Abstract
The 4-repeat (4R)-tauopathies can be clinically heterogeneous and difficult to diagnose. An FDG-PET pattern of hypometabolism has been previously reported in clinically suspected 4R-tauopathies. Considering that pathological confirmation has not been used as inclusion criteria in these studies, however, the possibility exists that atypical cases were excluded. We studied pathologically confirmed cases of 4R-tauopathies to determine if FDG-PET patterns of hypometabolism different than those previously described exist. We identified all autopsy confirmed 4R-tauopathies with FDG-PET imaging performed between 2010 and 2013 within the Mayo Clinic database. Clinical features and FDG-PET imaging were compared to a group of normal controls. Ten patients, seven of which had autopsy-confirmed progressive supranuclear palsy (PSP), were identified. We also identified two cases with globular glial tauopathy (GGT) and one case of corticobasal degeneration (CBD). The overall predominant imaging findings included bilateral caudate hypometabolism in nine cases, mild asymmetric thalamic hypometabolism in eight, midbrain hypometabolism in seven, and bilateral hypometabolism in the supplementary motor area in seven. No differences were observed between PSP and GGT. The one CBD case had asymmetric parietal hypometabolism that was not seen in the PSP and GGT cases. As previously described, 4R-tauopathies are associated with frontal, caudate, midbrain and thalamic hypometabolism on FDG-PET. This is the first report of FDG-PET in GGT, and although our series was limited, no features distinguish GGT from PSP. There was some evidence that parietal hypometabolism may be suggestive of CBD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mutation of the microtubule protein tau and its subsequent distribution throughout the brain is responsible for several neurodegenerative diseases. Within this spectrum of tau pathology exists a group characterized by sporadic 4-repeat (4R) tau neurofibrillary tangles. The sporadic 4R-tauopathies include corticobasal degeneration (CBD), progressive supranuclear palsy (PSP), and globular glial tauopathies (GGTs). Corticobasal degeneration pathology predominately affects the frontoparietal area, resulting in an asymmetric clinical syndrome that includes limb apraxia, alien-limb phenomena, myoclonus, and dystonia [1]. Progressive supranuclear palsy involves tau distribution predominantly affecting the basal ganglia, brainstem, cerebellum, and medial frontal cortex [2]. In PSP’s classic form, known as Richardson’s Syndrome [3], the dominant clinical features are symmetric parkinsonism, axial rigidity, early falls, supranuclear vertical gaze palsy, apathy, and a lack of response to levodopa. The GGTs are a relatively newly described group of neurodegenerative diseases that are characterized by widespread 4R immunoreactive globular oligodendroglial and astrocytic inclusions [4]. GGTs predominantly feature patterns of frontotemporal and corticospinal tract involvement [4].
The in vivo diagnosis of these 4R-tauopathies and other neurodegenerative diseases can be clinically challenging, and is becoming increasingly important with the future of targeted therapies. To aid the physician with the clinical diagnosis, the use of FDG-PET imaging has been examined by many previous studies to help differentiate between a spectrum of neurodegenerative diseases including Parkinson’s disease (PD), PSP, CBD, multiple system atrophy (MSA), dementia with Lewy bodies (DLB), and frontotemporal lobar degeneration (FTLD) [5–21]. Pathological confirmation was not used as inclusion criteria in any of these previous studies, which raises the question as to whether the described FDG-PET imaging findings are associated with the underlying pathology, or rather the clinical syndrome. This can be highlighted by the clinical heterogeneity resulting from PSP pathology, as there have been multiple pathologically confirmed atypical variants described in the literature that do not fit the typical Richardson’s Syndrome presentation. These variations include PSP-parkinsonism (PSP-P), pure akinesia with gait failure, progressive aphasia or apraxia of speech, and even a corticobasal syndrome due to PSP pathology [22].
Considering the overlapping and ambiguous features among many of the neurodegenerative diseases, our aim is to clarify the characteristic FDG-PET imaging findings of the sporadic 4R-tauopathies by using autopsy-confirmed cases. We hypothesize that pathologically confirmed cases of spontaneous 4R-tauopathies will collectively have different areas of hypometabolism on FDG-PET imaging than previously described in non-autopsy-confirmed studies.
Methods
Subjects
The Mayo Clinic Medical Records Linkage system was used to identify all patients seen in the Department of Neurology at Mayo Clinic in Rochester, MN by a degenerative specialist between 2005 and 2013 with an autopsy-confirmed spontaneous 4R-tauopathy who had FDG-PET imaging of the brain at the time of their clinical evaluation. Patient charts were reviewed and clinical features recorded. The study was approved by the Mayo institutional review board.
Imaging
All PET scans were acquired using a PET/CT scanner (GE Healthcare) operating in 3D mode. Subjects were injected with fluorodeoxyglucose in a dimly lit room with minimal auditory stimulation. An 8-min FDG scan was performed after a 30-min uptake period, which consisted of four 2-min dynamic frames following a low-dose CT transmission scan. Standard corrections were applied and frames were realigned if motion was present. The CortexID (GE Healthcare) software package was used and ran an automated analysis using 3D stereotactic surface projections. Activity for each subject’s scan was normalized to the pons and compared to an age-segmented normative database. The program provides 3D stereotactic surface projection images with a metabolic map using the Z scores as calculated for each surface pixel.
Results
There were ten patients identified that met criteria for our study, which included seven patients with autopsy-confirmed PSP, two patients with autopsy-confirmed GGT, and one patient with autopsy-confirmed CBD. Demographic features are provided in Table 1. The mean age of disease onset was 63.4 years (range 50–80). Five were female. The mean duration of disease from initial symptom onset to evaluation was 5.8 years (range 3–10 years). The mean Mini-Mental State Examination score was 24 (range 17–29).
The ten patients exhibited a wide variety of signs and symptoms (Table 2). Of note, both of the GGT patients presented with apraxia of speech [23] as their first symptom. Two of the seven PSP patients presented with a chief complaint of backward falls. Mild cognitive difficulties were reported as the initial symptom in another two of the seven PSP patients. The remaining three patients initially noticed symptoms of apathy, pseudobulbar affect, and task-specific tremors. The one CBD patient presented with the initial complaint of depression and irritability.
All eight patients who tried levodopa therapy showed no response in treating their parkinsonian features. Dysphagia or dysarthria was seen in eight of ten patients. Backward falls were documented in seven of ten patients, although only four of those seven patients had backward falls within the first year. A vertical supranuclear gaze palsy was identified in eight of ten patients; seven of the eight were noted at the initial visit. Significant apathy was experienced by seven of ten patients. Significant cravings for sweets and overeating of sweets was observed in three of ten patients. The final suspected diagnosis after evolution of symptoms and work-up was PSP in six of the seven PSP patients (the one that did not have suspected PSP did not follow up after initial work-up). Neither of the two GGT patients were suspected to have GGT; both were clinically suspected PSP variants. The one CBD patient was clinically suspected to have FTLD.
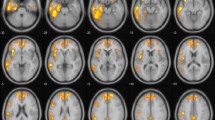
The FDG-PET CortexID maps for the ten subjects can be seen in Fig. 1. The predominate imaging findings included bilateral hypometabolism in the caudate in nine of ten patients (2–10), mild asymmetric hypometabolism in the thalamus in eight of ten patients (1–7, 10), and bilateral hypometabolism in the SMA seen in seven of ten patients (1, 2, 4, 5, 8, 9, 10). Other areas of hypometabolism included the premotor cortex in five patients (1, 5, 6, 9, 10), the precentral gyrus in one patient (6), the midbrain in seven patients (1, 2, 4, 5, 6, 7, 9), and the asymmetrical parietal cortex in one patient (10). There were no unique distinguishing features of hypometabolism in the GGT patients (8, 9) in comparison to the PSP patients. The one CBD patient had an asymmetric parietal hypometabolism that was not seen in the PSP or GGT patients.
Discussion
We have described FDG-PET imaging findings in patients with autopsy-confirmed PSP, GGT, and CBD. This is the first FDG-PET imaging report in patients with a GGT, and the first time an FDG-PET imaging study has focused on including only autopsy-confirmed sporadic 4R-tauopathies. With our findings, we conclude that there are recognizable FDG-PET imaging patterns of hypometabolism that can aid the clinician with the in vivo diagnosis of PSP, GGT, and CBD. In our study, these sporadic 4R-tauopathies predominantly show a recognizable pattern of hypometabolism bilaterally in the caudate and SMA, with asymmetric hypometabolism in the midbrain and thalamus. Although our numbers were small, we did not find consistent unique FDG-PET imaging findings to differentiate between PSP and GGT. This is not necessarily surprising given that some GGT cases have overlapping pathological features with PSP [24]. We did, however, find that our CBD patient had asymmetric hypometabolism of the parietal lobe, which has previously been described in multiple non-autopsy confirmed studies [9–12, 18–21].
The heterogeneity and overlapping features of neurodegenerative diseases have led to the use of PET imaging to help distinguish between them. The goal of this has been to find unique metabolic patterns among these various neurodegenerative diseases that can point towards the correct diagnosis. Due to this heterogeneity, though, pathologic confirmation should be considered the preferred gold standard when describing PET imaging findings in such patients. This heterogeneity has been repeatedly described in the literature, for example in 2010 when Dickson et al. [22] reviewed a collection of cases and studies showing a variety of atypical PSP presentations that consisted of cortical-predominant and brainstem-predominant PSP patterns that could be clinically confused with other neurodegenerative diseases. Because of the commonality of heterogeneity, it’s possible that there has been a skewed selection in prior FDG-PET studies, selecting for patients with more of a classic clinical presentation of a disease, whereas atypical or unsuspected presentations could be excluded or misclassified. This raises the possibility that the pathologically confirmed atypical and unsuspected presentations could have different patterns of metabolism that would be important to include in the collective PET imaging of these diseases.
There has been some attempt in previous studies to validate the clinical diagnosis with pathologic correlation. However, we could only find two previous studies in which this was done, but on an incomplete basis and as a side note. For example, in 2000 Foster et al. [8] showed pathological confirmation of disease in 4 of the 12 imaged patients with Richardson’s syndrome. Also, another study that attempted to establish PET imaging findings that could differentiate among PSP, MSA, and PD patients mentioned pathological examination done in 9 of the 167 enrolled patients, but the impression from PET imaging findings correctly correlated with pathological confirmation in only six of the nine patients examined [17]. These examples further support the importance of using pathologic confirmation as the gold standard in imaging studies.
Although our study is unique in its inclusion criteria, we have noticed that our results predominantly agree with previous findings of prominent hypometabolism in the medial frontal cortex, caudate, and thalamus of patients with PSP. We also observed mild involvement of the midbrain in a large number of the patients, consistent with previous studies [6–19, 21], known as the “pimple sign” [25]. We did notice a couple of patients with premotor cortex involvement, which was also specifically mentioned in previous PSP studies [6, 9–11, 17, 18]. As previously mentioned, our CBD patient demonstrated asymmetric parietal hypometabolism that is consistent with previous findings, but not quite to the predominant degree that was typically imaged in past studies [9–12, 18–21].
In conclusion, our study demonstrates FDG-PET imaging findings in patients with autopsy-confirmed PSP, GGT, and CBD. These findings may be helpful to clinicians in differentiating among multiple neurodegenerative diseases. Our findings show an asymmetric parietal involvement seen in our one CBD patient that was not seen in PSP and GGT patients, but there was no differentiating feature on PET imaging to distinguish between PSP and GGT 4R-tauopathies. We suggest that PET imaging findings between these two diseases be further studied in order to establish whether or not PET can help differentiate between them. Additional studies of the in vivo imaging of these and other autopsy-confirmed neurodegenerative diseases will help us better understand and more accurately diagnose patients and predict pathology.
References
Schneider JA, Watts RL, Gearing M, Brewer RP, Mirra SS (1997) Corticobasal degeneration: neuropathologic and clinical heterogeneity. Neurology 48(4):959–969
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44(11):2015–2019
Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, Holton JL, Revesz T, Lees AJ (2005) Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain J Neurol 128(Pt 6):1247–1258. doi:10.1093/brain/awh488
Ahmed Z, Bigio EH, Budka H, Dickson DW, Ferrer I, Ghetti B, Giaccone G, Hatanpaa KJ, Holton JL, Josephs KA, Powers J, Spina S, Takahashi H, White CL 3rd, Revesz T, Kovacs GG (2013) Globular glial tauopathies (GGT): consensus recommendations. Acta Neuropathol. doi:10.1007/s00401-013-1171-0
Coulier IM, de Vries JJ, Leenders KL (2003) Is FDG-PET a useful tool in clinical practice for diagnosing corticobasal ganglionic degeneration? Mov Disord Off J Mov Disord Soc 18(10):1175–1178. doi:10.1002/mds.10498
Eckert T, Barnes A, Dhawan V, Frucht S, Gordon MF, Feigin AS, Eidelberg D (2005) FDG PET in the differential diagnosis of parkinsonian disorders. NeuroImage 26(3):912–921. doi:10.1016/j.neuroimage.2005.03.012
Foster NL, Gilman S, Berent S, Morin EM, Brown MB, Koeppe RA (1988) Cerebral hypometabolism in progressive supranuclear palsy studied with positron emission tomography. Ann Neurol 24(3):399–406. doi:10.1002/ana.410240308
Foster NL, Minoshima S, Johanns J, Little R, Heumann ML, Kuhl DE, Gilman S (2000) PET measures of benzodiazepine receptors in progressive supranuclear palsy. Neurology 54(9):1768–1773
Garraux G, Salmon E, Peigneux P, Kreisler A, Degueldre C, Lemaire C, Destee A, Franck G (2000) Voxel-based distribution of metabolic impairment in corticobasal degeneration. Mov Disord Off J Mov Disord Soc 15(5):894–904
Hellwig S, Amtage F, Kreft A, Buchert R, Winz OH, Vach W, Spehl TS, Rijntjes M, Hellwig B, Weiller C, Winkler C, Weber WA, Tuscher O, Meyer PT (2012) [(1)(8)F]FDG-PET is superior to [(1)(2)(3)I]IBZM-SPECT for the differential diagnosis of parkinsonism. Neurology 79(13):1314–1322. doi:10.1212/WNL.0b013e31826c1b0a
Hosaka K, Ishii K, Sakamoto S, Mori T, Sasaki M, Hirono N, Mori E (2002) Voxel-based comparison of regional cerebral glucose metabolism between PSP and corticobasal degeneration. J Neurol Sci 199(1–2):67–71
Juh R, Kim J, Moon D, Choe B, Suh T (2004) Different metabolic patterns analysis of Parkinsonism on the 18F-FDG PET. Eur J Radiol 51(3):223–233. doi:10.1016/s0720-048x(03)00214-6
Juh R, Pae CU, Kim TS, Lee CU, Choe B, Suh T (2005) Cerebral glucose metabolism in corticobasal degeneration comparison with progressive supranuclear palsy using statistical mapping analysis. Neurosci Lett 383(1–2):22–27. doi:10.1016/j.neulet.2005.03.057
Klein RC, de Jong BM, de Vries JJ, Leenders KL (2005) Direct comparison between regional cerebral metabolism in progressive supranuclear palsy and Parkinson’s disease. Mov Disord Off J Mov Disord Soc 20(8):1021–1030. doi:10.1002/mds.20493
Mishina M, Ishii K, Mitani K, Ohyama M, Yamazaki M, Ishiwata K, Senda M, Kobayashi S, Kitamura S, Katayama Y (2004) Midbrain hypometabolism as early diagnostic sign for progressive supranuclear palsy. Acta Neurol Scand 110(2):128–135. doi:10.1111/j.1600-0404.2004.00293.x
Srulijes K, Reimold M, Liscic RM, Bauer S, Dietzel E, Liepelt-Scarfone I, Berg D, Maetzler W (2012) Fluorodeoxyglucose positron emission tomography in Richardson’s syndrome and progressive supranuclear palsy-parkinsonism. Mov Disord Off J Mov Disord Soc 27(1):151–155
Tang CC, Poston KL, Eckert T, Feigin A, Frucht S, Gudesblatt M, Dhawan V, Lesser M, Vonsattel JP, Fahn S, Eidelberg D (2010) Differential diagnosis of parkinsonism: a metabolic imaging study using pattern analysis. Lancet Neurol 9(2):149–158. doi:10.1016/s1474-4422(10)70002-8
Teune LK, Bartels AL, de Jong BM, Willemsen AT, Eshuis SA, de Vries JJ, van Oostrom JC, Leenders KL (2010) Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov Disord Off J Mov Disord Soc 25(14):2395–2404. doi:10.1002/mds.23291
Tripathi M, Dhawan V, Peng S, Kushwaha S, Batla A, Jaimini A, D’Souza MM, Sharma R, Saw S, Mondal A (2013) Differential diagnosis of parkinsonian syndromes using F-18 fluorodeoxyglucose positron emission tomography. Neuroradiology 55(4):483–492. doi:10.1007/s00234-012-1132-7
Turaga SP, Mridula R, Borgohain R (2013) Cerebral glucose metabolism, clinical, neuropsychological, and radiological profile in patients with corticobasal syndrome. Neurol India 61(1):7–11. doi:10.4103/0028-3886.107916
Zhao P, Zhang B, Gao S (2012) 18F-FDG PET study on the idiopathic Parkinson’s disease from several parkinsonian-plus syndromes. Parkinsonism Relat Disord 18(Suppl 1):S60–S62. doi:10.1016/s1353-8020(11)70020-7
Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA (2010) Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23(4):394–400. doi:10.1097/WCO.0b013e32833be924
Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, Dickson DW, Jack CR Jr, Petersen RC (2006) Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain J Neurol 129(Pt 6):1385–1398. doi:10.1093/brain/awl078
Josephs KA, Katsuse O, Beccano-Kelly DA, Lin WL, Uitti RJ, Fujino Y, Boeve BF, Hutton ML, Baker MC, Dickson DW (2006) A typical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol 65(4):396–405
Botha H, Whitwell JL, Madhaven A, Senjem ML, Lowe V, Josephs KA (2013) The pimple sign of progressive supranuclear palsy syndrome. Parkinsonism Relat Disord. doi: 10.1016/j.parkreldis.2013.10.023 [Epub ahead of print]
Conflicts of interest
N. L. Zalewski: none. H. Botha: none. J. Whitwell: none. V. Lowe: none. Dr. Lowe: Received personal compensation for consulting from Bayer Pharmaceuticals; Research Grants from: GE Health Care, Siemens Molecular Imaging, AVID Radiopharmaceuticals, INC. D. Dickson: Consultant, Neotope, Inc., South San Francisco, CA. K. Josephs: none.
Ethical standard
The study was approved by the Mayo Clinic Institutional Review Board. All patients consented for their data to be used for research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zalewski, N., Botha, H., Whitwell, J.L. et al. FDG-PET in pathologically confirmed spontaneous 4R-tauopathy variants. J Neurol 261, 710–716 (2014). https://doi.org/10.1007/s00415-014-7256-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7256-4