Abstract
Parkinson’s disease (PD) is a progressive neurological disorder characterized by motor symptoms as well as severe deficits in olfactory function and microstructural changes in olfactory brain regions. Because of the evidence of asymmetric neuropathological features in early-stage PD, we examined whether lateralized microstructural changes occur in olfactory brain regions and the substantia nigra in a group of early-stage PD patients. Using diffusion tensor imaging (DTI) and the University of Pennsylvania Smell Identification Test (UPSIT), we assessed 24 early-stage PD patients (Hoehn and Yahr stage 1 or 2) and 26 healthy controls (HC). We used DTI and a region of interest (ROI) approach to study the microstructure of the left and right anterior olfactory structures (AOS; comprising the olfactory bulbs and anterior end of the olfactory tracts) and the substantia nigra (SN). PD patients had reduced UPSIT scores relative to HC and showed increased mean diffusivity (MD) in the SN, with no lateralized differences. Significant group differences in fractional anisotropy (FA) and MD were seen in the AOS, but these differences were restricted to the right side and were not associated with the primary side of motor symptoms amongst PD patients. No associations were observed between lateralized motor impairment and lateralized microstructural changes in AOS. Impaired olfaction and microstructural changes in AOS are useful for early identification of PD but asymmetries in AOS microstructure seem unrelated to the laterality of PD motor symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive neurological disorder that typically develops over decades. PD usually follows a defined clinical progression beginning with non-motor symptoms reflecting neuropathological changes in anterior olfactory structures and in the dorsal motor nucleus of the vagus (DMV [8]). This stage begins, on average, 2–5 years or more before motor symptoms of PD appear and is characterized by a loss of olfactory function in up to 90–95% of patients [17] and by accumulation of α-synuclein in neurites and cell bodies of the olfactory bulb and tract [7,8,9,10]. At this early stage (Braak stage 1–2), there are no changes in α-synuclein accumulation or neurotransmission in the nigrostriatal dopaminergic system and little or no evidence of motor symptoms.
Years to decades after its appearance in olfactory structures, α-synuclein accumulations appear in neurons and neurites of the substantia nigra and striatum, coincident with the appearance of the classical motor symptoms of PD (resting tremor, bradykinesia, rigidity and postural instability). At even later stages, α-synucleinopathy is evident in cortical regions, which coincides with disruption of cognitive functions [7, 9, 10, 31]. Thus, PD is characterized by the sequential accumulation of α-synuclein first in peripheral brain regions (olfactory bulb and tract/DMV), followed by the nigrostriatal system and finally in cortical regions. Although the mechanisms involved in this progression are unknown, there is support for the involvement of a prion-like propagation leading to these neuropathological changes [40]. The accumulation of Lewy bodies and Lewy neurites also follows a distinct chronology beginning with the gut (enteric nervous system), DMV, and primary olfactory regions (including the olfactory bulbs, anterior olfactory nucleus and possibly the olfactory epithelium [9]).
Halting the progression of the disease through early interventions is a goal of treatment for PD. This approach depends on identifying specific and reliable biomarkers which are not yet available for early stages of the disease (reviewed in [45]). Single-photon emission computed tomography (SPECT) is useful for imaging changes in dopamine transporter (DAT) activity in striatal dopaminergic pathways in PD. However, other neuroimaging methods are needed to identify pathology in brain regions affected at earlier stages of PD.
The presence of olfactory loss in patients with PD has now been documented in more than 100 published studies (e.g., [17, 24, 47]). Reduced olfactory discrimination, identification and detection threshold [17] has been observed in as many as 90% of PD patients [29]. In fact, so pervasive is this deficit that olfactory testing is now included in the International Parkinson’s and Movement Disorder Society revised diagnostic criteria for PD [5, 6]. However, olfactory deficits are not specific to PD and are also seen in other common neurodegenerative disorders, including those without features of PD such as Alzheimer’s disease (AD; reviewed in [2]) and those with PD features such as Lewy Body Disease (LBD; reviewed in [16]), and thus are not a specific biomarker for PD.
Few studies of PD have used MRI to examine the structure of the olfactory bulbs (e.g. [28]) or tracts (e.g. [14, 47, 50]). PD patients have shown decreased left and right olfactory bulb volumes, although results have been variable across studies [34]. Diffusion tensor imaging (DTI), implemented using magnetic resonance imaging (MRI), can be used to gauge morphological changes by detecting altered diffusion patterns of water molecules in brain tissue [36]. DTI analysis of early PD pathology has been performed for the substantia nigra and other parts of the basal ganglia [32, 33, 42].
The microstructure of the substantia nigra (SN) has been investigated frequently in PD patients using DTI (e.g., [32, 42, 47, 49, 53]). For example, one study observed abnormalities in diffusion within the entire SN in PD patients. SN mean diffusivity (MD) was also correlated with disease severity (as estimated by the Unified Parkinson’s Disease Rating Scale; UPDRS) and the extent of [18F] DOPA uptake in the putamen [49]. These results demonstrate that SN microstructural changes can be detected in PD patients, suggesting that they could help to distinguish PD at early disease stages and help to track disease progression.
A defining characteristic of early PD is the emergence of any two of the three cardinal motor symptoms on one side of the body [21] and this initial asymmetry represents stage 1 of the Hoehn and Yahr [26] scale. Early asymmetry of motor symptoms distinguishes PD from other symptomatically similar, but pathologically distinct conditions, such as multi-system atrophy and essential tremor [12]. Although bilateral motor symptoms emerge with disease progression, their relative severity remains asymmetric [19]. The underlying pathological loss of dopamine neurons in the SN pars compacta is also thought to be asymmetric [30].
In a previous study involving olfactory testing and DTI of the SN and AOS, we found that these measures distinguish early-stage PD patients from HC [47]. However, we did not examine the possibility of asymmetries in the DTI measures for these structures, and to our knowledge, there is no published information regarding potential lateralized differences in DTI measures of anterior olfactory structures in PD. The goal of this study was to reexamine olfactory functioning in patients with Parkinson’s disease, using DTI measures to examine structural and microstructural changes in anterior olfactory regions and the substantia nigra, with a focus on potential asymmetries.
Methods
Participants
This study was approved by the Nova Scotia Health Authority Research Ethics Board and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Patients were recruited from the Movement Disorders Clinic of the Nova Scotia Health Authority, in Halifax, NS, Canada by board-certified neurologists (KS, JRM, GP). Healthy control participants were recruited from the general population through advertisements on websites along with local print and electronic media. Exclusion criteria for PD patients were: signs of dementia, autonomic dysfunction, vertical gaze palsy and cerebellar signs on clinical examination (see [47]). HCs with an UPSIT score (see below) of lower than 30 were excluded. Given that age can affect DTI parameters [4], all possible efforts were made to ensure that the two subject groups were similar in age. All participants received an honorarium for participation. This study was approved by the Nova Scotia Health Authority Research Ethics Board and all participants signed informed consent prior to beginning any study procedures.
Twenty-six (12/14, M/F) HC [58.8 ± 6.2 years of age (mean ± SD)] and 24 (15/9, M/F) PD (61.9 ± 4.9 years) participants were recruited. Relevant demographic and related information is provided in Table 1. Twenty-two of the PD subjects were taking PD medications.
Demographic and disease-related information
Prior to olfactory testing and MRI scanning, participants completed a questionnaire regarding demographic information. Patients’ neurologists provided disease-related information, including Hoehn and Yahr [26] and UPDRS scores [20].
Olfactory testing: University of Pennsylvania Smell Identification Test (UPSIT)
All participants were administered the UPSIT, which consists of 40 microencapsulated odorants that are activated by scratching. For each test item, participants scratch the scent-impregnated strip to release the odorant, and select, from the four-choice array of answers, the smell that it most closely matches. Each correct answer carries a score of one point, with higher scores reflecting better performance.
MRI data collection
A GE Signa HDxt 1.5 T whole body magnet with an 8HRBrain coil located in the IWK Health Center, Halifax, NS, Canada, was used to obtain MRI images. The details of sequences used in our protocol are presented in Supplementary Table 1. The total scan time (including sequences not reported in this manuscript) was approximately 50 min. Parallel imaging was used while acquiring the scans (ASSET). An experienced MRI technologist visually inspected all MRI images for the presence of artifacts, both during the scan and immediately after scanning.
MRI data analysis
Data analysis was performed using FSL version 5.0.8 software [52]. The following preprocessing steps were carried out on all MRI scans prior to region of interest (ROI) analysis: brain data extraction (BET), eddy current correction, and motion artifact calculation. DTIFit was used to calculate tensor fractional anisotropy (FA), and radial (RD) and axial diffusivities (AD). RD and AD were averaged to calculate mean diffusivity (MD). ROI tracing was completed by NJ (and confirmed by TR and NK) who were blinded with respect to the participants’ group identity and olfactory test score.
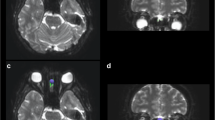
The SN was identified by first locating the red nucleus (RN), which was clearly visible on the T2 coronal images. The RN was traced on approximately 3–4 coronal slices (separate masks traced for the left and for the right halves; Supplementary Fig. 1). The SN was traced in the region inferior and slightly lateral to the RN (on the T2 coronal images), while avoiding the cerebral peduncles (also identifiable in the T2 coronal images). Identical coronal slices of the brain were used to trace both the RN and the SN.
The AOS was identified on coronal images; these dark regions were surrounded by cerebrospinal fluid (appearing bright on T2 images). The AOS identification protocol was similar to that used previously [1, 41]. The olfactory bulbs/tracts were identified on T2 coronal slices, with the AOS being traced on 2–4 consecutive T2 coronal slices on average; left and right halves were traced on separate masks (Supplementary Fig. 1).
Once completed, tracings for both structures were registered onto the DTI image, visually checked, and DTI measures were generated. Separate DTI measures (FA, MD, RD, AD) were generated for the left and right halves of each structure; the most commonly derived metrics used in previous studies (FA and MD) are discussed in this manuscript.
Motor impairment and lateralized microstructural changes
For each patient, the side of predominant motor symptoms was recorded as part of the Hoehn and Yahr assessment. From this, patients were subgrouped into ‘left’ or ‘right’ motor impairment groups. These two groups were compared across all DTI measures (T2 volume, FA and MD of the AOS and SN) using a two way, mixed design ANOVA (with side of predominant motor symptoms as between group factor and hemisphere; left vs right).
Statistical analysis
All statistical analyses were performed using SPSS (version 20.0, SPSS Inc., Chicago, IL). Two-way, mixed design ANOVAs were run for all lateralized measures with side (left, right) as the within-subject factor and group (PD, HC) as the between-group factor. Degrees of freedom were adjusted if the assumption of homogeneity of variance was violated (using Levene’s test). Significance for all analyses was set at 0.05. Post-hoc analyses, when performed, did not involve correcting for multiple comparisons, given the exploratory nature of the study [43, 48].
Results
Demographics and UPSIT scores
HC and PD patients did not differ significantly in age (F(1, 48) = 3.8, p = ns) nor on the proportion of males and females between the two groups (X 2(1) = 1.3, p = ns). However, the two groups differed significantly on UPSIT scores (HC > PD; F(1, 48) = 85.1, p < 0.001) (see Table 1; Fig. 1).
Anterior olfactory structure (AOS): T2 volume and DTI measures
T2 volume, FA, and MD of the AOS were examined on the left and right sides using a two-way ANOVA with side (left, right) as the within-subject factor and group (PD, HC) as the between-group factor. For T2 volume, there was no significant difference between groups [F(1, 48) = 1.1, p = ns] or sides [F(1, 48) = 1.0, p = ns], but the interaction between side and group was significant [F(1, 48) = 5.72, p < 0.05]. Nonetheless, post hoc tests failed to differentiate the two groups [Left t(48) = 1.7, p = ns; Right t(48) = 0.243, p = ns].
For AOS FA values, there was no significant difference between the two sides [F(1, 48) = 0.28, p = ns], nor did the groups differ significantly [F(1, 48) = 2.1, p = ns], but there was a significant side by group interaction [F(1, 48) = 4.4, p < 0.042]. In post hoc tests, there was no significant difference in FA values on the left between PD and HC [t(48) = 0.27, p = ns], but PD patients had significantly reduced FA values in the right AOS relative to HC [t(48) = 2.1, p < 0.043].
For AOS MD, there was no significant difference between the two sides [F(1, 48) = 0.14, p = ns], but there was significant difference between groups [F(1, 48) = 5.3, p < 0.03] and there was a significant side by group interaction [F(1, 48) = 6.1, p < 0.02]. In post hoc tests, there was no significant difference between groups in the left AOS [t(48) = 0.84, p = ns], but there was a significant difference between groups for the right AOS [PD > HC t(60) = 3.1, p < 0.003] (Fig. 2).
Diffusion tensor imaging analysis of the anterior olfactory structure (AOS) of healthy controls (HC) and early-stage Parkinson’s disease (PD) patients. a Tissue volume in cubic millimeters from T2-weighted images. b Fractional anisotropy (FA). c Mean diffusivity (MD) for the AOS. Data are mean ± SEM
Substantia nigra (SN): T2 volume and DTI measures
Similar two-way ANOVAs were computed for T2 volume, FA, and MD for the SN. For T2 volume, there were no significant differences for group [F(1, 48) = 0.33, p = ns], side [F(1, 48) = 2.8, p = ns] or the interaction between side and group [F(1, 48) = 0.00, p = ns]. There were also no significant differences for FA values in the SN for side [F(1, 48) = 2.8, p = ns], group [F(1, 48) = 0.56, p = ns] or the interaction between side and group [F(1, 48) = 0.45, p = ns].
For MD values in the SN, there was no significant main effect for side [F(1, 48) = 1.6, p = ns], nor for the interaction between side and group [F(1, 48) = 0.984, p = ns]; however, the MD values did differ significantly between groups [F(1, 48) = 4.2, p < 0.046] (PD > HC; Fig. 3).
Relationship between motor asymmetry and microstructural changes in AOS
PD patients with predominantly left motor symptoms were compared with those with predominantly right motor symptoms on the UPSIT. No differences were observed between these two groups [Left: 21.2(7.3) vs. Right 22.0(8.1); F(1, 22) = 0.72, p = ns]. Moreover, no differences were noted on any of the AOS DTI measures. For the SN, the left impairment group had significantly lower FA than did the right impairment group but this main effect was the only significant finding and no other differences were seen between motor impairment groups (see Table 2).
Discussion
We used DTI to assess microstructural changes in olfactory brain regions (AOS) and in the SN in early-stage PD patients together with assessment of olfactory function. We found that fractional anisotropy (FA) was decreased in the AOS of PD patients relative to HC, and this difference was attributable to the right side of the AOS, while the left side did not differ between groups. We also observed that mean diffusivity (MD) was increased in both the AOS and SN of PD patients relative to HC. As with FA, the group difference in MD in the AOS was attributable to the right side, while no lateralized difference was observed in MD of the SN. MD measures the average water diffusivity perpendicular and parallel to the axons and provides an estimate of the structural integrity of the neurons and myelin. MD increases [27] and elevated α-synuclein deposition [37] have been observed in PD patients in extra-olfactory regions, including the striatum, pallidum and thalamus, with greater MD and α-synuclein deposition seen in the later stages of PD [27, 37], consistent with more widespread pathology.
Structural changes, as estimated by the volumes of AOS and SN assessed in T2-weighted images did not differ between PD patients and HC and no left–right asymmetries were noted. Changes in AOS morphology in PD patients have been investigated previously. While some reports have described reduced volumes of olfactory bulbs and/or tracts [11, 14], others have not observed differences from controls [23, 28, 38, 41]. A recent meta-analysis of six studies related to olfactory bulb volume in PD concluded that there were significant decreases in volumes of both left and right olfactory structures, although there was a high degree of heterogeneity among studies [34]. Some differences among studies may be related to the use of automated programs to identify ROIs (e.g., [11, 14]) versus manual segmentation, and to differences in the stages and/or lengths of illness. The absence of significant volume differences in our results may be related to the early stage of disease in the patients studied and/or to the use of manual segmentation.
Consistent with our findings, previous studies using DTI have also observed differences in AOS microstructure between PD patients and controls, including differences in FA [47], MD [49], and apparent diffusion coefficient (ADC [50]), although these studies had not examined lateralized differences in these parameters. Studies of overall microstructural changes in the SN have reported varying results, including no differences between PD and HC [25], decreased FA [13, 42, 53], and increased FA in a subregion of the SN on the right side [33]. Meta-analyses related to the microstructural changes in SN have noted inconsistent findings across studies and found that net differences in FA are too small to be clinically useful in discriminating PD patients from controls [25, 51].
A significant difference between PD and HC was observed in FA and MD only in the right AOS, Olfactory deficits have been shown to be asymmetric in schizophrenia [22, 46], but not in Alzheimer’s disease or MCI [3]. Whether lateralized olfactory deficits occur in PD is still a matter of debate. While some studies reported greater left sided olfactory deficits [57], others have not demonstrated lateralized differences [18]. Unilateral lesions of the ventral striatum (nucleus accumbens and olfactory tubercule) in animals lead to contralateral olfactory deficits [54]. In humans, there is also evidence of a strong link between poor olfactory function and damage to right hemisphere structures (e.g., orbitofrontal cortex [56]). Whether lateralized anatomical changes in the AOS are related to olfactory deficits at later stages of PD remains to be determined.
Early in the course of PD, motor signs tend to be unilateral. Evidence that this may reflect asymmetric neuropathology comes from a post mortem study where more neuronal loss was observed in the SN contralateral to the affected body side [30]. A more recent examination of early onset PD patients using DTI found no differences in MD or in FA of the SN, when sides contralateral and ipsilateral to the side of initial symptom onset were compared [55]. In the current study, we found that patients with predominantly right sided motor signs had greater MD in the SN than those with predominantly left sided motor impairment; but no lateralized DTI asymmetries were noted.
Unlike prior studies, we also examined the association between motor asymmetry and structural and microstructural changes in olfactory brain regions. However, we found no differences in DTI measures in the AOS between the groups of PD patients with predominantly left versus predominantly right sided motor signs. These negative findings were seen despite the fact that lateralized microstructural changes were observed in the AOS when PD patients were compared to healthy controls. Thus, lateralized microstructural abnormalities were not associated with lateralized motor changes. Previously, Scherfler et al. [49] reported no correlations between objective olfactory testing and motor signs as measured by the UPDRS. Such findings seem consistent with the lack of differences in UPSIT scores between our PD subgroups as well as the absence of any apparent association between lateralized AOS DTI measures and laterality of motor signs in our sample of early stage PD patients.
Limitations and conclusions
Strengths of our study include our ability to accurately identify the AOS and SN on MRI in early-stage PD patients, our adequate number of participants and similar ages and sex distributions in each study group, and our analysis of potential lateralized changes in AOS and SN microstructure in early PD. Our study has limitations that should be noted. Using DTI imaging to investigate regions of the brain other than white matter tracts is challenging as DTI is best suited for examining the microstructural integrity of large white matter tracts in which the direction of relative diffusion can be readily observed. However, DTI has been successfully applied to studies of grey matter regions including the SN (e.g. [53]). A recently identified factor that may have contributed to inconsistencies across studies is the influence of ‘free water’ on diffusion parameters. Future studies will attempt to estimate DTI measures for both free water and cellular water to better resolve currently conflicting findings related to SN microstructure in PD [35, 39, 44]. One final limitation was our use of bilateral olfactory testing and it would be informative to conduct unilateral olfactory testing in future studies.
In conclusion, the results of the study confirm previous findings by our group [47] and others [49, 50] that early-stage PD is associated with both olfactory functioning deficits and microstructural changes on DTI of both AOS and SN. However, the current findings are unique in that they show that although lateralized differences in microstructural integrity of AOS may be seen in early PD relative to healthy individuals, these differences are not associated with the laterality of motor signs of PD. This finding potentially reflects the fact that structures in the AOS are among the first to show deposition of α-synuclein in PD patients, a process which appears to begin years before motor symptoms emerge [9, 15]. Thus, although asymmetries in AOS microstructure seem unrelated to the laterality of PD motor symptoms both DTI measures of AOS and impaired olfaction are early features of PD and further prospective studies using these techniques in sub-clinical, high-risk populations are required to determine their utility in identifying prodromal PD.
References
Altinayar S, Oner S, Can S, Kizilay A, Kamisli S, Sarac K (2014) Olfactory dysfunction and its relation olfactory bulb volume in Parkinson’s disease. Eur Rev Med Pharmacol Sci 18(23):3659–3664
Atik A, Stewart T, Zhang Z (2016) Alpha-synuclein as a biomarker for Parkinson’s disease. Brain Pathol 26(3):410–418
Bahar-Fuchs A, Chételat G, Villemagne VL, Moss S, Pike K, Masters CL, Rowe C, Savage G (2010) Olfactory deficits and amyloid-β burden in Alzheimer’s disease, mild cognitive impairment, and healthy aging: a PiB PET study. J Alzheimers Dis 22(4):1081–1087
Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M (2006) Influence of aging on gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology 66(4):535–539
Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, Gasser T, Goetz CG, Halliday G, Joseph L, Lang AE, Liepelt-Scarfone I, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G (2015) MDS research criteria for prodromal Parkinson’s disease. Mov Disord 30(12):1600–1611
Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, Goetz CG, Halliday GM, Hardy J, Lang AE, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschi G (2014) Time to redefine PD? Introductory statement of the MDS task force on the definition of Parkinson’s disease. Mov Disord 29(4):454–462
Braak H, Braak E, Bohl J, Reintjes R (1996) Age, neurofibrillary changes, A beta-amyloid and the onset of Alzheimer’s disease. Neurosci Lett 210(2):87–90
Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K (2006) Stanley Fahn lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21:2042–2051
Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318(1):121–134
Brodoehl S, Klingner C, Volk GF, Bitter T, Witte OW, Redecker C (2012) Decreased olfactory bulb volume in idiopathic Parkinson’s disease detected by 3.0-Tesla magnetic resonance imaging. Mov Disord 27(8):1019–1025
Brooks DJ, Pavese N (2011) Imaging biomarkers in Parkinson’s disease. Prog Neurobiol 95(4):614–628
Chan LL, Rumpel H, Yap K, Lee E, Loo HV, Ho GL, Fook-Chong S, Yuen Y, Tan EK (2007) Case control study of diffusion tensor imaging in Parkinson’s disease. J Neurol Neurosurg Psychiatry 78(12):1383–1386
Chen S, Tan HY, Wu ZH, Sun CP, He JX, Li XC, Shao M (2014) Imaging of olfactory bulb and gray matter volumes in brain areas associated with olfactory function in patients with Parkinson’s disease and multiple system atrophy. Eur J Radiol 83(3):564–570
Del Tredici K, Braak H (2016) Sporadic Parkinson’s disease: development and distribution of α-synuclein pathology. Neuropathol Appl Neurobiol 42(1):33–50
Doty RL (2012) Olfaction in Parkinson’s disease and related disorders. Neurobiol Dis 46(3):527–552
Doty RL, Deems DA, Stellar S (1988) Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 38:1237–1244
Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI (1992) Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 55(2):138–142
Djaldetti R, Ziv I, Melamed E (2006) The mystery of motor asymmetry in Parkinson’s disease. Lancet Neurol 5(9):796–802
Fahn S, Elton RL (1987) UPDRS program members. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB (eds) Recent developments in Parkinson’s disease, vol 2. Florham Park, Macmillan Healthcare Information, pp 153–163 (293–304)
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56(1):33–39
Good KP, Martzke JS, Milliken HI, Honer WG, Kopala LC (2002) Unirhinal olfactory identification deficits in young male patients with schizophrenia and related disorders: association with impaired memory function. Schizophr Res 56(3):211–223
Hakyemez HA, Veyseller B, Ozer F, Ozben S, Bayraktar GI, Gurbuz D, Cetin S, Yildirim YS (2013) Relationship of olfactory function with olfactory bulbus volume, disease duration and Unified Parkinson’s disease rating scale scores in patients with early stage of idiopathic Parkinson’s disease. J Clin Neurosci 20(10):1469–1470
Haehner A, Hummel T, Hummel C, Sommer U, Junghanns S, Reichmann H (2007) Olfactory loss may be a first sign of idiopathic Parkinson’s disease. Mov Disord 30 22(6):839–842
Hirata FC, Sato JR, Vieira G, Lucato LT, Leite CC, Bor-Seng-Shu E, Pastorello BF, Otaduy MC, Chaim KT, Campanholo KR, Novaes NP, Melo LM, Gonçalves MR, do Nascimento FB, Teixeira MJ, Barbosa ER, Amaro Jr E, Cardoso EF (2016) Substantia nigra fractional anisotropy is not a diagnostic biomarker of Parkinson’s disease: a diagnostic performance study and meta-analysis. Eur Radiol (epub ahead of print)
Hoehn M, Yahr M (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442
Kim HJ, Kim SJ, Kim HS, Choi CG, Kim N, Han S, Jang EH, Chung SJ, Lee CS (2013) Alterations of mean diffusivity in brain white matter and deep gray matter in Parkinson’s disease. Neurosci Lett 550:64–68
Kim JY, Lee WY, Chung EJ, Dhong HJ (2007) Analysis of olfactory function and the depth of olfactory sulcus in patients with Parkinson’s disease. Mov Disord 22(11):1563–1566
Katzenschlager R, Lees AJ (2004) Olfaction and Parkinson’s syndromes: its role in differential diagnosis. Curr Opin Neurol 17:417–423
Kempster PA, Gibb WR, Stern GM, Lees AJ (1989) Asymmetry of substantia nigra neuronal loss in Parkinson’s disease and its relevance to the mechanism of levodopa related motor fluctuations. J Neurol Neurosurg Psychiatry 52(1):72–76
Klingelhoefer L, Reichmann H (2015) Pathogenesis of Parkinson disease—the gut-brain axis and environmental factors. Nat Rev Neurol 11(11):625–636
Lenfeldt N, Hansson W, Larsson A, Nyberg L, Birgander R, Forsgren L (2013) Diffusion tensor imaging and correlations to Parkinson rating scales. J Neurol 260(11):2823–2830
Lenfeldt N, Larsson A, Nyberg L, Birgander R, Forsgren L (2015) Fractional anisotropy in the substantia nigra in Parkinson’s disease: a complex picture. Eur J Neurol 22(10):1408–1414
Li J, Gu C, Zhu L, Zhou Y, Huang H (2016) Changes in olfactory bulb volume in Parkinson’s disease: a systematic review and meta analysis. PLoS One 11(2):e0149286
Metzler-Baddeley C, O’Sullivan MJ, Bells S, Pasternak O, Jones DK (2012) How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage 59(2):1394–1403
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51(5):527–539
Mori F, Tanji K, Zhang H, Kakita A, Takahashi H, Wakabayashi K (2008) Alpha-synuclein pathology in the neostriatum in Parkinson’s disease. Acta Neuropathol 115(4):453–459
Mueller A, Abolmaali ND, Hakimi AR, Gloeckler T, Herting B, Reichmann H, Hummel T (2005) Olfactory bulb volumes in patients with idiopathic Parkinson’s disease a pilot study. J Neural Transm (Vienna) 112(10):1363–1370
Ofori E, Pasternak O, Planetta PJ, Burciu R, Snyder A, Febo M, Golde TE, Okun MS, Vaillancourt DE (2015) Increased free water in the substantia nigra of Parkinson’s disease: a single-site and multi-site study. Neurobiol Aging 36(2):1097–1104
Olanow CW, Brundin P (2013) Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov Disord 28(1):31–40
Paschen L, Schmidt N, Wolff S, Cnyrim C, van Eimeren T, Zeuner KE, Deuschl G, Witt K (2015) The olfactory bulb volume in patients with idiopathic Parkinson’s disease. Eur J Neurol 22(7):1068–1073
Péran P, Cherubini A, Assogna F, Piras F, Quattrocchi C, Peppe A, Celsis P, Rascol O, Démonet JF, Stefani A, Pierantozzi M, Pontieri FE, Caltagirone C, Spalletta G, Sabatini U (2010) Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain 133(11):3423–3433
Perneger TV (1998) What’s wrong with Bonferroni adjustments. BMJ 316:1236–1238
Planetta PJ, Ofori E, Pasternak O, Burciu RG, Shukla P, DeSimone JC, Okun MS, McFarland NR, Vaillancourt DE (2016) Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain 139(Pt 2):495–508
Postuma RB, Berg D (2016) Advances in markers of prodromal Parkinson disease. Nat Rev Neurol 12(11):622–634
Roalf DR, Turetsky BI, Ozwar K, Balderston CC, Johnson SC, Brensinger CM, Gur RE, Siegel SJ, Moberg PJ (2006) Unirhinal olfactory function in schizophrenia patients and first-degree relatives. J Neuropsychiatr Clin Neurosci 18:389–396
Rolheiser TM, Fulton HG, Good KP, Fisk JD, McKelvey JR, Scherfler C, Khan NM, Leslie RA, Robertson HA (2011) Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson’s disease. J Neurol 258(7):1254–1260
Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1:43–46
Scherfler C, Esterhammer R, Nocker M, Mahlknecht P, Stockner H, Warwitz B, Spielberger S, Pinter B, Donnemiller E, Decristoforo C, Virgolini I, Schocke M, Poewe W, Seppi K (2013) Correlation of dopaminergic terminal dysfunction and microstructural abnormalities of the basal ganglia and the olfactory tract in Parkinson’s disease. Brain 136(10):3028–3037
Scherfler C, Schocke MF, Seppi K, Esterhammer R, Brenneis C, Jaschke W, Wenning GK, Poewe W (2006) Voxel-wise analysis of diffusion weighted imaging reveals disruption of the olfactory tract in Parkinson’s disease. Brain 129(Pt 2):538–542
Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP (2013) Diffusion tensor imaging of nigral degeneration in Parkinson’s disease: a region-of-interest and voxel-based study at 3 T and systematic review of meta-analysis. NeuroImage Clin 3:481–488
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(S1):208–219
Vaillancourt DE, Spraker MB, Prodoehl J, Abraham I, Corcos DM, Zhou XJ, Comella CL, Little DM (2009) High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 72(16):1378–1384
Valle-Leija P, Drucker-Colín R (2014) Unilateral olfactory deficit in a hemiparkinson’s disease mouse model. NeuroReport 25(12):948–953
Wang J, Yang QX, Sun X, Vesek J, Mosher Z, Vasavada M, Chu J, Kanekar S, Shivkumar V, Venkiteswaran K, Subramanian T (2015) MRI evaluation of asymmetry of nigrostriatal damage in the early stage of early-onset Parkinson’s disease. Parkinsonsim Relat Disord 21:590–596
Zatorre RJ, Jones-Gotman M (1991) Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain 114:71–84
Zucco GM, Rovatti F, Stevenson RJ (2015) Olfactory asymmetric dysfunction in early Parkinson patients affected by unilateral disorder. Front Psychol 6:1020
Acknowledgements
Our sincere appreciation is extended to Mr. Carl Helmick. As well, Denise Lewis, Ceire Storey, Dana Song, Rebecca Hastings, and Rose Gan were instrumental in this project. This study was funded by Canadian Institutes of Health Research MOP-112645, Parkinson Society Canada, Dalhousie Department of Psychiatry Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Harry Robertson is the Director of Clinical Development, Neurodyn Inc.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joshi, N., Rolheiser, T.M., Fisk, J.D. et al. Lateralized microstructural changes in early-stage Parkinson’s disease in anterior olfactory structures, but not in substantia nigra. J Neurol 264, 1497–1505 (2017). https://doi.org/10.1007/s00415-017-8555-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8555-3