and 62 non-CJD).
The study was conducted according to the revised Declaration of Helsinki and Good Clinical Practice guidelines and approved by the “Area Vasta Emilia Centro” ethics committee. Informed consent was given by study participants or the next of kin.
All clinical data, including follow-up information, were acquired for each patient. When available, the results of EEG recordings and brain MRI studies, inclusive of fluid-attenuated inversion recovery (FLAIR) and/or diffusion-weighted (DW) sequences, were also obtained.
Molecular analysis of the PRNP gene was carried out in all subjects, as previously described [24]. Moreover, PrPSc typing and histotype classification were performed in all pathologically confirmed (i.e., definite) CJD cases according to established methodologies and consensus criteria [4, 9].
CSF biochemical analysis
CSF samples were obtained by lumbar puncture (LP) at the L3/L4 or L4/L5 level following a standard procedure, centrifuged in case of blood contamination, divided into aliquots and stored in polypropylene tubes at − 80 °C until analysis. Protein 14-3-3 detection by WB was performed as previously described [9]. The 14-3-3 gamma isoform was measured using a commercially available ELISA assay kit (Circulex 14-3-3 gamma ELISA kit, MBL, Woburn, MA) according to the manufacturer’s instructions. CSF t-tau and NfL levels were analyzed as previously described [18]. PrPSc seeding activity was detected by RT-QuIC using full-length (first generation assay or PQ-CSF) or truncated (second generation assay or IQ-CSF) hamster recombinant prion protein as a substrate, as previously described [9, 14].
14-3-3 WB and ELISA, t-tau, NfL and PQ-CSF were performed in all subjects, whereas IQ-CSF data were available for 78 CJD and 53 non-CJD cases [14]. The effects of pre- and analytical variables were also explored (supplementary methods).
Patient classification
Patients with prion disease were classified into diagnostic categories according to the new European criteria for CJD and related disorders [25]. Specifically, the group of prion disease (simplified as “CJD group”) consisted of 83 definite cases (80 sporadic CJD [sCJD], 2 genetic CJD, 1 VPSPr) and 20 cases carrying a pathogenic PRNP mutation. Probable sCJD cases were excluded from the study due to potential sampling bias.
The “non-CJD” group included: (1) patients in whom post-mortem examination excluded a prion disease (n = 33); (2) those showing a clinical evolution incompatible with a prion disease (e.g., improvement or stabilization at follow-up) (n = 7); and (3) those with an alternative definite clinical diagnosis that was strongly supported by neuroradiological and/or laboratory findings (n = 69) (Supplementary methods).
To focus on the problematic scenarios which still occur in clinical practice, we also compared the accuracy of each ELISA-based surrogate biomarker in cases with conflicting results at PQ-CSF and brain MRI (14 CJD and 13 non-CJD cases with negative PQ-CSF and positive brain MRI, 20 CJD cases with positive PQ-CSF and negative brain MRI results).
Statistical analyses
Statistical analysis was performed using IBM SPSS Statistics version 21 (IBM, Armonk, NY, USA). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) and accuracy of each diagnostic investigation were obtained. Receiver operating characteristic (ROC) analyses were performed to establish the sensitivity, specificity and cut-off value of 14-3-3 ELISA, t-tau and NfL assays. The optimal cut-off value for each biomarker was chosen using the maximized Youden index. Based on the distribution of values, data were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). For continuous variables, the Mann–Whitney U test or the t test was used to test differences between two groups, while the Chi-square test was adopted for categorical variables. Differences were considered statistically significant at p < 0.05.
Results
Demographics and results of RT-QuIC, brain MRI and EEG in the diagnostic groups
There were no differences regarding age, sex and disease duration between CJD and non-CJD cases (Table 1). In line with our previous studies [9, 14], PQ-CSF demonstrated an overall sensitivity of 82.5%, even though with lower values in the rarest subtypes (MV2K, MM2C and MM2T) than in the most common ones (MM(V)1 and VV2) (Table 1). Moreover, IQ-CSF demonstrated a higher diagnostic performance than PQ-CSF, reaching a 97.4% overall sensitivity and a full sensitivity for all definite sCJD subtypes (100%) except for the MM(V)1 group (Table 1). There were only two confirmed sCJD MM1 cases that tested negative with both PQ-CSF and IQ-CSF, although both showed a positive brain MRI. In the present cohort, both PQ-CSF and IQ-CSF gave no false-positive results among non-CJD subjects. More details about EEG and brain MRI findings are provided in the supplementary results.
Diagnostic accuracy of CSF surrogate biomarkers alone and in combination with RT-QuIC
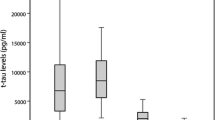
At the cutoff value of 1147 pg/ml, t-tau yielded the highest diagnostic value among the tested assays in terms of both sensitivity (91.3%) and specificity (78.9%) (Table 2).
The comparison of 14-3-3 measurement by ELISA and WB demonstrated that the former approach performed slightly better (Table 2). At the cut-off value of 23,400 AU/ml, the 14-3-3 positivity by ELISA matched the WB-positive results (91.5%. 108/118 of cases), but also correctly classified as positive 45.5% (10/22) of CJD cases that showed only a weakly positive or negative result by WB, while it only misclassified 5.6% (4/72) of the non-CJD cases that were correctly recognized as negative by WB.
At the cutoff point of 1847 pg/ml, NfL showed a higher sensitivity (97.1%) but a lower specificity (43.1%) than the other surrogate biomarkers (Table 2).
Other possible decision points for ELISA-based biomarkers with the respective statistics are shown in Supplementary Table 1.
We then built three clinically adapted algorithms in which we compared 14-3-3 ELISA, t-tau or NfL as fast first-step screening tests, followed by the RT-QuIC assay (IQ-CSF or PQ-CSF) as a second-step investigation. The results (Fig. 1 and Supplementary Fig. 1) showed that, at cut-off values favoring sensitivity over specificity (14-3-3 ELISA: 11,050 AU/ml; t-tau: 757 pg/ml; NfL: 1847 pg/ml), which is imposed by the need to select the vast majority of CJD cases during the pre-screening, t-tau maintained a higher specificity than NfL or 14-3-3 ELISA. The same findings were confirmed when the Youden Index calculated cut-offs were adopted (supplementary results).
Diagnostic algorithms based on CSF surrogate biomarkers followed by IQ-CSF. 14-3-3 ELISA (a), t-tau (b) or NfL (c) screening and confirmatory test by means of IQ-CSF. CJD Creutzfeldt–Jakob disease, ELISA enzyme-linked immunosorbent assay, IQ-CSF second-generation RT-QuIC, NfL neurofilament light chain protein, Sens sensitivity, Spec specificity, t-tau total tau protein
The data regarding the performance of CSF surrogate biomarkers according to prion disease molecular subtypes and non-CJD etiologies are shown in Supplementary results.
Comparison of the accuracy of diagnostic investigations in different clinical scenarios
Detailed sensitivity and specificity for each surrogate biomarker and other diagnostic investigations are reported in Table 3. In the consecutive case cohort (2011–2018), we confirmed the results obtained in the overall cohort. As expected, RT-QuIC (in particular IQ-CSF) was the best diagnostic investigation overall, whereas among surrogate biomarkers t-tau showed the higher diagnostic value in terms of both sensitivity and specificity, although NfL remained the most sensitive surrogate biomarker. In cases with positive MRI and negative PQ-CSF, once again, t-tau yielded the best accuracy in terms of both sensitivity (85.7%) and specificity (61.5%). At variance, in subjects with a negative MRI and a positive PQ-CSF, only NfL demonstrated 100% sensitivity and 100% specificity.
Discussion
Prion RT-QuIC, especially the improved second-generation assay (IQ-CSF), is emerging as the most powerful test for the in vivo diagnosis of CJD, given its virtually full specificity and relatively high sensitivity [9,10,11,12,13,14]. However, a limited number of laboratories currently perform this test, and most of them rely on the external supply of the recombinant prion protein substrate, which is a significant limit to its application on a larger scale. Moreover, only the interlaboratory reproducibility of PQ-CSF using a single source and batch of substrate assay has been tested to date [26], while no data are available for IQ-CSF and the use of different batches or sources of recombinant prion protein. Altogether, all these factors justify continuing the use of CSF surrogate markers in the diagnostic assessment of RPDs.
In the present study, we compared the diagnostic value of established and emerging surrogate CSF biomarkers in a large cohort of RPDs, well representative of the heterogeneous patient population that is submitted to CJD reference laboratories worldwide. Notably, we investigated for the first time the accuracy of CSF NfL in a large cohort of RPD cases with a definite or highly probable diagnosis.
The results confirmed that t-tau is the most accurate surrogate CSF biomarker for the diagnosis of CJD in terms of both sensitivity and specificity [8, 9, 13, 27, 28], a finding which we also reproduced in a prospective cohort. Given that previous comparison between t-tau and 14-3-3 performance used WB data for 14-3-3, we extended the analysis to the 14-3-3 ELISA assay. In comparison to western blot, ELISA demonstrated a slightly higher diagnostic accuracy, which is in addition to the advantage of the quantitation over the densitometric semiquantitative assessment. However, even the 14-3-3 ELISA showed a lower accuracy than the t-tau assay. Thus, the inclusion of 14-3-3 as the only surrogate CSF biomarker in current diagnostic criteria for probable CJD is unjustified [25]. Indeed, t-tau should be recommended over 14-3-3 or at least both t-tau and 14-3-3 be included. The lower performance of the 14-3-3 ELISA in the present cohort in comparison to previous studies [15, 16] is primarily explained by the inclusion in the latters of a relatively high percentage of typical CJD (MM(V)1 subtype) and non-CJD cases of neurodegenerative etiologies, which are notoriously associated with positive and negative 14-3-3 results, respectively. At variance, we analyzed the whole spectrum of CJD subtypes, as well as many patients with non-CJD RPDs etiologies that often test positive at the 14-3-3 assay (e.g., encephalitis/paraneoplastic syndromes and CNS malignancy).
NfL is an emerging surrogate biomarker of neurodegeneration [29]. While the CSF concentration of both t-tau and NfL biomarkers positively correlate with the extent of neuronal degeneration in a given period, NfL levels seem also significantly influenced by the degree of subcortical pathology (i.e., deep nuclei, brainstem and cerebellum) [18]. The latter feature may be one of the reasons of the very high sensitivity of NfL for the sCJD subtypes, including those which typically show low values of t-tau and negative protein 14-3-3 (e.g., sCJD MV2K, MM2C and gCJD E200K). Unfortunately, the low specificity of NfL significantly limits its role as an isolated test in the differential diagnosis of RPDs. Nevertheless, according to the present study, NfL may represent an alternative to t-tau as first-step assay for suspected CJD cases in the early phase of symptoms, when followed by specific tests such as RT-QuIC. Moreover, given the high NPV, a low concentration of CSF NfL in a patient with RPD would exclude with very high certainty the diagnosis of prion disease and induce the clinicians to consider other etiologies and the introduction of an ex-adiuvantibus therapy. Since current ultrasensitive immunoassay platforms also allow to reliably measure NfL levels in blood, with a good correlation with the concentration of the analyte in the CSF [17, 30], blood NfL may also provide a minimally invasive screening assay to estimate the degree of neural tissue damage with relatively low cost and a possible application to a broader population.
The inclusion of several etiologies of non-CJD RPDs, including a significant proportion of non-neurodegenerative cases, reflects our long-lasting experience as reference laboratory for the diagnosis of prion disease and represents another strength of our study [8, 15, 16, 18,19,20]. This choice probably affected the specificity of surrogate biomarkers, especially that of NfL and 14-3-3, but added more clinical reliability to the results.
One possible limit, instead, concerns the lack of brain MRI data, especially of DW/FLAIR sequences, in a significant proportion of cases, which may partially explain the apparent lower accuracy of brain MRI in our cohort in comparison with previous studies [6, 10]. Two additional factors may also have contributed to the relatively high number of false positive MRIs in our case series: (1) the inclusion of a few cases in which the suspicion of CJD was raised by the MRI findings despite the unspecific clinical picture, a scenario which has become more frequent in recent years because of the improved sensitivity of DW/FLAIR techniques and the higher awareness among neuroradiologists of CJD being associated with signal hyperintensities, and (2) the fact that, at variance with Zerr et al. [6], we did not revise the MRI scans, but relied on the actual reports from the local neuroradiologist, which might have increased the chance of false-positive findings due to limited operator experience with a rare disease such as CJD.
In conclusion, based on the present and previous studies, we recommend the use of CSF t-tau assay for those laboratories dealing with RPDs not performing RT-QuIC or still considering it a second-level confirmatory test due to relatively high costs or limited substrate availability. The single RT-QuIC assay would, instead, represent the best choice in laboratories in which the substrate for PQ-CSF or IQ-CSF is largely available and has been positively tested for high reproducibility.
References
Baiardi S, Rossi M, Capellari S, Parchi P (2019) Recent advances in the histo-molecular pathology of human prion disease. Brain Pathol 29:278–300
Parchi P, Giese A, Capellari S et al (1999) Classification of sporadic Creutzfeldt–Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 46:224–233
Parchi P, Strammiello R, Notari S et al (2009) Incidence and spectrum of sporadic Creutzfeldt–Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: an updated classification. Acta Neuropathol 118:659–671
Parchi P, de Boni L, Saverioni D et al (2012) Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: an inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol 124:517–529
Baiardi S, Capellari S, Bartoletti Stella A, Parchi P (2018) Unusual clinical presentations challenging the early clinical diagnosis of Creutzfeldt–Jakob disease. J Alzheimers Dis 64:1051–1065
Zerr I, Kallenberg K, Summers DM et al (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt–Jakob disease. Brain 132:2659–2668
Chohan G, Pennington C, Mackenzie JM et al (2010) The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt–Jakob disease in the UK: a 10-year review. J Neurol Neurosurg Psychiatry 81:1243–1248
Hamlin C, Puoti G, Berri S et al (2012) A comparison of tau and 14-3-3 protein in the diagnosis of Creutzfeldt–Jakob disease. Neurology 79:547–552
Lattanzio F, Abu-Rumeileh S, Franceschini A et al (2017) Prion-specific and surrogate CSF biomarkers in Creutzfeldt–Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol 133:559–578
Rudge P, Hyare H, Green A, Collinge J, Mead S (2018) Imaging and CSF analyses effectively distinguish CJD from its mimics. J Neurol Neurosurg Psychiatry 89:461–466
McGuire LI, Peden AH, Orrú CD et al (2012) Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt–Jakob disease. Ann Neurol 72:278–285
Groveman BR, Orrú CD, Hughson AG et al (2016) Extended and direct evaluation of RT-QuIC assays for Creutzfeldt–Jakob disease diagnosis. Ann Clin Transl Neurol 4:139–144
Foutz A, Appleby BS, Hamlin C et al (2017) Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Ann Neurol 81:79–92
Franceschini A, Baiardi S, Hughson AG et al (2017) High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Sci Rep 7:10655
Schmitz M, Ebert E, Stoeck K et al (2016) Validation of 14-3-3 protein as a marker in sporadic Creutzfeldt–Jakob disease diagnostic. Mol Neurobiol 53:2189–2199
Leitão MJ, Baldeiras I, Almeida MR et al (2016) Sporadic Creutzfeldt–Jakob disease diagnostic accuracy is improved by a new CSF ELISA 14-3-3γ assay. Neuroscience 322:398–407
Steinacker P, Blennow K, Halbgebauer S et al (2016) Neurofilaments in blood and CSF for diagnosis and prediction of onset in Creutzfeldt–Jakob disease. Sci Rep 6:38737
Abu-Rumeileh S, Capellari S, Stanzani-Maserati M et al (2018) The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. Alzheimers Res Ther 10:3
Zerr I, Schmitz M, Karch A et al (2018) Cerebrospinal fluid neurofilament light levels in neurodegenerative dementia: evaluation of diagnostic accuracy in the differential diagnosis of prion diseases. Alzheimers Dement 14:751–763
Kanata E, Golanska E, Villar-Piqué A et al (2019) Cerebrospinal fluid neurofilament light in suspected sporadic Creutzfeldt–Jakob disease. J Clin Neurosci 60:124–127
Grau-Rivera O, Gelpi E, Nos C et al (2015) Clinicopathological correlations and concomitant pathologies in rapidly progressive dementia: a brain bank series. Neurodegener Dis 15:350–360
Geschwind MD, Murray K (2018) Differential diagnosis with other rapid progressive dementias in human prion diseases. Handb Clin Neurol 153:371–397
Zerr I, Hermann P (2018) Diagnostic challenges in rapidly progressive dementia. Expert Rev Neurother 18:761–772
Jansen C, Parchi P, Capellari S et al (2012) Human prion diseases in the Netherlands (1998–2009): clinical, genetic and molecular aspects. PLoS ONE 7:e36333
ECDC (2017) https://www.cjd.ed.ac.uk/sites/default/files/criteria_0.pdf. Accessed 10 July 2019
McGuire LI, Poleggi A, Poggiolini I et al (2016) Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic Creutzfeldt–Jakob disease: an international study. Ann Neurol 80:160–165
Otto M, Wiltfang J, Cepek L et al (2002) Tau protein and 14-3-3 protein in the differential diagnosis of Creutzfeldt–Jakob disease. Neurology 58:192–197
Skillbäck T, Rosén C, Asztely F et al (2014) Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt–Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol 71:476–483
Gaetani L, Blennow K, Calabresi P et al (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90:870–881
Kovacs GG, Andreasson U, Liman V et al (2017) Plasma and cerebrospinal fluid tau and neurofilament concentrations in rapidly progressive neurological syndromes: a neuropathology-based cohort. Eur J Neurol 24:1326–e77
Acknowledgements
We sincerely thank Dr. Byron Caughey for providing the substrate for IQ-CSF RT-QuIC. This work was supported by the Italian Ministry of Health (“Ricerca Corrente”).
Author information
Authors and Affiliations
Contributions
Conceptualization: SA-R and PP; methodology, formal analysis and investigation: all authors; writing—original draft preparation: SA-R and PP; writing—review and editing: PP based on the critical revision of all authors—supervision: PP.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
The study was conducted according to the revised Declaration of Helsinki and Good Clinical Practice guidelines and approved by the “Area Vasta Emilia Centro” ethics committee (CE-AVEC: 18025, 113/2018/OSS/AUSLBO). Informed consent was given by study participants or the next of kin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abu-Rumeileh, S., Baiardi, S., Polischi, B. et al. Diagnostic value of surrogate CSF biomarkers for Creutzfeldt–Jakob disease in the era of RT-QuIC. J Neurol 266, 3136–3143 (2019). https://doi.org/10.1007/s00415-019-09537-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09537-0