Abstract
Invasive electroencephalography recordings with depth or subdural electrodes are necessary to identify the ictogenic area in some drug-resistant focal epilepsies. We aimed to analyze the safety profile of intracranial electrode implantation in a tertiary center and the factors associated with its complications. We retrospectively examined complications in 163 intracranial procedures performed in adult patients. Implantation methods included oblique depth stereotactic approach (n = 128) and medial–temporal depth stereotactic approach in combination with subdural strip placement (n = 35). 1201 depth macroelectrodes, 59 bundles of microelectrodes (in 30 patients) and 148 subdural electrodes were implanted. Complications were classified as major (requiring treatment or leading to neurological impairment) or minor. The rate of overall complications was 4.9 % (n = 8), with 3.1 % (n = 5) of major complications, though no permanent morbidity or mortality was recorded. Infection occurred in 1.2 % and hemorrhage in 3.7 % of patients. One hemorrhage occurred for every 225 electrodes implanted (4.4 ‰). Microelectrodes were not responsible for any complications. Overall and hemorrhagic complications were significantly associated with MRI-negative cases (7.3 and 6.3 % versus 0 %, p = 0.04). We believe that intracranial electrode implantation has a favorable safety profile, without permanent deficit. These risks should be balanced with the benefits of invasive exploration prior to surgery. Furthermore, this study provides preliminary evidence regarding the safety of micro-macroelectrodes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive EEG monitoring is essential for the localization and the delineation of the ictogenic area in a number of patients with drug-resistant focal epilepsy. The implantation of intracranial (IC) electrodes constitutes the first step of continuous long-term intracranial EEG (iEEG) recording [1]. Patients who are offered a surgical solution are severely disabled by the epileptic seizures that have not been sufficiently controlled by a well-conducted antiepileptic therapy. They are in a therapeutical dead-end and have no other options for seizure relief. Yet surgery can be beneficial with a good post-operative outcome. The post-operative rate of seizure-free patients is expected to be around 60 % [2] after invasive evaluation based on current literature. This therapeutical option appears all the more desirable that it is safe. Nonetheless, it has a certain morbidity and mortality [3] that should be discussed with the patient prior to surgery.
Very few studies focus on complications and adverse events regarding depth electrode implantation in epileptic patients for diagnostic video-EEG recordings. Moreover, to date, complications related to depth micro-macroelectrodes implantation have not been widely evaluated. Hence, we conducted a retrospective study on the morbidity and mortality of IC electrodes in all patients who were monitored with depth electrodes in our institution. The main purpose of this report was to describe and analyze the safety of IC electrode implantation using MRI-guided stereotactic surgery and the recording period with the electrodes in place. The secondary endpoint was to identify possible factors associated with an increased complication rate.
Patients and methods
Patients
We conducted a retrospective analysis of all adult patients who underwent implantation of depth electrodes for presurgical evaluation of drug-resistant focal epilepsy at the Pitié-Salpêtrière Hospital (Paris, France) between 1991 and July 2014. We also included patients who had subdural electrodes, in addition to the depth electrodes. Patients implanted only with subdural strips or grids were not included (n = 9). Presurgical non-invasive evaluation included: complete neurological and neuropsychological examination, scalp video-EEG recording of habitual seizures, brain MRI, and in some cases subtraction ictal single photon emission computed tomography (SPECT) co-registered to MRI and interictal positron emission tomography (PET). At this stage, a multidisciplinary team analyzed data drawn from these investigations if a surgical treatment was indicated without any further localizing presurgical investigations. Otherwise and when a unique or strongly predominant ictogenic area is expected from non-invasive data, iEEG recording was decided case by case. It was mainly indicated in MRI-negative patients, in case of discordance between electroclinical data and imaging, in large or multifocal lesions or in suspected multifocal epilepsy. In several patients, iEEG were scheduled for more than one of these reasons. All these indications were corresponded to about 1 in 5 surgical candidates. A few patients were implanted twice mainly to delimit more precisely the boundaries of the ictogenic area for the second implantation. Data regarding age, gender, relevant medical history, seizure semiology, number and location of implanted electrodes, electrophysiological localization of the epileptogenic focus, duration of invasive recording, post-implantation brain MRI and CT scan and complications were collected and analyzed. All the adverse events during implantation, iEEG recording, and post-explantation survey (if related to the intracranial procedure) were considered to be complications.
Surgical methodology
Electrodes
Implantations were performed with standard platinum intracranial [depth (i.e., intracerebral) and subdural] macroelectrodes (Adtech Medical Instrumentation, Racine, Wisconsin, USA). We used Spencer® probe intracerebral electrodes, with 4–12 contacts (1 mm of diameter), with intercontact spacing of 10–5 mm. They were inserted using a semi-rigid stylet. Since 2010, we implanted micro-macroelectrodes in combination with classical macroelectrodes [4]. For micro-macroelectrodes (Adtech Medical Instrumentation), we used Benke-Fried® depth electrodes, with 8 macro contacts (1.3 mm of diameter), with intercontact spacing of 10–5 mm. They were inserted using a semi-rigid stylet; then an 8-contact micro-wire bundle was introduced into the macroelectrodes and wires extruded distally with a defined length (between 3 and 10 mm). For some cases, we used subdural strips [Adtech Medical Instrumentation; 6 or 8 large platinum contacts (6 mm diameter)] in combination with depth electrodes.
Intracranial implantation procedure
The implantation plan was designed according to two models: (1) double obliquity methodology using depth electrodes implanted orthogonally or obliquely to the cortical surface, along lateral-medial trajectories (oblique approach, Fig. 1); or (2) Spencer’s methodology using, on each side if necessary, three or four subdural electrode strips for polar, lateral and basal sampling (Fig. 2a, b), and one longitudinal hippocampal depth electrode introduced posteriorly along a parasagittal direction with the amygdala as the target (Fig. 2c) [5–7]. Spencer’s methodology was in use in our center between 1991 and 2004. We first started using the double obliquity methodology in 1995, in combination with Spencer’s methodology, which was stopped in 2004.
Plans of depth implantations (with the double obliquity method) conducted on MRI. a–c Planning illustrations for a left pericentral implantation (in the same patient). a 3D reconstruction of Leksell frame MR indicators and of patient’s head and brain in transparency. Depth electrode n°7 is planned in left medial parietal lobe. b Overall plan of depth electrodes in upper and medial pericentral regions (parietal, central and precentral). c Simulation of depth electrode n°7 (left medial parietal) and its relationships with vessels. The electrode tip is at 4.2 mm (as measured by the distance tool, formed of a white line between two crosses) from the vessel situated on the other side of the falx cerebri. The 1 mm electrode thickness is depicted on the image. d–f Planning illustrations for a right frontal and temporal lobe implantation (in the same patient). d Large frontal and temporal lobe planning with 13 depth electrodes. e Simulation of right temporal lobe depth electrode n°3 exploring hippocampal head, insula and first temporal gyrus. e, f Electrode n°3 is 4.5 mm away from the insular artery. f Brain display perpendicular to the planned n°3 electrode axis showing neighborhood structures along the trajectory. The 1 mm electrode thickness is depicted on the image
Spencer’s depth implantation (with the double obliquity method) and subdural strips. a 3D skull CT reconstruction showing symmetrical exploration of the temporal lobes with subdural strips and Spencer’s depth electrodes (view of the base of skull from above). Temporal lobe electrodes on the left side: n°1 polar subdural, n°2 anterior basal subdural, n°3 posterior basal subdural and n°4 Spencer’s depth electrode (recording the medial temporal and occipital structures). b 3D skull CT reconstruction of the left side showing the subdural electrodes emerging from the burr hole. c Post-implantation axial MRI control passing through the 2 Spencer’s electrodes. Only n°1, 2 and 4 electrodes are seen. Electrode n°2 (anterior basal subdural) is seen twice: the 1st contact of the tip is anterior and medial and the last ones are more posterior and lateral, the others are below the image plane
No robot-assisted methods were used. Our surgical procedures consisted of two steps: stereotactic MRI and electrode implantation. All the patients were placed in a Leksell-G stereotactic frame, and underwent a 3-Dimensional (3D) Spoiled Gradient Recalled (SPGR) gadolinium MRI and a 3D Time of Flight (3DTOF) on a 1.5 Tesla MR scanner (Signa, General Electric, Milwaukee, Wisconsin, USA) after intravenous administration of gadolinium contrast material [0.01 mmol/kg gadopentetate dimeglumine (Magnevist, Berlex Laboratories, Inc., Montville, New Jersey, USA)]. Scans were obtained using the following parameters:
-
3D TOF MULTISLABS GADO used a 256 × 224 matrix. The field of view (FOV) was 22 cm, with, zero-filled in k-space to produce 256 × 256 pixel images. Repetition time (TR) was 22 ms, and echo time (TE) was 4.1 ms. Section thickness and spacing were 1.5 mm respectively.
-
Axial T1-weighted SPGR used a 128 × 128 matrix. The FOV was 22 cm, with, zero-filled in k-space to produce 256 x 256 pixel images. TR was 9.024 ms, and TE was 1.988 ms. Section thickness and spacing were 1.5 and 1.3 mm, respectively. Since 2008, we only use a SPGR sequence but we increase the intravenous administration of gadolinium contrast material (0.012 mmol/kg).
Once these data were acquired, the depth trajectories were planned according to the cortical areas to be explored. They were defined by the neurophysiologist (CA), together with the neuroradiologist (DH) and reviewed by the neurosurgeons (SC and HB). Stereotactic coordinates for each electrode were calculated with the General Electrics software®, and more recently with the Brainlab software® (Brainlab AG, Feldkirchen, Germany). The calculation protocol for depth electrodes followed 4 steps: 3D reconstruction of the brain, 3D positioning of each electrode while controlling adjacent structures (vessels and eloquent areas), 3D control of the ongoing electrode plan in space with brain transparency to achieve regular distribution of electrodes and avoid electrode overlapping, retroactive placement modification of previously planned electrodes if necessary. The electrode path was carefully chosen so as to avoid injury to superficial and deep critical veins and arteries. This was simultaneously controlled millimeter by millimeter in the three planes of space (3D view), and in the perpendicular and parallel oblique views of the electrode trajectory. Furthermore we took great care, when possible, to minimize entry into the ventricular system and risking the tearing of subependymal veins. The Brainlab software® gave a better display for evaluating distances between electrodes and anatomical structures with a visualization of the actual diameter of the electrode.
After returning to the operative room, under general anesthesia, patients were locally shaved and surgery was carried out under standard aseptic operative conditions with antibiotic prophylaxis (cefazolin 2 g). Patients were positioned half-sitting, and the stereotactic arc was used to plan the incision. A stab incision was made and a 2 mm twist-drill hole was performed according to previously calculated coordinates. An intracerebral electrode was then introduced through the drill hole to the target and secured to the scalp using a suture or more recently a bone screw. This process was repeated according to the number of necessary electrodes. When Spencer’s methodology was used, the subdural strips were introduced through a single temporal 2 cm burr hole.
Postoperative management
Patients were monitored at least 6 h in the recovery unit. Postoperative CT scan and 3-dimensional 1.5-Tesla MRI were then performed to precisely locate the electrodes and rule out immediate complications, prior to transfer to the epileptology unit. The IC electrodes were then connected to the data recording system, and antiepileptic treatment was slightly decreased until enough seizures were acquired to allow a diagnosis, or until an adverse event occurred requiring electrodes removal. A CT scan was systematically performed after electrodes removal to exclude intracranial bleeding. Antibiotics were only dispensed during the invasive recording period if infection was suspected.
Classification of complications
We considered complications occurring during the implantation and the iEEG recording period together. In the light of current literature on complications of IC implantation procedures [2], we used two severity scales, to allow for direct comparison with previous studies: (1) major/minor scale: a complication was defined as major when medical or surgical treatment was required and/or when a neurologic impairment occurred; it was otherwise considered as minor. (2) Graded scale: with composite items including a prevailing surgical component [2, 8]:
-
Grade 0: no complication.
-
Grade 1: complication visible only on CT/MRI; or transient complication that did not require treatment.
-
Grade 2: transient complication that resolved completely but required treatment, or revision of electrodes, or preterm ending of monitoring.
-
Grade 3: persisting neurologic deficit >12 months.
-
Grade 4: patient’s death related to the invasive workup.
Furthermore, several adverse events (meningeal syndrome, low-grade fever, headaches, pneumocephalus) can occur after electrode implantation or removal, but they were considered as expected mild functional disturbances and not as complications.
Statistical Analysis
Comparisons between the groups with or without complications were assessed using Fisher exact test or Mann–Whitney test as appropriate, regarding different variables (age at implantation, gender, number of electrodes, presence of subdural electrodes, duration of recording, implantation methodology and scheme, number of lobes affected by the implantation, side of implantation and cryptogenic epilepsy). Significance was set at a p value of 0.05. Data are reported as means plus/minus standard deviation unless stated otherwise.
Results
Patient demographics
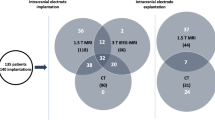
One hundred and sixty-three (163) stereotactic implantations of depth electrodes were performed in 157 adult patients, of whom six patients were explored twice. Mean age at surgery was 32.4 ± 9.2 years (range, 15.1–60.2); the sex ratio male/female was 1.14. Mean age at the time of the first seizure was 14.1 years (±6.7, range, 3.0–38.8 years). All patients had drug-resistant focal epilepsy, which was severe and/or disabling with a mean seizure frequency of 13 seizures per week (±21; range, 1–100). Most of our patients had negative MRI exploration (cryptogenic epilepsy) (n = 96, 58.9 %). Epileptogenic lesions comprised hippocampal sclerosis (n = 27, 16.6 %), focal cortical dysplasias (n = 14, 8.6 %), posttraumatic lesions (n = 9, 5.5 %), porencephalic cysts (n = 3, 1.8 %), cavernomas (n = 3, 1.8 %), dysembryoplastic neuroepithelial tumors (n = 2, 1.2 %) and other forms of cortical malformations (n = 9, 5.5 %), as disclosed at the neuropathological examination.
Surgery
The double obliquity approach was used in 128 cases (78.5 %) and Spencer’s methodology in 35 cases (21.5 %). A total of 1201 depth electrodes and 59 bundles of microelectrodes were implanted over a period of 23 years, ranging from 1 to 13 per patient. The overall median number of depth electrodes was 8 electrodes per procedure. In general, implantation with the double obliquity technique comprised a higher number of electrodes (mean: 9.0 ± 2.4; median: 10). A total of 148 subdural electrodes were implanted when Spencer’s methodology was used, ranging from 2 to 6 per patient (mean: 4.2 ± 1.4; median: 4).
Collapsed over all procedures, the exploration was unilateral in 108 cases (66.3 %), with 58 right and 50 left hemispheric explorations, respectively, and bilateral in 55 procedures. Sixty-three percent (n = 102) of the patients were explored for temporal lobe epilepsy, 21.5 % (n = 35) for frontal lobe epilepsy, 8.0 % (n = 13) for occipital lobe epilepsy, 5.5 % (n = 9) for parietal lobe epilepsy and 2.4 % (n = 4) for insular epilepsy. The mean duration of prolonged continuous 24 h video-iEEG monitoring was 15.6 ± 4.2 days (range 7–23 days).
Overall complications
The implantation for iEEG was uneventful for 155 (95.1 %) of the procedures (Fig. 3a). Complications were considered as major for five procedures (3.1 %, Fig. 3b), leading to a prolonged hospital stay in 4 patients (2.5 %), and minor in three patients (1.8 %). On a composite graded scale, 1.8 % (n = 3) had a grade 1 complication and 3.1 % (n = 5) had a grade 2 complication (Fig. 3C). Complications related to iEEG procedures are summarized in Table 1. Of the eight patients who experienced adverse events, surgery had to be performed in one case to evacuate an epidural hematoma (Fig. 4a–c). The iEEG recording was shortened in three cases (because of hemorrhage, infection or anesthesia shock). Neither permanent neurologic deficit nor death (grade 3 and 4, respectively), occurred with implantation and invasive recording. Micro-electrodes (in 30 patients) were not at the origin of adverse events (patient 8 had a complication related to a macroelectrode with a high probability, and not to a micro-macroelectrode).
Major complications. Cerebral imaging of the major hemorrhagic and infectious complications classified as grade 2. a–c Epidural hematoma requiring surgical evacuation. CT scan (a) and MRI T2* imaging (b) within 2 h after implantation. The most basal orbitofrontal electrode (b) does not seem to penetrate the dura. c Postoperative CT scan after surgical evacuation of the epidural hematoma. d, e Bilateral frontotemporal implantation (d) complicated by subarachnoid hemorrhage (e predominating in right sylvian fissure) causing localized vasospasm and ultimately right insular stroke. f, g Bilateral temporal implantation with fever and headache. Native CT scan showing subtle subdural hematoma of the right cerebellar tent, probably due to an initial lumbar puncture on day 8 (f). Iodine injected CT scan on day 22 (g) showing multiple right temporal abscesses
Regarding expected adverse events, after removal of the electrodes, we observed pneumocephalus in 87.2 % of cases and pneumoventricle in 9.3 % of cases that often led to headaches. Low-grade fever and afebrile meningeal syndrome (stiff neck, headaches, nausea/vomiting and photophobia) were frequently observed after the removal of electrodes fixed with screws, possibly due to slightly larger drill holes in this procedure.
Hemorrhage
Hemorrhagic complications related to iEEG procedures were observed in six patients (3.7 %), with a variable degree of severity. Grade 2 hemorrhagic adverse events occurred in three cases: an epidural, a subarachnoid and a subdural hemorrhage, respectively. One patient became less responsive during post-implantation MRI scanning, the latter showing a frontal lobe epidural hematoma (grade 2). The surgical evacuation entailed removal of 3/12 electrodes, but did not reveal any intra-operatively visible laceration of blood vessels. Post-implantation MRI scan (Fig. 4a, b) also suggested that the most basal orbitofrontal electrode had not penetrated the dura, causing excessive separation of the frontobasal dura. This electrode was a macroelectrode, and not a micro-macroelectrode implanted in this patient. The patient recovered immediately following evacuation (Fig. 4c). Furthermore the remaining electrodes allowed the localization the ictogenic area. A second implantation was achieved (without complications) to define its posterior boundary using interhemispheric subdural strips (not included in this report).
Two patients developed subarachnoid hemorrhage, one of which was moderate (grade 1), and the other one severe (grade 2). The latter, occurring in a 46-year-old female, caused localized vasospasm at distance of the temporal depth electrodes, which resulted in a right insular stroke (Fig. 4d, e). She experienced transient aphasia that had completely recovered at the follow-up evaluation at 15 months.
One patient developed an acute subdural hematoma without mass effect, causing a status epilepticus, which ultimately required an unplanned removal of all the electrodes and management in intensive care unit (grade 2). Then she recovered completely.
Lastly, two patients showed small and asymptomatic intracerebral hemorrhage visible on post-implantation CT scan (grade 1).
Given the total number of implanted electrodes, the rate of hemorrhage per implanted electrode in this series was 4.4 ‰, i.e., one hemorrhage per 225 electrodes implanted.
Infection
In our series, there were two cases (1.2 %) of infection: one patient with intracerebral temporal abscesses (grade 2) and one with meningitis (grade 2). The former was afebrile at discharge from the surgical unit on day 3 and all throughout video-EEG monitoring. Due to low-grade fever and new onset headaches on day 8, a lumbar puncture, with the electrodes in place, was performed after an unremarkable brain CT scan (glucose and proteins normal, negative GRAM staining, four cells, 370 red blood cells) and caused a subtle subdural hematoma of the cerebellar tent seen on the CT scan the next day (Fig. 4f). On day 22, because of hyperthermia and subcutaneous abscesses close to the electrode holes, a new CT scan revealed multiple temporal lobe abscesses requiring electrode removal, surgical drainage and antibiotic therapy (Fig. 4g). Coagulase-negative staphylococcus was determined to be the pathogenic agent. He experienced no neurological deficit and recovered totally from infection.
In the case of meningitis occurring on day 11 of video-EEG monitoring, Pseudomonas aeruginosa was determined as the pathogenic agent, electrodes were removed and antibiotic treatment was efficient.
Factors associated with complications
Comparisons between the groups with or without complications are listed in Table 2. MRI-negative epilepsy was found to be significantly associated with overall (p = 0.04; Fisher exact test) and hemorrhagic (p = 0.04; Fisher exact test) complications. The number of depth electrodes was not an independent predictor of overall complications (9.6 ± 2.3 versus 7.2 ± 3.6, p = 0.11; Mann-Whitney test). Further analysis also indicated a greater number of electrodes in the MRI-negative epilepsy subgroup (8.4 ± 3.1 versus 5.9 ± 3.9, p < 0.001; Mann-Whitney test).
There was no difference in complications between the two-implantation methods (double obliquity and Spencer’s), and between implantations with or without subdural electrodes (p = 0.53; Fisher exact test, Fig. 3d).
Discussion
We report implantation complications in a large series of iEEG recordings with depth electrodes for diagnostic purpose in epilepsy. Our complication rate (4.9 %) is in the range of those reported in the literature, between 1 and 15.9 % in adult populations [2, 3, 6, 9–15] (Table 3). And our rate of major complications (3.1 %) is also in line with those previously published, between 0.0 and 6.4 % (Table 3). However, there was heterogeneity between the different studies regarding the classification of complications related to different procedures, especially when appreciating the nature of major complications. In this study, we carefully analyzed each case and classified them with two severity scales to allow an objective comparison with previously published series. The incidence of minor intracranial bleedings could also be misjudged, as blood-sensitive postoperative imaging was not obtained in all cases [13]. It would indeed be fruitful to use the same severity scales across all studies [2, 8]. New studies, as ours, are thus mandatory to better assess the complications of diagnostic IC procedures in epilepsy. The multiplication of series has also offered the possibility to evaluate more properly the incidence of exceptional events, such as “death” evaluated at 0.17 % (3/1748 patients) [11, 13, 14].
Our study was not tailored to examine correctly the differences in complications between depth versus subdural iEEG, as each case in the subdural group had one or two depth electrodes. Hence, we did not find, unlike others, lower global complications in depth implantations compared to those with subdural electrodes (Table 4) [2, 8, 16–28]. The low number of subdural electrodes per patient (as well as depth electrodes) and the exclusion of patients with grids could potentially explain why complication rates seemed even lower in that subdural group. For subdural grids, a meta-analysis showed a prevalence as high as 5.3 % for infections and 4.0 % for intracranial hemorrhages in a pooled series of 2542 cases [29]. It is possible that the craniotomy to insert grids offers a large gateway for bacterial penetration. Interestingly, this difference between intracerebral and subdural grid implantations was still present within the same team who practiced both procedures, underscoring that it is not only a matter of team skill [2, 27]. Our low rate of complications and the absence of death and permanent neurological deficit are probably due both to accurate planning and surgery and the low invasiveness of the small drill holes performed to implant the depth electrodes.
In this series, micro-macroelectrodes were not at the origin of complications. However, our study was not tailored and sufficiently powered to study this outcome statistically. Hence, this report, while not providing conclusive data on this issue, provides preliminary evidence for safety of micro-macroelectrodes.
Intracranial bleeding complications
In our series, the rate of hemorrhagic complications (3.7 %) was comparable to those reported in previous publications [2, 3, 13]. Increased number of electrodes has been shown to be associated with increased frequency of intracranial bleedings [2, 27, 29] or not [3, 30]. In our study overall surgical complications (n = 7) mostly consisting of intracranial bleedings (n = 6) showed a trend towards an association with a greater number of electrodes (p = 0.11). This might be due to the too small number of complications. Nonetheless, unlike many reports [2, 13, 25, 27, 30], we found a higher rate of overall and hemorrhagic complications in MRI-negative epilepsies. Moreover, in this sub-population, the mean of intracerebral electrodes number was higher than in the remaining population, supporting eventually the role of the number of electrodes.
The literature also suggests that a higher age (>65 years) is associated with an increased risk for hemorrhagic complications during epilepsy surgery [20, 29]. Maybe our patients’ age range was too narrow to find this association. Valproate treatment with thrombocytopenia was also found to be another risk factor associated with hemorrhage [31] and has to be corrected before invasive exploration.
We observed a postoperative frontal lobe epidural hematoma in a patient investigated with frontal depth electrodes for frontal lobe epilepsy. As no source of hemorrhage was found intraoperatively, such as a lacerated vessel, the most likely alternative explanation, supported by MRI, is that the hematoma was due to an excessive separation of the frontobasal dura during the insertion of a frontal electrode. As the dura is adherent to the skull at the vertex, the latter has been proposed as a better insertion site to target the fronto-orbital cortex, but, in our opinion, this pathway would explore too scarcely this large cortical region [32, 33].
Infection complications
Regarding infectious complications, it is necessary to differentiate neurological infections and superficial infections. Two (1.2 %) of our patients had neurological infections, whereas there were no wound infections. This rate is close to the ones reported by Serletis et al. (1 %, superficial infections) [14], Wellmer et al. (0.8 %, meningitis) [2] and Cardinale et al. (0.4 %, encephalitis) [13] with depth electrodes. In several series using subdural grids or strips, infections emerged as the most common type of complications and, therefore, infection rates were much higher (5.3–14.9 %) [8, 18, 21, 29, 34]. Inversely, only a few studies reported lower infection rates with subdural grids or strips (0.7–1.1 %) [22, 27].
In one prospective series, the infection rate was shown to increase if subdural strips and grids remained implanted for more than 2 weeks or if more than 10 electrode cables were used [18]. In our 2 patients, the infection arose late during the recording (11 and 22 days). In studies using depth electrodes such as ours, these parameters were not significantly associated with a higher risk of infection, probably in relation to a low rate of infections causing a lack of power to our study.
Concerning our case with multiple abscesses, two causes were discussed: (1) the contamination by the sub-cutaneous infection through the electrode hole(s) favored by CSF leakage following lumbar puncture or (2) a direct contamination during the implantation. In favor of the first explanation were the occurrence of a subdural hematoma evoking a loss of cortical sulcal CSF (hence a suction of extracranial fluid) and the timing of abscesses that developed after the lumbar puncture according to the CT scan survey. There was no firm evidence for either explanation. This, however, modified the recommendations in our center contraindicating lumbar punctures with implanted electrodes.
Interestingly, the only two infections of our series occurred consecutively in 2007. Until this date, we used to change the head bandage every 2 days during the monitoring. A change in our postoperative management resulted from these serious adverse events. Since then, the patients keep the same head bandage until the removal of the electrodes to avoid contamination and breaking of electrodes. Since the application of this protocol, we have not experienced infections.
Conclusions
Stereotactic and subdural strip implantation is generally a safe procedure for the exploration of severe, drug-resistant focal epilepsies. In our experience mainly with depth electrodes, sometimes associated with subdural strips, there was only 5 % of global complications, without permanent neurological deficit or death. This was probably due in part to the improvements of the electrode planning software that has enhanced the visibility of blood vessels on 3-dimensional brain MRI slices. This low complication rate and the absence of long-term complications and death are encouraging and should make this procedure more acceptable, in view of the subsequent surgical treatment that has a good chance of providing worthwhile post-operative improvement. This is meaningful in the discussion of the benefits/risks ratio with the epileptic patient who have to make a decision for invasive presurgical explorations.
The single factor associated with implantation complications was the “MRI negativity” which is probably linked to the number of electrodes. If so, this does not add any further information since electrodes are definitely at the origin of implantation complications and do not help us better understand the reason of complications. No other relevant factors were in question for electrode complications. This fact outlines the role of the technical aspects in implantation safety, which is most likely linked to the quality of implantation tools and of the skill of the surgical and medical staff. Hence, progress in implantation safety will happen through with technical improvements.
References
Engel J Jr, Henry TR, Risinger MW, Mazziotta JC, Sutherling WW, Levesque MF, Phelps ME (1990) Presurgical evaluation for partial epilepsy: relative contributions of chronic depth-electrode recordings versus FDG-PET and scalp-sphenoidal ictal EEG. Neurology 40(11):1670–1677
Wellmer J, von der Groeben F, Klarmann U, Weber C, Elger CE, Urbach H, Clusmann H, von Lehe M (2012) Risks and benefits of invasive epilepsy surgery workup with implanted subdural and depth electrodes. Epilepsia 53(8):1322–1332. doi:10.1111/j.1528-1167.2012.03545.x
Hader WJ, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Wiebe S, Kwon CS, Jette N (2013) Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia 54(5):840–847. doi:10.1111/epi.12161
Stacey WC, Kellis S, Greger B, Butson CR, Patel PR, Assaf T, Mihaylova T, Glynn S (2013) Potential for unreliable interpretation of EEG recorded with microelectrodes. Epilepsia 54(8):1391–1401. doi:10.1111/epi.12202
Spencer SS, Spencer DD, Williamson PD, Mattson R (1990) Combined depth and subdural electrode investigation in uncontrolled epilepsy. Neurology 40(1):74–79
Fernandez G, Hufnagel A, Van Roost D, Helmstaedter C, Wolf HK, Zentner J, Schramm J, Elger CE (1997) Safety of intrahippocampal depth electrodes for presurgical evaluation of patients with intractable epilepsy. Epilepsia 38(8):922–929
Enatsu R, Bulacio J, Najm I, Wyllie E, So NK, Nair DR, Foldvary-Schaefer N, Bingaman W, Gonzalez-Martinez J (2014) Combining stereo-electroencephalography and subdural electrodes in the diagnosis and treatment of medically intractable epilepsy. J Clin Neurosci Off J Neurosurg Soc Australasia 21(8):1441–1445. doi:10.1016/j.jocn.2013.12.014
Hamer HM, Morris HH, Mascha EJ, Karafa MT, Bingaman WE, Bej MD, Burgess RC, Dinner DS, Foldvary NR, Hahn JF, Kotagal P, Najm I, Wyllie E, Luders HO (2002) Complications of invasive video-EEG monitoring with subdural grid electrodes. Neurology 58(1):97–103
Munari C (1987) Depth electrode implantation at Hôpital Sainte Anne, Paris. Surgical treatment of the epilepsies. Raven Press Ltd, New York, pp 583–588
Munari C, Hoffmann D, Francione S, Kahane P, Tassi L, Lo Russo G, Benabid AL (1994) Stereo-electroencephalography methodology: advantages and limits. Acta Neurol Scand Suppl 152:56–67 (discussion 68–59)
Guenot M, Isnard J, Ryvlin P, Fischer C, Ostrowsky K, Mauguiere F, Sindou M (2001) Neurophysiological monitoring for epilepsy surgery: the Talairach SEEG method. StereoElectroEncephaloGraphy. Indications, results, complications and therapeutic applications in a series of 100 consecutive cases. Stereotact Funct Neurosurg 77(1–4):29–32
Bekelis K, Desai A, Kotlyar A, Thadani V, Jobst BC, Bujarski K, Darcey TM, Roberts DW (2013) Occipitotemporal hippocampal depth electrodes in intracranial epilepsy monitoring: safety and utility. J Neurosurg 118(2):345–352. doi:10.3171/2012.9.JNS112221
Cardinale F, Cossu M, Castana L, Casaceli G, Schiariti MP, Miserocchi A, Fuschillo D, Moscato A, Caborni C, Arnulfo G, Lo Russo G (2013) Stereoelectroencephalography: surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery 72(3):353–366. doi:10.1227/NEU.0b013e31827d1161 (discussion 366)
Serletis D, Bulacio J, Bingaman W, Najm I, Gonzalez-Martinez J (2014) The stereotactic approach for mapping epileptic networks: a prospective study of 200 patients. J Neurosurg. doi:10.3171/2014.7.JNS132306
Gonzalez-Martinez J, Mullin J, Vadera S, Bulacio J, Hughes G, Jones S, Enatsu R, Najm I (2014) Stereotactic placement of depth electrodes in medically intractable epilepsy. J Neurosurg 120(3):639–644. doi:10.3171/2013.11.JNS13635
Swartz BE, Rich JR, Dwan PS, DeSalles A, Kaufman MH, Walsh GO, Delgado-Escueta AV (1996) The safety and efficacy of chronically implanted subdural electrodes: a prospective study. Surg Neurol 46(1):87–93
Behrens E, Schramm J, Zentner J, Konig R (1997) Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery 41(1):1–9
Wiggins GC, Elisevich K, Smith BJ (1999) Morbidity and infection in combined subdural grid and strip electrode investigation for intractable epilepsy. Epilepsy Res 37(1):73–80 (discussion 9–10)
Lee WS, Lee JK, Lee SA, Kang JK, Ko TS (2000) Complications and results of subdural grid electrode implantation in epilepsy surgery. Surg Neurol 54(5):346–351
Rydenhag B, Silander HC (2001) Complications of epilepsy surgery after 654 procedures in Sweden, September 1990-1995: a multicenter study based on the Swedish National Epilepsy Surgery Register. Neurosurgery 49(1):51–56 (discussion 56–57)
Burneo JG, Steven DA, McLachlan RS, Parrent AG (2006) Morbidity associated with the use of intracranial electrodes for epilepsy surgery. Can J Neurol Sci Le J Can des Sci Neurol 33(2):223–227
Fountas KN, Smith JR (2007) Subdural electrode-associated complications: a 20-year experience. Stereotact Funct Neurosurg 85(6):264–272. doi:10.1159/000107358
Lee JH, Hwang YS, Shin JJ, Kim TH, Shin HS, Park SK (2008) Surgical complications of epilepsy surgery procedures: experience of 179 procedures in a single institute. J Korean Neurosurg Soc 44(4):234–239. doi:10.3340/jkns.2008.44.4.234
Van Gompel JJ, Worrell GA, Bell ML, Patrick TA, Cascino GD, Raffel C, Marsh WR, Meyer FB (2008) Intracranial electroencephalography with subdural grid electrodes: techniques, complications, and outcomes. Neurosurgery 63(3):498–505. doi:10.1227/01.NEU.0000324996.37228.F8 (discussion 505–496)
Wong CH, Birkett J, Byth K, Dexter M, Somerville E, Gill D, Chaseling R, Fearnside M, Bleasel A (2009) Risk factors for complications during intracranial electrode recording in presurgical evaluation of drug resistant partial epilepsy. Acta Neurochir (Wien) 151(1):37–50. doi:10.1007/s00701-008-0171-7
Placantonakis DG, Shariff S, Lafaille F, Labar D, Harden C, Hosain S, Kandula P, Schaul N, Kolesnik D, Schwartz TH (2010) Bilateral intracranial electrodes for lateralizing intractable epilepsy: efficacy, risk, and outcome. Neurosurgery 66(2):274–283. doi:10.1227/01.NEU.0000363184.43723.94
Hedegard E, Bjellvi J, Edelvik A, Rydenhag B, Flink R, Malmgren K (2014) Complications to invasive epilepsy surgery workup with subdural and depth electrodes: a prospective population-based observational study. J Neurol Neurosurg Psychiatry 85(7):716–720. doi:10.1136/jnnp-2013-306465
Fountas KN (2011) Implanted subdural electrodes: safety issues and complication avoidance. Neurosurgery clinics of North America 22(4):519–531. doi:10.1016/j.nec.2011.07.009
Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA (2013) Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: a systematic review and meta-analysis. Epilepsia 54(5):828–839. doi:10.1111/epi.12073
Blauwblomme T, Ternier J, Romero C, Pier KS, D’Argenzio L, Pressler R, Cross H, Harkness W (2011) Adverse events occurring during invasive electroencephalogram recordings in children. Neurosurgery 69(2):169–175. doi:10.1227/NEU.0b013e3182181e7d (discussion ons175)
Nasreddine W, Beydoun A (2008) Valproate-induced thrombocytopenia: a prospective monotherapy study. Epilepsia 49(3):438–445. doi:10.1111/j.1528-1167.2007.01429.x
Talairach J, Bancaud J, Bonis A, Szikla G, Trottier S, Vignal JP, Chauvel P, Munari C, Chodkievicz JP (1992) Surgical therapy for frontal epilepsies. Adv Neurol 57:707–732
Talairach J, Tournoux P, Musolino A, Missir O (1992) Stereotaxic exploration in frontal epilepsy. Adv Neurol 57:651–688
Simon SL, Telfeian A, Duhaime AC (2003) Complications of invasive monitoring used in intractable pediatric epilepsy. Pediatr Neurosurg 38(1):47–52
Acknowledgments
This work received support from the program “Investissements d’avenir” ANR-10-IAIHU-06. The authors thank Prof. Pierre Levy and Dr. Alister Rogers for advice and discussion concerning the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Mathon, B., Clemenceau, S., Hasboun, D. et al. Safety profile of intracranial electrode implantation for video-EEG recordings in drug-resistant focal epilepsy. J Neurol 262, 2699–2712 (2015). https://doi.org/10.1007/s00415-015-7901-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7901-6