Abstract
Studies of the relationship between Alzheimer’s disease (AD) and single nucleotide polymorphism (SNP) T/C in intron 2 of the cholesterol-24S-hydroxylase gene (CYP46A1) have reported inconsistent results. To confirm the association between the CYP46A1 T/C polymorphism and AD risk, a meta-analysis containing 4,875 AD cases and 4,874 controls from 21 case–control studies was performed. There were 16 studies involving Europeans, four studies with Asians and one study with Africans. The combined results of overall analysis showed that the CYP46A1 T/C polymorphism increased the risk of AD significantly in recessive model [CC versus CT + TT, odds ratio (OR) = 1.20, 95 % confidence interval (CI) = 1.04–1.38, p = 0.01]. On subgroup analysis by ethnicity, similarly significant differences in recessive model were also found in Europeans. Another analysis of the synergistic effect of the CYP46A1 T/C polymorphism and the ε4 allele of the apolipoprotein E gene (APOE ε4) was performed in eight studies with available stratified information. The results revealed that the presence of APOE ε4 allele could strengthen the effect of CC genotype on AD risk, and the reverse was also true. In conclusion, our meta-analysis has successfully proved that CC genotype of the CYP46A1 T/C polymorphism could increase the risk of AD, and this effect would be weakened in APOE ε4 non-carriers and strengthened in APOE ε4 carriers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer's disease (AD) is a common, complex genetic disorder with a prevalence rate of 5–10 % and an incidence that increases exponentially after the age of 65 years. Familial autosomal dominant AD, caused by mutations in the amyloid precursor protein (APP), presenilin-1 (PSEN1) or presenilin-2 (PSEN2) genes [1], just accounts for ≤2 % of all cases. A majority of AD cases manifest as sporadic late onset form (LOAD), typically with onset above the age of 65 years. Many candidate genes have been studied in recent decades, the ε4 allele of the apolipoprotein E gene (APOE ε4) remains the one unquestionable risk factor for LOAD until 2009 [2]. In the last 5 years, an increasing number of genome-wide association studies (GWASs) in AD were performed; other novel genes for LOAD, such as clusterin (CLU), phosphatidylinositol-binding clathrin assembly protein (PICALM) and complement receptor 1 (CR1), have been identified [3–6]. However, these genes could not account for all the genetic components of AD, indicating that other genes are involved in the etiology of sporadic AD.
Recent evidence has suggested that cholesterol metabolism participates in the pathogenesis of Alzheimer’s disease [7]. The mechanism mainly considered is that high intracellular cholesterol concentrations increase the amyloidogenic processing of amyloid precursor protein (APP), leading to greater amyloid-beta (Aβ) production [8, 9]. Brain cholesterol is synthesized locally and then transported into circulation through the blood brain-barrier (BBB). The elimination of cerebral cholesterol involves two mechanisms, dependent of APOE and cholesterol 24-hydroxylase (CYP46). APOE is the main transporter of cholesterol in the neuronal cell membrane, and CYP46 is a key enzyme catalyzing formation of altered cholesterol which can pass the BBB.
CYP46A1 is located in 14q32.1, and this gene encodes CYP46, a member of the cytochrome P450 superfamily of enzymes. This enzyme is expressed in the brain, where it converts cholesterol to 24S-hydroxycholesterol. While cholesterol cannot pass the BBB, 24S-hydroxycholesterol is the major cholesterol elimination product of the brain because it is assumed to pass the BBB to the circulation via ATP-binding cassette transporters (ABCA1), becoming the origin of more than 90 % of plasma 24S-hydroxycholesterol [10].
A single nucleotide polymorphism (SNP) T/C (rs754203) in intron 2 of CYP46A1 has been identified and reported to be significantly associated with increased risk for AD. According to the reports, the frequency of the CYP46A1 T allele and TT genotype was significantly higher in AD patients from Switzerland, Greece, and Italy than in controls [11]. In contrast, another study reported that the CYP46A1 C allele might act as a risk factor for AD in Italian patients [12]. Publications about this subject around the world are controversial. Among the studies about the association between the CYP46A1 T/C polymorphism and AD risk, several studies have reported the synergistic effect of CYP46A1 T/C and APOE ε4 on AD risk [12, 13]. However, results in different studies have been inconsistent.
In view of the contradictory results previous reported, we conducted a meta-analysis by collecting and sorting the previous published studies, with the aim to report the association between the CYP46A1 T/C polymorphism and AD. In addition, we also analyzed the synergistic effect of CYP46A1 T/C and APOE ε4 on AD risk in order to better understand the mechanism of effect of APOE ε4 on AD risk.
Materials and methods
Literature search
We searched all published papers (before 2012) in the databases of PubMed, Medline and Embase. The keywords used were as follows: “polymorphism”, “CYP46” or “Cholesterol 24S-hydroxylase”, and “Alzheimer’s disease” or “AD”. Abstracts and unpublished reports were not included. Special consideration was dedicated to case–control studies. To avoid a possible loss of any relevant article, an additional control was performed through the references cited in identified articles, through the link ‘‘related articles’’ offered in the PubMed database, and through the references of review articles. Besides, we had referred to “Gene overview of all published AD-association studies for CYP46A1” in the AlzGene database [14, 15].
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) case–control study to evaluate the association between the CYP46A1 T/C polymorphism and risk of AD, (2) useful data including genotype number or frequency given, and (3) genotype distribution of control population in Hardy–Weinberg equilibrium (HWE). The exclusion criteria were as follows: (1) studies with insufficient genotype number or frequency not included, (2) genotype distribution of control population not in HWE, and (3) if the same population was included as in previous study, only the most recent study or the study with larger sample size was included in our meta-analysis.
Data extraction
For each study, information was extracted including first author, year of publication, country of publication, number and ethnicity of study population, AD diagnosis criteria, and genotype number in cases and controls. In addition, when cases and controls were stratified by the APOE ε4 carrier status, the distribution of CYP46A1 T/C in both statuses would be extracted. Data were extracted independently by three investigators. Agreement was reached after discussion for conflicting data.
Statistical analysis
Odds ratio (OR) and 95 % CI were used to assess the association between the polymorphism of CYP46A1 T/C and AD risk. Five different genetic models were used in our analysis: dominant model (CC + CT vs. TT), recessive model (CC vs. CT + TT), homozygote comparison (CC vs. TT), and heterozygote comparison (CT vs. CC; CT vs. TT). Heterogeneity was measured using the Q statistic. Pooled ORs were calculated using a fixed-effects model (p > 0.1) or a random-effects model (p ≤ 0.1). To evaluate ethnicity-specific effects, subgroup analysis was performed based on the ethnicity of study population.
In the analysis of the synergistic effect of CYP46A1 T/C and APOE ε4, the studies including stratification of the APOE ε4 allele carrier status would be conducted in another meta-analysis. Five genetic models were used again in both APOE ε4 carrier statuses. Publication bias was analyzed by Begg’s funnel plot and Egger’s test. All above statistical analysis were performed using Review Manager 5.0 and Stata 11.0.
Results
Study characteristics
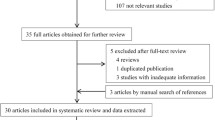
A total of 86 references were identified in PubMed, Medline, Embase and AlzGene database, of which 42 were excluded for duplication. After careful reading of abstracts and titles, six reviews, two studies written in Chinese [17, 18] and 11 references not involving in the relationship between AD and CYP46A1 were excluded. After careful reading of full text, two references not studying the SNP T/C were excluded [19, 20]. Moreover, one study was excluded because it was a prospective study and the population was divided into improved or stable group and deteriorated group according to the outcome of a two-year follow up [16]. In addition, distribution of the CYP46A1 T/C polymorphism was unavailable in two studies [21, 22]. Finally, 21 studies of 20 references containing 4,875 AD cases and 4,874 controls were included in our meta-analysis. AD cases in these 21 studies were probable or possible. One study was only included in the recessive model calculation [23]. The populations came from different countries, including Italy, the USA, Germany, the UK, Zurich, Switzerland, Finland, Sweden, Scotland, France, Hungary, Poland, Spain and China. There were 16 studies involving Europeans, four studies involving Asians (only China) and one study involving Africans (African American) (Table 1). Besides, eight studies containing 1,721 AD cases and 1,454 controls were stratified by the APOE ε4 carrier status and the stratified data was available. (Table 1).
Effect of CYP46A1 T/C polymorphisms on AD risk
A meta-analysis of the CYP46A1 T/C polymorphism association with AD risk was executed in all of included studies. The combined results of the overall analysis showed that there was significant positive association between the CYP46A1 T/C polymorphism and AD risk in the recessive model (CC vs. CT + TT, OR = 1.20, 95 % CI = 1.04–1.38, p = 0.01; results shown in Table 2; forest plot shown in Fig. 1). However, there were no significant associations in other genetic models (results seen in Table 2, forest plots not shown, available on request).
Meta-analysis of 21 case–control studies about the CYP46A1 T/C polymorphism and the risk for AD using the fixed-effects model. The genotype effect for CC vs. CT + TT was estimated for each study. The pooled OR for CC versus CT + TT was also calculated. The OR and 95 % CI for the effect for all comparisons are plotted on the graph. Studies are arranged chronologically based on the year of publication
On subgroup analysis by ethnicity of study population, increased AD risk among Europeans for CC genotype was just found in recessive model (CC versus CT + TT). In the other genetic models, there was no significant association between the CYP46A1 T/C polymorphism and AD risk on subgroup analysis (Table 2).
In conclusion, only the CC genotype of the CYP46A1 T/C polymorphism was found to increase the risk of AD significantly. But this phenomenon just appeared among Europeans, but not Asians and Africans.
Publication bias was analyzed by Begg’s funnel plot, the shape of which appeared to be approximately symmetrical (CC vs. CT + TT), and Egger’s test did not show any evidence of publication bias (t = 0.02, p = 0.986 for CC vs. CT + TT). Begg’s funnel plot and Egger’s test showed that there was no obvious publication bias in overall analysis (Fig. 2).
Synergistic effect of the CYP46A1 T/C polymorphism and APOE ε4 allele on AD risk
Distribution of the CYP46A1 T/C polymorphism stratified by the APOE ε4 carrier status was available in eight studies (Table 1), which were conducted into a meta-analysis in five different genetic models same as described above. CC genotype was proved to be an increased risk for AD in these eight studies once more (results of five genetic models shown in Table 3; forest plot of recessive model shown in Fig. 3a). In addition, promoted role of APOE ε4 was found to play in AD patients in every study whose APOEε4 is available.
Meta-analysis of eight case–control studies for which data of APOEε4 is available about the CYP46A1 T/C polymorphism and the risk for AD using the fixed-effects model. The genotype effect for CC versus CT + TT was estimated for each study. The pooled OR for CC versus CT + TT was also calculated. The OR and 95 % CI for the effect for all comparisons are plotted on the graph. Studies are arranged chronologically based on the year of publication. a Meta-analysis in eight studies whose APOEε4 is available (CC vs. CT + TT), b meta-analysis in eight studies whose APOEε4 is available (APOEε4 positive +CC vs. CT + TT), c meta-analysis in eight studies whose APOEε4 is available (APOEε4 negative +CC vs. CT + TT)
We have made a further analysis of the synergistic effect of the CYP46A1 T/C polymorphism and APOE ε4 allele on AD risk and got three findings as follows: (1) there was no significant association between the CYP46A1 T/C polymorphism and AD risk in every model when we just analyzed the APOE ε4 carriers (Table 3). Thus, we thought that the presence of the APOE ε4 allele was a larger risk factor for AD than the CYP46A1 T/C polymorphism, even when considered as an independent risk factor. (2) The CC genotype could also increase the risk of AD in APOE ε4 non-carriers, but its effect was weakened significantly by the absence of the APOE ε4 allele (Table 3, and the result of genetic model APOE ε4 negative +CC versus CT + TT, shown in Fig. 3b). (3) Accordingly, the presence of the APOE ε4 allele was proved to strengthen the effect of CC genotype on AD risk in the model of APOE ε4 positive +CC versus CT + TT (Fig. 3b).
Discussion
Alzheimer’s disease is one of the most disabling and burdensome health conditions worldwide. As the world’s population ages, we face a looming epidemic of AD. It is predicted that by the year 2050, worldwide prevalence of AD could grow to 106.8 million. [40] Effective interventions may significantly reduce the prevalence and incidence of Alzheimer’s disease and ultimately reduce the global burden of the disease. But unfortunately, no effective treatment is available for AD up to now, because of the limited understanding of the etiology of AD, especially the sporadic type. Many studies argue that the physiopathology of AD is complex and multifactorial, but mainly attributed to genetic and environmental factors.
The genetics of AD has taken impressive steps forwards in recent decades. To date, more than 600 genes have been linked to the disorder [41]. However, only a few of them are supported by a sufficient level of evidence. Meanwhile, cholesterol metabolism is believed to play a role in the development of AD according to some epidemiology research and biochemical studies [7, 42]. Thus, association between cholesterol related genes has drawn more attention in recent years, especially CYP46A1, whose encoding enzyme participates in one of the most important mechanisms for the elimination of excess brain cholesterol.
A SNP T/C (rs754203) in intron two of CYP46A1 has been studied in various populations. However, the different results of various studies were contradictory. Llorca et al. [43] have recently reported the negative association between the CYP46A1 T/C polymorphism and AD even stratified by APOE ε4 status in a meta-analysis, containing 13 case–control studies (date of publication from 2002 to 2005), with 3,409 AD cases and 3,209 controls. We conducted this comprehensive genetic meta-analysis after 5 years because of more published studies and the importance of CYP46A1 for AD risk.
Our results were completely contrary to previous report by Llorca et al. [43]. A total of 21 case–control studies containing 4,875 AD cases and 4,874 controls were included in our meta-analysis. The results confirm the association between CYP46A1 T/C polymorphism and AD risk. Our results indicate that the CC genotype of CYP46A1 T/C could increase the risk of AD in the recessive model (CC versus CT + TT, OR = 1.20, 95 % CI = 1.04–1.38, p = 0.01) on overall analysis. When stratifying by ethnicity, we obtained similar results just in Europeans in the recessive model (CC versus CT + TT, OR = 1.22, 95 % CI = 1.05–1.43, p = 0.01), but no association was found in either Asians or Africans at all. This result suggests that homozygous CC had a more obvious effect on an individual’s phenotype than did CT and TT, so individuals with the CC genotype could have higher risk of AD. In spite of negative results in non-Europeans, sufficient evidence could confirm the role of the CC genotype of CYP46A1 T/C in clinical evaluation, because only four studies involving Asians and only one study involving Africans were included in our meta-analysis. To clarify this kind of association in Asians and Africans, more studies are required.
Most of the genetic association studies on AD have either evaluated single genetic variation at one single time or have analyzed multiple variations independently, rather than estimating the size of the effect associated with gene–gene interaction. As a result, little information as to whether the impact of genetic variants on AD risk is simply the sum of their individual effects or whether more complex interactive effects are available. To assess the synergistic effect of the CYP46A1 T/C polymorphism and APOE ε4 allele on AD Risk, we further conducted meta-analysis in the studies with available stratified information (eight studies, containing 1,721 AD cases and 1,454 controls). Three important findings were obtained: (1) the presence of the APOEε4 allele was a lager risk factor for AD than the CYP46A1 T/C polymorphism, even likely to be an independent risk factor; (2) the absence of the APOE ε4 allele could weaken the effect of the CC genotype on AD risk, but could not counteract it completely; (3) accordingly, the presence of the APOE ε4 allele could strengthen the effect of the CC genotype on AD risk.
The CYP46A1 T/C polymorphism could increase the risk of AD with the synergistic influence of the APOE ε4 allele, mainly due to the same metabolic pathways of cholesterol in the brain. Cholesterol in the brain, containing approximately one-fourth of total body cholesterol, is a major component of myelin and of neuronal and glial membranes [44]. It is synthesized in situ and cannot be degraded locally. Excess cholesterol must be conversed by CYP46 into 24S-hydroxycholesterol which easily traverses the blood–brain barrier (BBB) first [10], and then transports on lipoproteins that resemble HDL particles, which can circulate in the cerebrospinal fluid and bring the 24S-hydroxycholesterol to BBB. By this process, approximately 6–7 mg of cholesterol leaves the human brain each day. During this process, APOE and several members of the adenosine triphosphate cassette transporter family, such as ABCA1, are believed to play major roles in regulating lipid and lipoprotein homeostasis in the central nervous system (CNS). But as one, both of the two cholesterol related genes play essential roles in elimination of cholesterol in the brain, finally modulating Aβ deposition and clearance.
Some limitations of our meta-analysis should be acknowledged. First, the sample sizes in most of the included studies were small, which could increase the probability of false positives or false negatives. Second, many factors participate in the progression of AD, and we did not carry out subgroup analyses based on other factors such as age, gender, sex, education attainment or blood cholesterol level due to a lack of sufficient sample size. Third, the influence of bias in this analysis could not be completely excluded, because studies with positive results are easier to publish. Moreover, we have not tested the publication bias of meta-analysis in the eight studies stratified by the APOE ε4 carrier status, because Egger’s test usually requires at least ten studies to detect it.
In conclusion, our meta-analysis provides evidence that the CYP46A1 T/C polymorphism could increase the risk of AD in recessive model (CC versus CT + TT). The CC genotype of the CYP46A1 T/C may be a predictor for use in clinical evaluation of AD. Besides, we also confirmed the synergistic effect of the CYP46A1 T/C polymorphism and APOE ε4 allele that the presence of the APOE ε4 allele could strengthen the effect of CC genotype on AD risk, and the reverse was also true. Nevertheless, further studies with large sample size, especially in subgroup analysis, should be done to confirm our findings.
References
St George-Hyslop PH (2000) Genetic factors in the genesis of Alzheimer’s disease. Ann NY Acad Sci 924:1–7
Bertram L (2009) Alzheimer’s disease genetics current status and future perspectives. Int Rev Neurobiol 84:167–184
Lambert JC, Amouyel P (2011) Genetics of Alzheimer’s disease: new evidences for an old hypothesis? Curr Opin Genet Dev 21:295–301 (Epub 1 Mar 2011)
Lambert JC, Heath S, Even G et al (2009) Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 41:1094–1099 (Epub 6 Sept 2009)
Sleegers K, Lambert JC, Bertram L et al (2010) The pursuit of susceptibility genes for Alzheimer’s disease: progress and prospects. Trends Genet 26:84–93 (Epub 18 Jan 2010)
Seshadri S, Fitzpatrick AL, Ikram MA et al (2010) Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 303:1832–1840
Martins IJ, Berger T, Sharman MJ et al (2009) Cholesterol metabolism and transport in the pathogenesis of Alzheimer’s disease. J Neurochem 111:1275–1308
D’Errico G, Vitiello G, Ortona O et al (2008) Interaction between Alzheimer’s Abeta (25–35) peptide and phospholipid bilayers: the role of cholesterol. Biochim Biophys Acta 1778:2710–2716 (Epub 28 Jul 2008)
Vitiello G, Grimaldi M, Ramunno A et al (2010) Interaction of a beta-sheet breaker peptide with lipid membranes. J Pept Sci 16:115–122
Bjorkhem I, Lutjohann D, Diczfalusy U et al (1998) Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res 39:1594–1600
Papassotiropoulos A, Streffer JR, Tsolaki M et al (2003) Increased brain β-amyloid load, phosphorylated tau, and risk of Alzheimer disease associated with an intronic CYP46 polymorphism. Arch Neurol 60:29–35
Borroni B, Archetti S, Agosti C et al (2004) Intronic CYP46 polymorphism along with ApoE genotype in sporadic Alzheimer disease: from risk factors to disease modulators. Neurobiol Aging 25:747–751
Tedde A, Rotondi M, Cellini E et al (2006) Lack of association between the CYP46 gene polymorphism and Italian late-onset sporadic Alzheimer’s disease. Neurobiol Aging 27:773.e1–773.e3 (Epub 1 Aug 2005)
Alzheimer research forum (2009) (updated 29 Jan 2010) Gene overview of all published AD-association studies for CYP46A1. Available at http://www.alzgene.org/geneoverview.asp?geneID=105
Bertram L, McQueen MB, Mullin K et al (2007) Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet 39:17–23
Fu BY, Ma SL, Tang NL et al (2009) Cholesterol 24-hydroxylase (CYP46A1) polymorphisms are associated with faster cognitive deterioration in Chinese older persons: a two-year follow up study. Int J Geriatr Psychiatry 24:921–926
He XM, Zhang ZX, Zhang JW et al (2006) An intronic cholesterol 24S-hydroxylase polymorphism associated with Alzheimer disease. Chin J Neurol 39:44–47
Wang F, Jia JP (2007) Correlation of cholesterol 24-hydroxylase and ATP-binding cassette transporter A1 polymorphisms with Alzheimer’s disease. Zhonghua Yi Xue Za Zhi 87:614–618
Li Y, Chu LW, Wang B et al (2010) CYP46A1 functional promoter haplotypes decipher genetic susceptibility to Alzheimer’s disease. J Alzheimers Dis 21:1311–1323
Kölsch H, Lütjohann D, Jessen F et al (2009) CYP46A1 variants influence Alzheimer’s disease risk and brain cholesterol metabolism. Eur Psychiatry 24:183–190 (Epub 16 Mar 2009)
Vega GL, Weiner MF (2007) Plasma 24S hydroxycholesterol response to statins in Alzheimer’s disease patients: effects of gender, CYP46, and ApoE polymorphisms. J Mol Neurosci 33:51–55
Shibata N, Kawarai T, Lee JH et al (2006) Association studies of cholesterol metabolism genes (CH25H, ABCA1 and CH24H) in Alzheimer’s disease. Neurosci Lett 391:142–146 (Epub 12 Sep 2005)
Helisalmi S, Vepsäläinen S, Koivisto AM et al (2006) Association of CYP46 intron 2 polymorphism in Finnish Alzheimer’s disease samples and a global scale summary. J Neurol Neurosurg Psychiatry 77:421–422
Desai P, DeKosky ST, Kamboh MI (2002) Genetic variation in the cholesterol 24-hydroxylase (CYP46) gene and the risk of Alzheimer’s disease. Neurosci Lett 328:9–12
Kölsch H, Lütjohann D, Ludwig M (2002) Polymorphism in the cholesterol 24S-hydroxylase gene is associated with Alzheimer’s disease. Mol Psychiatry 7:899–902
Chalmers KA, Culpan D, Kehoe PG et al (2004) APOE promoter, ACE1 and CYP46 polymorphisms and beta-amyloid in Alzheimer’s disease. Neuroreport 15:95–98
Combarros O, Infante J, Llorca J, Berciano J (2004) Genetic association of CYP46 and risk for Alzheimer’s disease. Dement Geriatr Cogn Disord 18:257–260 (Epub 29 Jul 2004)
Ingelsson M, Jesneck J, Irizarry MC, Hyman BT, Rebeck GW (2004) Lack of association of the cholesterol 24-hydroxylase (CYP46) intron 2 polymorphism with Alzheimer’s disease. Neurosci Lett 367:228–231
Johansson A, Katzov H, Zetterberg H et al (2004) Variants of CYP46A1 may interact with age and APOE to influence CSF Abeta42 levels in Alzheimer’s disease. Hum Genet 114:581–587 (Epub 18 Mar 2004)
Kabbara A, Payet N, Cottel D (2004) Exclusion of CYP46 and APOM as candidate genes for Alzheimer’s disease in a French population. Neurosci Lett 363:139–143
Wang B, Zhang C, Zheng W et al (2004) Association between a T/C polymorphism in intron 2 of cholesterol 24S-hydroxylase gene and Alzheimer’s disease in Chinese. Neurosci Lett 369:104–107
Golanska E, Hulas-Bigoszewska K, Wojcik I et al (2005) CYP46: a risk factor for Alzheimer’s disease or a coincidence? Neurosci Lett 383:105–108 (Epub 21 Apr 2005)
Juhász A, Rimanóczy A, Boda K et al (2005) CYP46 T/C polymorphism is not associated with Alzheimer’s dementia in a population from Hungary. Neurochem Res 30:943–948
Fernández Del Pozo V, Alvarez Alvarez M, Fernández Martínez M et al (2005) Polymorphism in the cholesterol 24S-hydroxylase gene (CYP46A1) associated with the APOEpsilon3 allele increases the risk of Alzheimer’s disease and of mild cognitive impairment progressing to Alzheimer’s disease. Dement Geriatr Cogn Disord 21:81–87 (Epub 9 Dec 2005)
Li Y, Chu LW, Chen YQ et al (2006) Intron 2 (T/C) CYP46 polymorphism is associated with Alzheimer’s disease in Chinese patients. Dement Geriatr Cogn Disord 22:399–404 (Epub 8 Sept 2006)
Ma SL, Tang NL, Lam LC, Chiu HF (2006) Polymorphisms of the cholesterol 24-hydroxylase (CYP46A1) gene and the risk of Alzheimer’s disease in a Chinese population. Int Psychogeriatr 18:37–45
Wang F, Jia J (2007) Polymorphisms of cholesterol metabolism genes CYP46 and ABCA1 and the risk of sporadic Alzheimer’s disease in Chinese. Brain Res 1147:34–38 (Epub 8 Feb 2007)
Golanska E, Hulas-Bigoszewska K, Sieruta M et al (2009) Earlier onset of Alzheimer’s disease: risk polymorphisms within PRNP, PRND, CYP46, and APOE genes. J Alzheimers Dis 17:359–368
Ghebranious N, Mukesh B, Giampietro PF et al (2011) A pilot study of gene/gene and gene/environment interactions in Alzheimer disease. Clin Med Res 9:17–25 (Epub 3 Aug 2010)
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 3:186–191
Olgiati P, Politis AM, Papadimitriou GN, De Ronchi D, Serretti A (2011) Genetics of late-onset Alzheimer’s disease: update from the alzgene database and analysis of shared pathways. Int J Alzheimers Dis 832379 (Epub 10 Dec 2011)
Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K (2005) Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64:277–281
Llorca J, Rodríguez-Rodríguez E, Dierssen-Sotos T et al (2008) Meta-analysis of genetic variability in the beta-amyloid production, aggregation and degradation metabolic pathways and the risk of Alzheimer’s disease. Acta Neurol Scand 117:1–14 (Epub 14 Sept 2007)
Dietschy JM, Turley SD (2001) Cholesterol metabolism in the brain. Curr Opin Lipidol 12:105–112
Conflicts of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work. Six authors of this paper are willing to provide their autographs if necessary.
Ethical standard
All human studies must state that they have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, L., Yin, Z., Liu, J. et al. CYP46A1 T/C polymorphism associated with the APOEε4 allele increases the risk of Alzheimer’s disease. J Neurol 260, 1701–1708 (2013). https://doi.org/10.1007/s00415-012-6690-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6690-4