Abstract
Although diffusion tensor imaging (DTI) and the magnetization transfer ratio (MTR) have been extensively studied in multiple sclerosis (MS), it is still unclear if they are more effective biomarkers of disability than conventional MRI. MRI scans were performed on 117 participants with MS in addition to 26 healthy volunteers. Mean values were obtained for DTI indices and MTR for supratentorial brain and three white matter tracts of interest. DTI and MTR values were tested for correlations with measures of atrophy and lesion volume and were compared with these more conventional indices for prediction of disability. All DTI and MTR values correlated to an equivalent degree with lesion volume and cerebral volume fraction (CVF). Thalamic volumes correlated with all indices in the optic radiations and with mean and perpendicular diffusivity in the corpus callosum. Nested model regression analysis demonstrated that, compared with CVF, DTI indices in the optic radiations were more strongly correlated with Expanded Disability Status Scale and were also more strongly correlated than both CVF and lesion volume with low-contrast visual acuity. Abnormalities in DTI and MTR are equivalently linked with brain atrophy and inflammatory lesion burden, suggesting that for practical purposes they are markers of multiple aspects of MS pathology. Our findings that some DTI and MTR indices are more strongly linked with disability than conventional MRI measures justifies their potential use as targeted, functional system-specific clinical trial outcomes in MS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It has become increasingly clear that quantitative MRI techniques are needed to measure aspects of pathologic change in multiple sclerosis (MS) that are not adequately explained by more conventional measures of active inflammation (gadolinium enhancement), lesion burden, and brain atrophy [1]. Quantitative analysis of diffusion tensor imaging (DTI) and magnetization transfer ratio (MTR) data has shown measurable changes in normal-appearing white matter and lesions [2–4]. Alterations in the diffusion properties of water, as measured by DTI, have been proposed as markers of neurodegeneration and demyelination in axonal pathways [5–7], and MTR has been proposed as a marker of the myelin content of tissues [8], although neither type of imaging is pathologically specific under most clinical conditions. Nevertheless, both DTI and MTR indices correlate with disability [9–11] and thus may be clinically relevant. Recently, we have quantified longitudinal changes in white matter tract-specific DTI/MTR values over time periods, with proposed effect sizes that are reasonable for clinical trials [12].
While it is clear that these quantitative techniques are sensitive to aspects of MS pathology not adequately captured by more conventional techniques, it is not clear if the additional information provided actually increases our ability to measure overall disease burden and predict disability. Demonstration of this additional predictive ability would render DTI and MTR more useful and clinically interpretable as clinical trial outcomes. In this study, we performed an analysis on a cohort of participants with MS who had been studied with conventional and DTI/MTR MRI techniques. We aimed to examine the relationship of DTI/MTR outcomes to conventional MRI measures and also to determine if the addition of these newer techniques adds to our ability to explain disability in MS.
Methods
Standard protocol approvals and patient consents
Protocols were approved by the Institutional Review Boards at the Johns Hopkins University School of Medicine and the Kennedy Krieger Institute, who comply with all of the ethical standards of the 1964 Declaration of Helsinki. Written, informed consent was obtained from all participants.
Participants
Participants with MS were recruited from the Johns Hopkins MS Center. Individuals with diagnoses of relapsing–remitting (RRMS), secondary progressive (SPMS), and primary progressive (PPMS) MS were enrolled; the diagnosis was assigned by the treating physician. Data were collected from April 2005 through July 2009. Scans performed within 30 days of a clinical relapse or steroid administrations were excluded. Scans from a healthy volunteer population were also analyzed to demonstrate that the DTI and MTR findings were due to MS pathology.
MRI protocol and image analysis
The MRI acquisition protocol has been fully described elsewhere [13, 14], and only the essential details are presented here. On a 3-T Philips scanner, we obtained the following whole-brain sequences at the specified acquired resolution without gaps: DTI (2.2 mm isotropic); magnetization transfer (1.5 × 1.5 × 2.2 mm); proton-density/T2-weighted and fluid-attenuated inversion recovery (FLAIR) (0.8 × 0.8 × 2.2 or 4.4 mm for some of the FLAIR scans) and three-dimensional (3D) magnetization-prepared rapid acquisition with gradient echo (MPRAGE) (1.1 mm isotropic).
The details of our image analysis protocol also have been previously reported [13, 14]. In brief, MTR and diffusion-weighted images were coregistered, and the diffusion tensor was estimated to generate maps of fractional anisotropy (FA), mean diffusivity (MD), parallel diffusivity (λ ║), and perpendicular diffusivity (λ ⊥). To reduce the influence of distortion at the periphery, particularly the temporal lobes, we restricted our analysis to Montreal Neurological Institute (MNI-152) coordinates 54 ≤ z ≤ 124. This restricted the analysis to supratentorial brain only, from the mid-temporal lobes to the centrum semiovale. Also a small section of the anterior frontal lobes (y ≤ 37) was removed, because it is particularly susceptible to distortion on DTI scans. Cerebrospinal fluid was masked out by excluding voxels with MD ≥1.7 μm2/ms. DTI indices and MTR were calculated as mean values across this entire supratentorial area, in addition to within specific tracts of interest. For tract analysis, the optic radiations, corticospinal tracts, and corpus callosum were analyzed in an automated fashion via linear registration to a normalized DTI atlas. This atlas was created by conventional tractography [manual region of interest (ROI) drawing with tractography between these ROIs] upon 26 healthy volunteers with probability-based tract reconstruction, as described previously [12, 13]. These tracts were chosen because of their clinical and functional relevance and because of existing reliability data for these same tracts via the same methodology in an overlapping dataset [13]. Whole tracts (without lesions removed) were analyzed in this study, because prior work on this dataset has demonstrated that T2 lesions only account for 1–2 % of DTI-derived tract profiles, and removal of lesions in order to examine only “normal-appearing white matter” does not meaningfully alter mean tract index values or analyses performed with these values [12, 14]. As described in our prior report [12], all values obtained were adjusted to account for known data shifts over time due to scanner upgrades/modifications.
For brain segmentation, we used the Lesion-TOADS algorithm [15, 16] (http://www.nitrc.org/projects/toads-cruise/). This algorithm uses the MPRAGE and FLAIR scans to account for and segment T2 lesions while also automatically segmenting whole-brain and multiple white and gray matter structures. All segmentation results were visually inspected for errors by an experienced observer (D.M.H.). Three cases required custom parameter settings, and one case required manual editing of the lesion mask. To account for intersubject variability in head size, the segmented volumes were normalized to each individual’s intracranial volume. Thus, brain volume is reported as the cerebral volume fraction (CVF), in addition to cerebral white and gray matter fractions (WMF and GMF, respectively) and normalized brain substructure and total T2 lesion volumes (T2LV).
Disability measures
All disability assessments were performed within 30 days of scan date. Neurologic examinations were performed to determine the Expanded Disability Status Scale (EDSS) [17] score. The timed 25-foot walk, 9-hole peg test, and paced auditory serial addition test with 3 second delay (PASAT-3) were administered to determine the Multiple Sclerosis Functional Composite (MSFC) score [18]. z-Scores for individual test results and the MSFC total score were calculated according to the recommendations of the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force, using the Task Force dataset for normalization [19, 20]. Lower z-scores correspond to greater disability. Low-contrast visual acuity (1.25 %), which has been demonstrated to be more sensitive to MS-related optic nerve damage than standard visual acuity [21, 22], was used as a measure of visual function.
Statistical analysis
All statistical analyses were performed in Stata 10.1 IC (StataCorp, College Station, Texas). Comparisons between subgroups for demographic, clinical, and MRI data were performed by Student’s t test (means) and Mann–Whitney test (medians). Correlation analysis was performed by examining partial correlations, adjusted for age and sex. Multivariate linear regression (also adjusted for age and sex) was used to further probe these relationships. Comparisons between subgroups for the strength of relationship between MRI and disability data was performed in a multivariable regression model with interaction terms. To determine if the values for the DTI-derived MRI indices could be best predicted by brain volume (CVF) versus lesion burden (T2LV) and WMF versus GMF, regression analysis was performed for each of these individually, and then compared (as a nested model) with a combined model via the likelihood ratio test. To determine if the DTI-derived indices could add to the ability of CVF and T2LV to predict disability, a similar nested model regression approach was used with significance assessed by the likelihood ratio test. Because of the large number of analyses performed, a significance level of p < 0.01 was used for the correlation and regression analyses to reduce the false discovery rate.
Results
Scans from 117 MS cases (66 RRMS, 30 SPMS, and 21 PPMS) were analyzed, in addition to 26 healthy volunteers. Demographic and clinical data are described in Table 1. The healthy volunteer population was significantly younger than the full MS population, but not distinguishable from the RRMS population. As expected, disease duration was longest in those with SPMS [mean 20.9 years, standard deviation (SD) 10.0 years], and EDSS, MSFC, and low-contrast visual acuity were all significantly worse in those with SPMS and PPMS compared with RRMS.
Mean values for normalized structural volumes, DTI indices, and MTR can be found in Supplementary Table 1. CVF and gray matter, caudate, putamen, and thalamic volumes were all reduced in those with MS compared with healthy volunteers, whereas WMF was not. All of the DTI indices (except FA in corticospinal tract) and MTR were pathologically altered (reduced FA and MTR and increased diffusivities) in comparison with the healthy volunteers. Although there were no significant differences in the supratentorial brain analysis, most tract-specific indices were more abnormal in those with SPMS compared with RRMS.
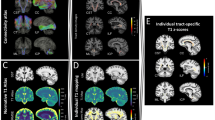
To explore if DTI and MTR abnormalities are markers of global, gray matter, white matter, and/or lesion pathology, relationships of the DTI indices and MTR with the segmented volumes were explored by correlation analysis, the results of which are provided in Table 2. MTR and all DTI indices except FA in the corticospinal tract (which was not abnormally low in MS; see Supplementary Table 1) demonstrated robust correlations with CVF, T2LV, and WMF, with most correlation coefficients >0.5. A visual representation of this relationship is presented in Fig.1a, b with optic radiations MD as an example. More modest correlations were found between GMF and DTI indices (again, except for FA in the corticospinal tract) and MTR, with correlation coefficients between 0.28 and 0.58. Values for MTR and DTI indices did not correlate with caudate or brainstem volume, and only supratentorial FA correlated with cerebellum volume (r = 0.26). All DTI indices and MTR in the optic radiations correlated modestly with thalamus volume, as did MD and λ ⊥ in the corpus callosum.
Relationship between mean diffusivity in the optic radiations and atrophy, lesion load, and disability. The graphs demonstrate the relationship between mean diffusivity in the optic radiations (as an example) and cerebral volume fraction (a), T2 lesion volume (b), EDSS (c), MSFC (d), and low-contrast visual acuity (e). The line of best fit was obtained by plotting values as predicted by a regression model adjusted for age and sex. R 2 values represent the proportion of variability in the dependent variable explained by the independent variable
A nested model regression analysis was performed to evaluate whether the DTI and MTR indices were most associated with measures of atrophy (CVF) or lesion burden (T2LV). For all indices except FA, λ ⊥, and MTR in the corticospinal tract, DTI and MTR values were predicted to a greater degree in a regression model containing both CVF and T2LV, as opposed to one or the other independently (Table 3).
A similar approach was used to evaluate if MTR and the DTI indices were more strongly related to gray or white matter pathology (Table 3). Although R 2 values (measure of degree of variability in DTI and MTR predicted by regression model) were generally greater for WMF, addition of GMF to the regression model added significant additional predictive ability. This was not the case for supratentorial FA and corticospinal tract λ ║, λ ⊥, and MTR, for which addition of GMF to the regression model did not add significant predictive information beyond that provided by WMF. The opposite was found for corticospinal tract FA, in which addition of WMF to the regression model did not add predictive information beyond that provided by GMF.
Most of the DTI indices and MTR in supratentorial brain, corpus callosum, and optic radiations were significantly correlated with disability in all MS patients, as measured by EDSS and MSFC scores (Table 4). A visual representation of this relationship is shown in Fig. 1c–e, with optic radiations MD as an example. In the corticospinal tract, only λ ⊥ correlated with EDSS and only MTR correlated with MSFC scores. Low-contrast visual acuity did not correlate with DTI or MTR values in supratentorial brain or corpus callosum, but did correlate with all indices except λ ║ in the optic radiations. Low-contrast visual acuity also correlated with corticospinal tract FA, λ ⊥, and MTR. Subgroup analysis revealed similar patterns of correlation in those with RRMS, and to a degree, PPMS. In SPMS, the DTI-derived indices did not significantly correlate with disability measures, and in fact, a number of indices actually had trends towards reciprocal relationships to disability compared with RRMS and PPMS; For example, whereas MD in the optic radiations in RRMS and PPMS had positive correlations with EDSS (ρ = 0.41 and 0.47, respectively), this index negatively correlated (ρ = −0.26) in SPMS.
A nested model regression approach was used to determine if DTI values and MTR in relapsing–remitting MS provided additional information toward the prediction of disability beyond that provided by CVF or T2LV (Table 5; Supplementary Table 3). DTI values and MTR in the supratentorial brain and corpus callosum, in addition to most values in the optic radiations and corticospinal tract, did not add any further significant predictive value for disability prediction beyond that provided by CVF. Exceptions to this were optic radiation MD and λ ⊥ for prediction of EDSS and optic radiation FA and λ ⊥ and corticospinal tract λ ⊥ and MTR for prediction of low-contrast visual acuity. None of the DTI indices or MTR was able to predict EDSS or MSFC scores beyond that predicted by T2LV alone. Values for FA and λ ⊥ in the optic radiations and corticospinal tract added significant predictive value for low-contrast visual acuity to T2LV alone. None of the DTI indices or MTR was able to add significant additional predictive value for EDSS and MSFC scores beyond CVF and T2LV combined. However, optic radiation FA and λ ⊥ were able to add additional predictive value for low-contrast visual acuity beyond CVF and T2LV combined.
Discussion
This study supports the links previously seen between DTI and MTR abnormalities and disability in MS [10, 11, 13], as demonstrated by the correlations between EDSS and MSFC and most of the measured indices, and with low-contrast visual acuity for some indices in the optic radiations and corticospinal tract. We went one step further to compare the relative strength of correlation with disability for the DTI indices and MTR with conventional MRI measures. This analysis showed that DTI values in the optic radiations may improve prediction of EDSS over brain volume alone and may also improve prediction of low-contrast visual acuity over brain volume and lesion burden, both individually and combined. The fact that it was the optic radiations that stood apart from the other tracts in comparison with conventional MRI for prediction of EDSS is an interesting finding requiring future replication. While visual disability does impact the EDSS score to a degree, it is more likely that the predominantly periventricular nature of this tract may make it more likely to represent global MS disease processes better than other tracts. Regardless, the ability to predict disability to a greater extent than the individual conventional MRI measures argues for further investigation into the use of optic radiations DTI as a surrogate marker in clinical trials. Further supporting this notion is our prior report demonstrating that this analysis technique can measure longitudinal change with variability small enough to allow for reasonable sample sizes for detection of treatment effects [12]. The strong link seen between optic radiation damage and visual disability in this study supports our prior work demonstrating the ability of tract-based DTI analysis to probe structure–function relationships [9]. Integration of more disability-specific tract-based measures into clinical trials, as opposed to global markers of pathology (such as T2LV and brain atrophy), may help resolve the clinicoradiologic paradox noted previously in observational and therapeutic studies [1].
As opposed to conventional MRI outcomes in MS, DTI-based tract-specific measures have the particular advantage of probing structure–function relationships and may provide evidence for cellular injury along an entire functional pathway. This notion is supported by our observation that damage to all of the DTI indices and MTR in the optic radiations correlated with thalamic atrophy, and these values specifically correlated with low-contrast visual acuity. Mechanistic links between damage to thalamocortical pathways and thalamic atrophy have been previously established in MS and have been demonstrated to be independent of damage to unrelated white matter tracts [23]. Whether MS-related thalamic atrophy is primary and damage to connected pathways occurs through secondary Wallerian degeneration or vice versa has still yet to be clearly established. It is unclear if our results represent the quantification of death of neuronal cell bodies of the optic radiations originating in the thalamus, transsynaptic degeneration, or if the structure and disability correlations are simply stronger for cases in which pathology has occurred throughout the entire visual pathway. Regardless, our results demonstrate that structure–function relationships can be probed in this manner across an entire functional pathway, with clinical relevance.
Our findings also provide additional information as to what underlying disease processes may be responsible for alterations in DTI and MTR values. We were able to demonstrate robust correlations between white matter tract and supratentorial DTI and MTR and CVF, T2LV, and GMF/WMF, consistent with prior reports [10, 24]. Cluing towards the structural specificity of this type of analysis, beyond the correlations seen between thalamic volume and all indices in the optic radiations and corpus callosum MD and λ ⊥, no correlations were found with other brain substructures, such as caudate and brainstem. Further analysis demonstrated that the variability of DTI values and MTR was best predicted by a combined model including T2LV and CVF, which was superior to either independently. Also, neither white nor gray matter atrophy was superior to the other in predicting the DTI values or MTR.
These findings have implications for our understanding of the causes of DTI and MTR abnormalities in MS; For example, if MTR abnormalities were purely influenced by lesion-localized demyelination, it would be expected that values would be best predicted by lesion volume. The additional predictive value of brain atrophy towards these values, however, clues towards the additional influence of more global neurodegenerative processes and axonal injury, both of which have been shown to alter MTR in autopsy studies [8, 25]. Additionally, the fact that DTI values are influenced by both CVF and T2LV likely means that these values are influenced by multiple processes, including demyelination, axonal transection, secondary Wallerian degeneration, and inflammation. The fact that lesion volume was not found to be the sole determinant of DTI and MTR values is consistent with multiple studies that have demonstrated DTI and MTR abnormalities in normal-appearing white matter [26–28]. Also, the observed independent predictive abilities of gray and white matter volumes toward DTI abnormalities lends credence to the idea that these values may be influenced by secondary neurodegeneration and demyelination stemming from both axonal transection in white matter lesions and primary gray matter pathology, both of which have been independently demonstrated in pathologic studies [29–31].
Also, although λ ║ has been proposed as a measure of axonal degeneration and λ ⊥ has been proposed as a measure of demyelination [5–7], the fact that supratentorial atrophy, gray matter volume, white matter volume, and lesion volume all play significant independent roles in influencing these values demonstrates that these indices are also likely not pure measures and may be influenced to varying degrees by degenerative, inflammatory, and demyelinating pathology. Although we have demonstrated here that DTI and MTR are not purely influenced by demyelination or axonal degeneration, it may turn out that these measures still prove superior to conventional measures in evaluation of future therapies aimed at demyelination or neuroregeneration [32, 33].
The pathologic alterations noted in this study in nearly all of the DTI and MTR indices are consistent with prior literature [27, 34]. These alterations were more profound in participants with SPMS when compared with RRMS, which is also consistent with prior reports [28, 35]. Despite this evidence of more significant pathology in axonal pathways, no correlations were found between these imaging outcomes and disability measures in participants with SPMS. This was not the case for those with RRMS, in whom such correlations could be found. While this may have been an issue of the smaller sample size of this subgroup, statistical comparisons found significant and mostly reciprocal differences between those with RRMS and PPMS compared with SPMS in a number of the tract-specific indices and supratentorial MTR. This disconnect between more severe DTI and MTR abnormalities and disability in SPMS may represent a basement effect, in which pathologic alterations of axonal pathways in MS are responsible for disability earlier in the disease course, until a critical threshold, after which other aspects of MS pathology become more prominent determinants of disability. Thus, in SPMS, many of the axonal tracts being measured may have already lost function, yet they may continue to experience further pathologic change, either due to the disease process or age-related changes. Alternately, these reciprocal relationships may be the result of the influence of inflammation on DTI values, with increases in diffusivity occurring in the RRMS population due to inflammatory change and tissue edema, correlating with worsening disability. Conversely, because of a reduction in central nervous system (CNS) inflammation in the later stages of secondary progressive phase of MS, diffusivity values may be reducing in late SPMS due to a pseudonormalization effect. This would also correlate with worsening disability due to the expected worsening of disability seen clinically in SPMS.
It should be noted that our methodology of summary statistics derived from automated, probability-based tract identification differs from other studies in which DTI and MTR were measured by whole-brain histogram techniques, ROI analysis, or voxel-based morphometry. While our technique may provide simple, easy to interpret values in an automated manner, and has been shown to correlate well with disability and be stable enough to evaluate for longitudinal change [12, 13], it is nonetheless difficult to compare these results directly with studies of those types. Additionally, the stronger correlations with physical disability seen for the optic radiations and corpus callosum over the corticospinal tracts may have been influenced by the anatomy of these tracts, which allow for superior automated tractography. Because of their simple anatomy and sheet-like architecture, these tracts are less susceptible to registration errors, resulting in more reproducible tract identification than the corticospinal tract [13]. Lastly, definitive conclusions in regards to our MS subtype analysis should be tempered with an acknowledgement of the reduced sample sizes of the progressive subtypes compared with RRMS. Regardless, we feel that the comprehensive nature of this analysis and the large cohort analyzed mean that the results contribute substantially to our understanding of the role of DTI and MTR in measuring pathology and its relationship to disability in multiple sclerosis.
References
Barkhof F (2002) The clinico-radiological paradox in multiple sclerosis revisited. Curr Opin Neurol 15(3):239–245
Filippi M et al (2001) Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology 56(3):304–311
Filippi M et al (1995) A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology 45(3 Pt 1):478–482
Filippi M, Rocca MA, Comi G (1998) Magnetization transfer ratios of multiple sclerosis lesions with variable durations of enhancement. J Neurol Sci 159(2):162–165
DeBoy CA et al (2007) High resolution diffusion tensor imaging of axonal damage in focal inflammatory and demyelinating lesions in rat spinal cord. Brain 130(Pt 8):2199–2210
Song SK et al (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26(1):132–140
Zhang J et al (2009) Diffusion tensor magnetic resonance imaging of wallerian degeneration in rat spinal cord after dorsal root axotomy. J Neurosci 29(10):3160–3171
Schmierer K et al (2007) Quantitative magnetization transfer imaging in postmortem multiple sclerosis brain. J Magn Reson Imaging 26(1):41–51
Reich DS et al (2009) Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol 66(8):998–1006
Giorgio A et al (2010) Relationships of brain white matter microstructure with clinical and MR measures in relapsing-remitting multiple sclerosis. J Magn Reson Imaging 31(2):309–316
Agosta F et al (2006) Magnetization transfer MRI metrics predict the accumulation of disability 8 years later in patients with multiple sclerosis. Brain 129(10):2620–2627
Harrison DM et al (2011) Longitudinal changes in diffusion tensor-based quantitative MRI in multiple sclerosis. Neurology 76(2):179–186
Reich DS et al (2010) Automated vs. conventional tractography in multiple sclerosis: variability and correlation with disability. Neuroimage 49(4):3047–3056
Ozturk A et al (2010) MRI of the corpus callosum in multiple sclerosis: association with disability. Mult Scler 16(2):166–177
Shiee N et al (2010) A topology-preserving approach to the segmentation of brain images with multiple sclerosis lesions. Neuroimage 49(2):1524–1535
Shiee N et al (2012) Revisiting brain atrophy and its relationship to disability in multiple sclerosis. PLoS ONE 7(5):e37049
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452
Cutter GR et al (1999) Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 122(5):871–882
Fischer JS et al (1999) The multiple sclerosis functional composite measure (MSFC): an integrated approach to MS clinical outcome assessment. National MS Society Clinical Outcomes Assessment Task Force. Mult Scler 5(4):244–250
Rudick R et al (1997) Recommendations from the National Multiple Sclerosis Society Clinical Outcomes Assessment Task Force. Ann Neurol 42(3):379–382
Baier ML et al (2005) Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology 64(6):992–995
Balcer LJ, Frohman EM (2010) Evaluating loss of visual function in multiple sclerosis as measured by low-contrast letter acuity. Neurology 74(Suppl 3):S16–S23
Henry RG et al (2009) Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J Neurol Sci 282(1–2):61–66
De Stefano N et al (2002) MR correlates of cerebral atrophy in patients with multiple sclerosis. J Neurol 249(8):1072–1077
Petzold A, Tozer DJ, Schmierer K (2011) Axonal damage in the making: neurofilament phosphorylation, proton mobility and magnetisation transfer in multiple sclerosis normal appearing white matter. Exp Neurol 232(2):234–239
Pulizzi A et al (2007) Determinants of disability in multiple sclerosis at various disease stages: a multiparametric magnetic resonance study. Arch Neurol 64(8):1163–1168
Ceccarelli A et al (2007) Normal-appearing white and grey matter damage in MS. A volumetric and diffusion tensor MRI study at 3.0 Tesla. J Neurol 254(4):513–518
Vrenken H et al (2010) Diffusely abnormal white matter in progressive multiple sclerosis: in vivo quantitative MR imaging characterization and comparison between disease types. Am J Neuroradiol 31(3):541–548
Tsunoda I et al (2003) Axonal injury heralds virus-induced demyelination. Am J Pathol 162(4):1259–1269
Trapp BD et al (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338(5):278–285
Bodini B et al (2009) Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: an in vivo study with TBSS and VBM. Hum Brain Mapp 30(9):2852–2861
van den Elskamp IJ et al (2010) Lesional magnetization transfer ratio: a feasible outcome for remyelinating treatment trials in multiple sclerosis. Mult Scler 16(6):660–669
Fox RJ et al (2011) Measuring myelin repair and axonal loss with diffusion tensor imaging. Am J Neuroradiol 32(1):85–91
Fink F et al (2010) Comparison of diffusion tensor-based tractography and quantified brain atrophy for analyzing demyelination and axonal loss in MS. J Neuroimaging 20(4):334–344
Preziosa P et al (2011) Intrinsic damage to the major white matter tracts in patients with different clinical phenotypes of multiple sclerosis: a voxelwise diffusion-tensor MR study. Radiology 260(2):540–550
Acknowledgments
The MRI data was acquired through National MS Society Tissue Repair grant TR3760A3 and through a grant from EMD Serono. Research support was also obtained through NINDS grant K99NS064098 from the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, and NINDS grant R01NS070906.
Conflicts of interest
Dr. Calabresi has received research support and consultation fees from EMD Serono. Otherwise, the authors declare no financial relationships with the supporting entities of this study.
Ethical standards
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harrison, D.M., Shiee, N., Bazin, PL. et al. Tract-specific quantitative MRI better correlates with disability than conventional MRI in multiple sclerosis. J Neurol 260, 397–406 (2013). https://doi.org/10.1007/s00415-012-6638-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6638-8