Abstract
Invasive treatment for Gilles de la Tourette syndrome has shown interesting results in a number of published reports; it seems to be evolving into a promising therapeutic procedure for those patients demonstrating disabling clinical pictures who are refractory to conservative treatments. There are important issues concerning the stimulated brain target, with different nuclei currently under investigation. Our group asked in this pilot study whether Tourette syndrome could be treated by tailoring specific brain targets for specific symptoms. Deep brain stimulation for Tourette syndrome may thus in the future be tailored and patient specific, utilizing specific target regions for individual clinical manifestations. In our early experience we did not adequately address non-motor clinical symptoms as we only used a thalamic target. More recently in an obsessive compulsive disease cohort we have had success in using the anterior limb of the internal capsule and nucleus accumbens region as targets for stimulation. We therefore explored the option of a “rescue” procedure for our Tourette patients with persistent obsessive-compulsive disorder following ventralis oralis/centromedianus-parafascicularis (Vo/CM-Pf) deep brain stimulation. Following two cases where rescue anterior limb of internal capsule/nucleus accumbens leads were employed, we performed two additional procedures (anterior limb of the internal capsule plus ventralis oralis/centromedianus-parafascicularis and anterior limb of the internal capsule alone) with some mild improvement of comorbid obsessive–compulsive disorder, although the number of observations in this case series was low. Overall, the effects observed with using the anterior limb of the internal capsule either alone or as a rescue were less than expected. In this report we detail our experience with this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are a number of recent studies addressing the use of deep brain stimulation (DBS) for neuropsychiatric indications [1–8], however little is known about the anterior limb of the internal capsule/nucleus accumbens (ALIC/NA) target specifically for Tourette syndrome (TS). Additionally, there is sparse information available supporting the utilization of multiple brain targets to address medically refractory symptoms of TS and for behavioral manifestations [10–14]. Previously, collaborators to our study group published a small series of severe tremor patients who successfully underwent multiple DBS leads which were placed in the same hemisphere [15, 16] in order to tailor therapy into multiple brain circuits. Additionally and recently, Houeto et al. [14] published a case of a TS patient who was simultaneously and intentionally implanted with bilateral thalamic DBS, and bilateral ventral (non-motor) pallidal DBS. This unique approach allowed them to assess the effects of each target independently and to assess for synergy between the two sets of DBS leads. The use of multiple leads by Houeto (more recent follow-up evaluation of three patients treated at multiple targets with improvements of up to 96% at the YGTSS with bilateral Gpi DBS in one and 64% in another) [17] and also reported by Ackermans [18] have potentially opened the door for cases of TS (with coexistent and severe comorbidities), to be addressed by the targeting of multiple brain regions in the same hemisphere. Multiple leads could therefore be used to specifically address problematic symptoms. Our recently published experience on 18 cases with TS DBS has revealed that the situation of excellent tic control may coexist with a complete failure to address obsessive compulsive features (OCD). In several of our previously reported cases, tic was well controlled with Vo/CM-Pf DBS, however severe OCD persisted and was disabling. We have attempted in several patients to use rescue DBS leads (adding bilateral ALIC/NA region DBS leads to the existing Vo/CM-Pf stimulation). Additionally in two patients with TS we attempted de novo ALIC/NA DBS plus Vo/CM-Pf region DBS, and ALIC/NA DBS as monotherapy, respectively. We report the results of our open-label clinical experience with these approaches in the hope that this data will assist the refinement of these approaches in the future.
Methods
Thirty-two TS patients have been treated to date with bilateral DBS (Neurochirurgia Funzionale, Istituto Galeazzi IRCCS Milan) at the Vo/CM-Pf or at the anterior globus pallidus internus (Gpi). Potential patients were drawn from thousands of TS patients from our specialty clinic. All potential subjects were screened vigorously by a multidisciplinary team of a neurologist, neurosurgeon, neuropsychologist, and psychiatrist. Indication for treatment received ethical approval, and treatment was followed post-DBS by a neurologist (MP) and a neuropsychologist (AB). The assessment battery included a ten point scale for the patient to rate TS-influenced social function (visual analogue scale, VAS [19]), the Beck Depression Inventory (BDI) [20], the State-Trait Anxiety Inventory (STAI) [21], the Yale-Brown Obsessive–Compulsive Scale (YBOCS) [22, 23], the Yale Global Tic Severity Scale (YGTSS) [24], and the Diagnostic Confidence Index (DCI) [25]. All the patients received a complete clinical, neurological, and neuropsychological evaluation together with routine blood tests, as well as chest X-rays if above age 40. All patients signed an informed consent detailing the risks of the DBS procedure and the experimental nature of the DBS trial. We recruited two patients from our initial cohort of existing Vo/CM-Pf DBS implants for a potential rescue procedure after documenting persisting obsessionality despite good tic reduction. Following the improvement of the clinical picture in these two patients, two more patients were drawn from our clinics for de novo implantation (one patient had de novo Vo/CM-Pf plus ALIC/NA DBS, and a final patient had only ALIC/NA DBS). To qualify for DBS surgery patients had to complete a 6-month drug therapy trial with conventional and atypical antipsychotic medications, and they must have failed to show significant clinical improvement. Considering the wide range of clinical manifestations in TS patients, we followed patients with monthly serial evaluations during which the therapeutic regimen could be modified. A trial of at least one typical and one atypical neuroleptic medication was performed in all patients. All TS patients had to be tried on adequate doses of a dopamine depletor (tetrabenazine (TBZ)) 25 mg three times per day) [26] and a selective serotonin reuptake inhibitor (SSRI, fluvoxamine 25 up to 100 mg) was also administered for obsessive–compulsive comorbidity, alone or in association with clorimipramine (25 up to 100 mg per day). When present [27], attention deficit-hyperactive disorder (ADHD) was treated with clonidine 75–150 mg per day or guanfacine 5 mg or higher per day. After arriving at an optimal drug regimen (on the basis of adverse effects and best symptom improvement), patients were instructed to continue drug therapy throughout their hospitalization, DBS procedure, and postoperative follow-up evaluations. Drugs were then modified on the basis of further amelioration of the clinical picture. Informed consent was signed by all patients acknowledging that risks and benefits of additional or de novo DBS leads had been addressed. We utilized rescue leads in the first two patients who still had severe and disabling behavioural manifestations of TS despite Vo/CM-Pf region DBS. In the third patient we attempted bilateral DBS of both regions simultaneously (because of the severe behavioural features).
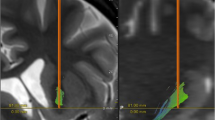
The DBS procedure for implantation of the Vo/CM-Pf has been previously detailed and published [28]. A 2 mm thick contrast-enhanced T1 axial and T1 sagittal image and a T2 coronal MRI was fused with a stereotactic 3 mm slice thickness CT scan on a neuronavigational device (Medtronic, StealthStation TREON). A Cosman–Roberts–Wells frame (CRW) was utilized, however microelectrode recording was not used on all cases. An MR angiography study was performed in an effort to avoid puncturing blood vessels. Post-operative MRI was obtained in order to document adequate positioning of the stimulating leads (Fig. 1). Postoperative imaging was fused with neuronavigational documentation in order to obtain a comparison between the planned and the implanted leads. A second procedure under general anaesthesia was performed 3 days following lead placement in order to connect the stimulating leads with a Kinetra pulse-generator. The pulse-generator was positioned in a subclavicular or abdominal subcutaneous pouch.
Case 1
A right-handed 25-year-old male with a 13-year history of schooling and without a family history of neurological diseases reported his initial symptoms of TS appeared prior to age 10. His original presentation included a clinical picture that was characterized by OCD, impulse-control disorder, aggressiveness, self injurious behavior (SIB) (self-inflicted punches on the forehead), complex motor/phonic tics of trunk, face and arms. The degree of social impairment was severe.
The following pharmacological trial was instituted: Pimozide 4 mg PO BID for 1 month, then sulpiride 400 mg/day for 1 month, then TBZ 75 mg combined with aripiprazole 15 mg for 4 months, sertraline 100 mg for 6 months. His drug trials produced unbearable side effects: the SSRI resulted in somnolence, and clinically significant parkinsonism was revealed on neurological examination as a result of neuroleptic use.
He underwent bilateral Vo/CM-Pf region DBS in November 2004. Baseline characteristics, together with follow-up evaluations, are detailed in Table 1. Thirty months following bilateral Vo/CM-Pf DBS, the overall clinical picture improved (YGTSS from 79 to 52) and the self injurious behavior resolved. Despite these benefits the patient remained unsatisfied and disabled. His depressive symptoms also worsened (BDI 15–33) and his OCD (YBOCS 23–28) remained relatively unchanged. In September 2007, 34 months after his first DBS, bilateral ALIC/NA stimulation was performed to address the OCD, depressive, and behavioural features. Significant clinical benefit was not evidenced in previous tic symptoms (although motor tic scores improved), and although OCD/depression scores improved from September 2007, the improvement was only mildly clinically relevent.
Case 2
A 31-year-old right-handed female, with an 8-year history of post-high school education and an uncertain family history of TS presented for management. She reported having a 6-year-old child diagnosed with OCD, ADHD as well as motor and phonic tics (a diagnosis of TS was not formally made). Prior to age 10 she presented with impulsiveness, rage, attention deficit, OCD, and non-obscene socially inappropriate behavior (NOSI). Features of the clinical picture, and their evolution are reported in Table 2. The pharmacological trial included haloperidol 6 mg/day for 2 months, sulpiride 200 mg, and tiapride 300 mg combined with TBZ 75 mg/day. A satisfying outcome from drug and behavioral treatments was not achieved. Haloperidol was unbearable for her due to sleepiness, confusion, and apathy. Other neuroleptics resulted in amenorrhea and galactorrhea. She was periodically admitted to a social reintegration unit in a hospital. Written reports revealed she was unable to successfully socialize and integrate with other people in daily life and to complete short term tasks. Vo/CM-Pf was implanted in May 2006 for symptoms which included significant OCD and motor tics of the face, trunk and arms (YGTSS 79/100, see Table 2). In spite of an improvement of tics and perceived quality of life, her OCD, depression and anxiety states remained incompatible with normal social integration. Her expectations were not met. Rescue DBS at ALIC/NA was discussed and she consented. The tic manifestations mildly improved and the OCD comorbidity also mildly improved since June 2007 (date of the rescue procedure) (see Table 2).
Case 3
A right-handed 37-year-old man with 13 years of schooling and no family history of TS had his first symptoms before age 10. Because of his peculiar behavior he was initially misdiagnosed as having a personality disorder and attention deficit disorder (ADD), but not TS. He had many years which included difficulty in integrating into society, and he had many admissions to neurologic and psychiatric wards as a result of highly aggressive behavioural outbursts with multiple complex motor tics. His OCD was severe. The most disabling feature of his TS was self injurious behavior which was manifested by cutting himself with a knife. The summary of his data is reported in Table 3. The following pharmacological trial was instituted: haloperidol 6 mg/day, adding aripiprazole for 3 months, and combining this with tiapride 300 mg. He also had a course of botulinum toxin and tramadol. His medication regimen resulted in unacceptable levels of parkinsonism (rigidity and bradykinesia) and sedation. None of the many medicines attempted resulted in reasonable benefits. In particular, neuroleptics resulted in parkinsonism; pimozide elicited high levels of anxiety, jaw dryness, rigidity, drowsiness; and the SSRI resulted in erectile dysfunction and sleepiness. The severe motor manifestations and SIB led to the decision to implant the Vo/CM-Pf thalamic region DBS bilaterally. However, because of the severe OCD and behavioural manifestations the ALIC/NA was also implanted during the same surgical procedure following patient consent.
Following surgery, a protocol was followed to test each set of DBS leads: the thalamic and nucleus accumbens leads were turned on separately in different time epochs. The Vo/CM-Pf region DBS appeared clinically better for treatment of the tics and self injurious behaviours. The ALIC/NA DBS leads proved better for the treatment of the OCD and behavioural features (38–15, 60% improvement on YBOCS). One year following the surgery, the OCD and depressive symptoms were found to be reduced (38–15 YBOCS, 29–16 BDI). The patient was able to reintegrate into society, and was able to independently operate a car. His drug therapy was discontinued, and he maintained an ability to stay gainfully employed.
Table 3 presents the results of post-DBS evaluations with both the pulse-generators (ALIC/NA and Vo/CM-Pf) turned on.
Case 4
A right-handed 47-year-old man with 13 years of education presented with symptoms of complex motor and phonic tics, coprolalia, ADHD, and severe disabling OCD. His social functioning and integration were severely compromised by aggressiveness and by NOSI. He had separated from his family because of difficulty with social interaction and inappropriate behavior, and he was admitted multiple times into psychiatric inpatient facilities for treatment of his behavioural issues. His official TS diagnosis was made during his fourth decade when he was admitted to our TS unit, although a definite history of a full-blown TS clinical picture was evident since the age of 18. His baseline characteristics of TS and their evolution over time are reported in Table 4. He started pharmacologic treatment with haloperidol 6 mg, then pimozide 8 mg and the addition of tiapride 300 mg, and finally the addition of TBZ 100 mg and aripiprazole 15 mg. All drugs and behavioral therapies were unsuccessful in treating the motor and the behavioural manifestations (baseline YBOCS was 35, and STAI was 54). He had unbearable side effects which included: sleepiness, weight gain, parkinsonism and galactorrhea from neuroleptics and erectile dysfunction seen as a result of the SSRI. The patient elected for de novo ALIC/NA DBS only. Following 10 months of DBS, the patient’s ability to control impulses and OCD were improved (YBOCS 35–16), while anxiety and depressive symptoms were largely unchanged. The patient has now, however been living independently and has improved in his ability to integrate into society. Although he is still severely affected by the TS, his values on the YGTSS have shown a progressive trend toward improvement. The changes might be at least in part due to his decreased levels of anxiety and obsessionality, even if significantly associated correlation has not been observed between the improvements in the different scales (Spearman’s rho not significant).
Table 5 summarizes characteristics and lead information from each of the four cases.
Discussion
The data derived from our case series suggest that the improvements in OCD, mood and behavioural features using the ALIC/NA target either as a rescue lead, or as de novo therapy did not seem to achieve the same magnitude of benefits previously reported in medication resistant OCD patients [11, 12, 29], or in patients with a refractory TS worsened by a severe comorbid OCD. (Recently in a work by Kuhn et al. in 2006, they reported an improvement of up to 41% in the YGTSS after ALIC-NA DBS for a refractory TS 26-year-old patient demonstrating severe OCD with self-aggressive tics [30, 31].) One rescue lead did, however, reveal benefits in OCD plus tics, and OCD improved with simultaneous Vo/CM-Pf and ALIC/NA and with de novo ALIC/NA implantation. It is important to point out that in cases 1 and 2 (rescue leads) the addition of the ALIC-NA target did seem to mildly improve motor tic scores. Despite disappointing improvements in the scores on standardized examinations it should be noted there were clear clinical changes evident in select patients as well as improvements in social reintegration.
The results, although somewhat disappointing, do raise the important issues of patient selection, simultaneous versus staged procedures, and setting more stringent follow-up procedures. Rescue DBS was indicated after failure of improvement in social re-integration in spite of objectively assessed good response over tic manifestations: this failure was attributed (by the patient, caregivers, and examiners) to the persistence of an obsessional comorbidity (recognized in the preoperative period and documented as failing to respond to the first DBS procedure) rather than to fluctuations in the overall clinical picture of the patient. Such subjective features as social reintegration, which seemed improved in this study, may prove hard to capture with validated and available scales. All of these issues will need to be addressed in future studies.
ALIC/NA DBS in the setting of TS, rather than in plain OCD, may prove to be an inferior approach based on the results of our study. To date, sham and blinded testing of the ALIC/NA target are being performed after proper ethical approval. If ALIC/NA DBS is proven to be inferior, we may look to the parallel non-motor basal ganglia circuitry for other regions in the brain that may also provide viable target options. These may include, for example, the cingulate area 25, the ventral GPi, the putamen, and the orbitofrontal cortex. Future technologies (responsive stimulators, better lead designs, scheduled stimulation) along with tailoring the target to be more symptom specific may be potential approaches for future studies.
Despite the somewhat disappointing results of the rescue leads in this series and in the de novo ALIC implantation, it is not clear that this approach should be immediately abandoned. The notion of using multifocal DBS to tailor therapy in medically refractory and severely disabled patients with TS may in the future lead to more rational treatment. Improvements in technology, targeting and patient selection will be needed in order to enhance results and hopefully to offer new hope for this subgroup of severe TS patients. Centers performing DBS for TS should remain cautious, use ethical panels to screen patients, and employ informed consent driven research protocols. The courage of patients with disabling TS should not replace a cautious approach to this therapy. Our patients in this clinical series were drawn from a setting in Milan where we follow thousands of patients from all over Italy, and these patients represented a handful of the worst and most disabled. We would like to caution centers against routinely employing rescue leads and bilateral simultaneous implantations of multiple brain targets in TS, even if an argument could be raised for the efficacy of ALIC/NA on comorbid features in less-disabled patients. Finally, our smaller experience proved much less successful than our previous larger experience with Vo/CM-Pf-only region DBS for addressing the motor and vocal manifestations of TS but we hope future improvements and better powered studies will enhance these outcomes.
References
Lujan JL, Chaturvedi A, McIntyre CC (2008) Tracking the mechanisms of deep brain stimulation for neuropsychiatric disorders. Front Biosci 13:5892–5904. doi:10.2741/3124
Larson PS Neurotherapeutics 2008 Deep brain stimulation for psychiatric disorders, vol 5. No. 1
Greenberg BD, Askland KD, Carpenter LL (2008) The evolution of deep brain stimulation for neuropsychiatric disorders. Front Biosci 13:4638–4648. doi:10.2741/3029
Kopell BH, Greenberg BD (2008) Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry. Neurosci Biobehav Rev 32(3):408–422. doi:10.1016/j.neubiorev.2007.07.004 (review)
Wichmann T, Delong MR (2006) Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 52(1):197–204. doi:10.1016/j.neuron.2006.09.022 (review)
Giacobbe P, Kennedy SH (2006) Deep brain stimulation for treatment-resistant depression: a psychiatric perspective. Curr Psychiatry Rep 8(6):437–444. doi:10.1007/s11920-006-0048-5 (review)
Aouizerate B, Cuny E, Martin-Guehl C, Guehl D, Amieva H, Benazzouz A, Fabrigoule C, Allard M, Rougier A, Bioulac B, Tignol J, Burbaud P (2004) Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive–compulsive disorder and major depression. Case report. J Neurosurg 101(4):682–686
Brito GN (1997) A neurobiological model for Tourette syndrome centered on the nucleus accumbens. Med Hypotheses 49(2):133–142. doi:10.1016/S0306-9877(97)90218-8
Sturm V, Lenartz D, Koulousakis A, Treuer H, Herholz K, Klein JC, Klosterkotter J (2003) The nucleus accumbens: a target for deep brain stimulation in obsessive–compulsive- and anxiety-disorders. J Chem Neuroanat 26(4):293–299. doi:10.1016/j.jchemneu.2003.09.003 (review)
Greenberg BD, Gabriels LA, Malone DA Jr, Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS, Malloy PF, Salloway SP, Giftakis JE, Rise MT, Machado AG, Baker KB, Stypulkowski PH, Goodman WK, Rasmussen SA, Nuttin BJ (2008) Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive–compulsive disorder: worldwide experience. Mol Psychiatry [Epub ahead of print]
Okun MS, Mann G, Foote KD, Shapira NA, Bowers D, Springer U, Knight W, Martin P, Goodman WK (2007) Deep brain stimulation in the internal capsule and nucleus accumbens region: responses observed during active and sham programming. J Neurol Neurosurg Psychiatry 78(3):310–314. doi:10.1136/jnnp.2006.095315
Greenberg BD, Malone DA, Friehs GM, Rezai AR, Kubu CS, Malloy PF, Salloway SP, Okun MS, Goodman WK, Rasmussen SA (2006) Three-year outcomes in deep brain stimulation for highly resistant obsessive–compulsive disorder. Neuropsychopharmacology 31(11):2384–2393. doi:10.1038/sj.npp.1301165 (Erratum in: Neuropsychopharmacology. 31 November 2006 11:2394)
Rauch SL, Dougherty DD, Malone D, Rezai A, Friehs G, Fischman AJ, Alpert NM, Haber SN, Stypulkowski PH, Rise MT, Rasmussen SA, Greenberg BD (2006) A functional neuroimaging investigation of deep brain stimulation in patients with obsessive–compulsive disorder. J Neurosurg 104(4):558–565. doi:10.3171/jns.2006.104.4.558
Houeto JL, Karachi C, Mallet L, Pillon B, Yelnik J, Mesnage V, Welter ML, Navarro S, Pelissolo A, Damier P, Pidoux B, Dormont D, Cornu P, Agid Y (2005) Tourette’s syndrome and deep brain stimulation. J Neurol Neurosurg Psychiatry 76(7):992–995. doi:10.1136/jnnp.2004.043273
Foote KD, Seignourel P, Fernandez HH, Romrell J, Whidden E, Jacobson C, Rodriguez RL, Okun MS (2006) Dual electrode thalamic deep brain stimulation for the treatment of posttraumatic and multiple sclerosis tremor. Neurosurgery 58(4)(Suppl 2):ONS-280–ONS-285 (discussion ONS-285–ONS-286)
Foote KD, Okun MS (2005) Ventralis intermedius plus ventralis oralis anterior and posterior deep brain stimulation for posttraumatic Holmes tremor: two leads may be better than one: technical note. Neurosurgery 56(2(Suppl)):445. doi:10.1227/01.NEU.0000157104.87448.78 discussion E445
Welter ML, Mallet L, Houeto JL, Karachi C, Czernecki V, Cornu P, Navarro S, Pidoux B, Dormont D, Bardinet E, Yelnik J, Damier P, Agid Y (2008) Internal pallidal and thalamic stimulation in patients with Tourette syndrome. Arch Neurol 65(7):952–957. doi:10.1001/archneur.65.7.952
Ackermans L, Temel Y, Cath D, van der Linden C, Bruggeman R, Kleijer M, Nederveen P, Schruers K, Colle H, Tijssen MA, Visser-Vandewalle V (2006) Dutch Flemish Tourette Surgery Study Group. Deep brain stimulation in Tourette’s syndrome: two targets? Mov Disord 21(5):709–713. doi:10.1002/mds.20816
De Boer AG, Van Lanschot JJ, Stalmeier PF, Van Sandick JW, Hulscher JB, De Haes JC, Sprangers MA (2004) Is a single-item visual analogic scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res 13(2):311–320. doi:10.1023/B:QURE.0000018499.64574.1f
Beck AT, Rush AI, Shaw BF, Emery G (1987) Terapia cognitiva della depressione Boringhieri, Torino
Bertolotti G, Michelin P, Sanavio E, Simonetti G, Vidotto G, Zotti AM “CBA 2.0 Cognitive Behavioural Assessment” 1987 (4th edn) “Organizzazioni Speciali” Firenze
Goodman WK, price LH, rasmussen SA (1989) The Yale-Brown Obsessive Compulsive Scale: I. Development, use and reliability. Arch Gen Psychiatry 46(11):1006–1011
Goodman WK, Price LH, Rasmussen SA (1989) The Yale-Brown Obsessive Compulsive Scale: II. Validity. Arch Gen Psychiatry 46(11):1012–1016
Leckman JF, Riddle MA, Hardin MT (1989) The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of severity. Am Acad Child Adolesc Psychiatry 28:566–573. doi:10.1097/00004583-198907000-00015
Robertson MM, Banerjee S, Kurlan R, Cohen DJ, Leckman JF, McMahon W, Pauls DL, Sandor P, van de Wetering BJ (1999) The Tourette syndrome diagnostic confidence index: development and clinical associations. Neurology 53(9):2108–2112
Porta M, Sassi M, Cavallazzi M, Fornari M, Brambilla A, Servello D (2008) Tourette’s syndrome and role of tetrabenazine : review and personal experience. Clin Drug Investig 28(7):443–459. doi:10.2165/00044011-200828070-00006
American psychiatric association (2000) Diagnostic and statistical manual of mental disorders, 4th edn. APA, Washington DC. Text revised (DSM-IV-TR)
Servello D, Porta M, Sassi M, Brambilla A, Robertson MM (2008) Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry 79(2):136–142. doi:10.1136/jnnp.2006.104067
Nuttin BJ, Gabriels LA, Cosyns PR et al (2003) Long-term electrical capsular stimulation in patients with obsessive–compulsive disorder. Neurosurgery 52:1263–1272. doi:10.1227/01.NEU.0000064565.49299.9A (discussion 1272–1264)
Flaherty AW, Williams ZM, Amirnovin R, Kasper E, Rauch SL, Cosgrove GR, Eskandar EN (2005) Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: technical case report. Neurosurgery 57(4(Suppl)):E403. doi:10.1227/01.NEU.0000176854.24694.95 (discussion E403)
Kuhn J, Lenartz D, Mai JK, Huff W, Lee SH, Koulousakis A, Klosterkoetter J, Sturm V (2007) Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome. J Neurol 254(7):963–965
Acknowledgments
The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. We wish also to acknowledge Ms. Olga Lee Rachello for English language supervision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Servello, D., Sassi, M., Brambilla, A. et al. De novo and rescue DBS leads for refractory Tourette syndrome patients with severe comorbid OCD: a multiple case report. J Neurol 256, 1533–1539 (2009). https://doi.org/10.1007/s00415-009-5159-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-009-5159-6