Abstract
To investigate the usefulness of 18F-FP-CIT PET for assessing the severity of Parkinson’s disease (PD) at various clinical stages, 41 patients with PD were divided into early (Hoehn&Yahr I-II, n = 23) and advanced (Hoehn&Yahr III-IV, n = 18) subgroups. 18F-FP-CIT PET was performed in these patients and 12 normal subjects. 18F-FP-CIT uptake in striatal subregions and its correlation with UPDRS were first evaluated by ROI analysis, and between-group differences were also analyzed by Statistical Parametric Mapping (SPM). Our results showed that striatal 18F-FP-CIT binding were significantly reduced to 70.9% (caudate), 46.8% (anterior putamen) and 24.0% (posterior putamen) in early PD compared with that of the control, and to 52.0%, 34.5% and 16.5% correspondingly in advanced PD, respectively. There was significant negative correlation between total motor UPDRS score of all parkinsonian patients and 18F-FP-CIT uptake in caudate nucleus (r = −0.53, p < 0.001), anterior putamen (r = −0.53, p < 0.001) and posterior putamen (r = −0.61, p < 0.001). SPM comparison of 18F-FP-CIT uptake between early or advanced PD and the control group showed significant decline in striatum, predominantly localized on the contralateral side and in the dorsal-posterior putamen. These results indicate that 18F-FP-CIT PET can serve as a suitable biomarker to represent the severity of PD in early and advanced stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past decade, in vivo imaging of the nigrostriatal dopaminergic system offered the opportunity of being an objective method of measuring the severity and progression of PD. Dopaminergic dysfunction in PD has been visualized with positron emission tomography (PET) or single photon emission computed tomography (SPECT) by using various tracers [1–6]. Among them, two major approaches were used. 18F-dopa uptake in the striatum reflects the density of striatal dopaminergic terminals and the conversion of 18F-dopa to and storage as 18F-dopamine. However, it has been suggested that 18F-dopa assessment might overestimate the number of striatal dopaminergic nerve terminals in early stage of PD, because the loss of dopaminergic synapses is partially compensated for by increased dopamine turnover in the surviving terminals [2, 7–10]. An alternative radiotracer binding target is dopamine transporter (DAT), which has been visualized by 11C-cocaine [11], 11C-RTI-32 [12], 11C-CFT [13], 18F-CFT [14] and 18F-FP-CIT [5]. FPCIT is a highaffinity cocaine analog binding specifically to DAT. When it is labeled by 18F, it has fast kinetics and high signal-to-noise ratios and is suitable for PET neuroimaging [5,15,16]. Previous work indicated that DAT imaging might be more sensitive in detecting early stage of PD than 18F-dopa imaging [7, 17]. There has been, however, some controversy over whether DAT imaging might be used as a biomarker for assessing the severity and progression of PD, and for evaluating the efficacy of neuroprotective treatments [3, 18–20]. In longitudinal studies, DAT ligands with both PET and SPECT have demonstrated no significant correlation between the reduction in striatal DAT and the change in clinical severity in PD [19]. In cross-sectional studies, on the other hand, DAT levels were inversely correlated with the severity of motor dysfunction in early PD [3, 21].
The aim of the present study was to investigate the usefulness of 18F-FP-CIT PET for assessing severity of PD at various clinical stages, and to provide more validation on this imaging modality for the biomarker of the severity of PD.
Subjects and Methods
Patients
Forty one patients with idiopathic PD at various disability stages were enrolled. All were over 30 years old and met step I and step II criteria according to the PD society brain bank [22]. They were divided into 2 subgroups according to the Hoehn&Yahr (HY) stage: (1) 23 patients with early PD (56.2 ± 8.1 years old, 2.2 ± 1.4 years duration [mean ± SD], HY I-II). Of these 23 patients, 4 patients were less than 50 years old and defined as early-onset PD, 13 patients were rated stage I and 10 stage II. Thirteen initially had a favorable response to levodopa therapy and were taking levodopa(321 ± 226 mg), the other 10 patients were receiving dopamine agonists, amantadine, selegiline, or anticholinergic medications. (2) 18 patients with advanced PD (59.1 ± 7.6 years old, 6.9 ± 3.3 years duration, HY III-IV). Of them, 3 patients were early-onset PD, 7 patients were rated stage III and 11 stage IV. All of the 18 patients were on treatment with levodopa(492 ± 246 mg) with a favourable response initially, together with dopamine agonists, amantadine, selegiline, or anticholinergic medications in various combinations. The two groups were comparable in the age distribution and clinical features(subscores in bradykinesia, rigidity and tremor).
The severity of motor symptoms was evaluated using the motor part of the Unified Parkinson’s Disease Rating Scale (UPDRS) just before PET scanning when the patients were considered as “off medication” after antiparkinsonian drugs were discontinued for at least 6 hours.
The control group consisted of 12 neurologically normal and age-matched volunteers (58.8 ± 7.2 years). This study was approved by the Ethics Committee of Huashan Hospital and a written informed consent was obtained from each subject.
Radiochemistry and PET Imaging
18F-FP-CIT was synthesized by electrophilic fluorination of FP-CIT(N-3–fluoropropyl-2-β-carboxymethoxy-3-β-(4-iodophenyl) nortropane) (National Key Laboratory of Nuclear Medicine, Wuxi, Jiangsu, P.R.China) according to the method reported previously [5]. The preparative separation was made with high performance liquid chromatography, and the fraction containing the product was evaporated to dryness. Finally, the residues were dissolved in phosphate buffered saline (pH 7) and sterile filtrated. The radiochemical purity was better than 98%.
A whole body PET scanner (ECAT EXACT HR plus, Siemens) was used to simultaneously collect 63 imaging planes with a 3D resolution of 4.4 mm full width at half maximum (FWHM) with filtered back projection (FBP). Prior to the emission scan, each patient underwent a transmission scan for 10 minutes using a removable gallium-68 ring source to correct for photon attenuation. On average, 148 MBq (4.0 mCi) of 18F-FP-CIT was injected intravenously. The scan duration of each frame was 10–15 mins. The data were acquired in 3D mode.
The regions of interest (ROI) were identified and drawn on the caudate, putamen, and cerebellum in each hemisphere on MR images (1.5 T) realigned according to the PET image using the surface fitting method [23]. The four adjacent slices where the striatum was best seen was used for ROI analysis. The putamen was divided into anterior and posterior halves along its longitudinal axis. The ROIs were then copied onto the PET image and the uptake of 18F-FP-CIT was calculated as a (region-cerebellum)/cerebellum ratio at 120 minutes after injection.
Statistical analysis
The mean 18F-FP-CIT uptake of left and right hemispheric structures were averaged before performing statistical analysis. The 18F-FP-CIT uptake in parkinsonian patients at early stage and advanced stage was judged relative to control values using ANOVA. The correlation between UPDRS and 18F-FP-CIT uptake in striatal subregions was evaluated by calculating Pearson correlation coefficients using SPSS. P-values less than 0.05 was considered statistically significant.
In addition, we used statistical parametric mapping software (SPM99, Wellcome Department of Cognitive Neurology, London, UK) in MATLAB 5.3 (MathWorks, Natick, MA, USA) to perform between-group comparisons. Before statistical analysis the images of parkinsonian patients were reoriented so that the striatum contralateral to the symptomatic side was always on the left of the brain. Each individual PET scan was normalized into a standard stereotactic space [24], using the published brain template for 18F-FP-CIT binding [25]. The images were then smoothed using an isotropic 10-mm kernel to account for variation in striatum and individual variability in structure-function relationships. 18F-FP-CIT binding difference was examined for all voxels over the whole brain and appropriate linear contrasts were used to derive the between-group (unpaired) t statistic. The SPM{t}s were then transformed to the unit normal distribution (SPM{Z}) and converted to Z scores. Only the regions which exceeded a threshold of Puncorrected < 0.05 (Z score > 3.1) were accepted as significant. This was acceptable as our study was considered hypothesis driven in order to confirm binding deficits in the striatum.
Results
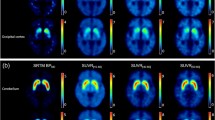
We found significant reduction in DAT binding in the caudate and putamen of PD patients as compared with those in healthy controls (Fig. 1, Table 1). In 23 patients with early PD (H & Y Stage 1.43 ± 0.51; motor UPDRS rating 22.0 ± 7.0), 18F-FP-CIT binding was significantly reduced (p < 0.01) to 70.9% (caudate), 46.8% (anterior putamen) and 24.0% (posterior putamen) of the normal mean. In 18 patients with advanced PD (H & Y Stage 3.61 ± 0.5; motor UPDRS rating 36.7 ± 7.4), striatal 18F-FP-CIT binding was significantly decreased (p < 0.01) to 52.0%, 34.5% and 16.5% respectively of the corresponding subregions in the control group. This gave rise to relative 18F-FP-CIT binding reductions between advanced and early PD of 18.9%, 12.3% and 7.5% in the caudate, anterior and posterior putamen (p < 0.01).
Images of 18F-FP-CIT uptake at the level of striatum in a healthy control and two PD patients at various stages. A: healthy control, 18F-FP-CIT PET image overlaid on an MR image; B: healthy control; C: early PD with HY stage I; D: advanced PD with HY stage IV. PD patients display progressive losses of striatal DAT binding over time compared to healthy controls
There was significant negative correlation (see Fig. 2) between total motor UPDRS scores in all 41 patients with PD and 18F-FP-CIT uptake in the caudate nucleus (r = −0.53, p < 0.001), anterior putamen (r = −0.53, p < 0.001) and posterior putamen (r = −0.61, p < 0.001), indicating that the more pronounced the disability, the lower the 18F-FP-CIT uptake in each striatal subregion.
SPM analysis showed significant decline of 18F-FP-CIT uptake in early (Fig. 3A) PD relative to the control group (p < 0.01) and advanced PD (Fig. 3B) comparable with the control group (p < 0.01). The reductions occurred in both the contralateral and ipsilateral striatum, localized predominantly on the contralateral side and in the dorsal-posterior putamen (Table 2). There were no regions with increased 18F-FP-CIT binding in PD patients.
Discussion
By means of ROI and SPM analyses in this study, we showed that 18F-FP-CIT uptake was significantly decreased in the parkinsonian striatum, with the greatest loss in the posterior putamen contralateral to the more affected limbs. Additionally 18F-FP-CIT uptake in each striatal subregion was negatively correlated with motor UPDRS score in PD patients with various clinical severity, indicating that it might be used as a suitable biomarker to represent the motor disability of PD.
ROI method was first used in data analysis of this study. We drew ROIs by coregistering each patient’s individual MRI to their DAT PET scan. This process not only improved the accuracy of ROI placement, but also allowed separation of the putamen into its anterior and posterior parts where DAT binding is severely reduced in advanced PD. In healthy control, the uptake ratio of 18F-FP-CIT in caudate, anterior putamen and posterior putamen was comparable quantitatively with those reported in Rinne’s [3], Ma’s [16] and Carbon’s [26] research. In the patients with PD in the present study, considering that the age difference between early and advanced PD was 2.9 years, the annual decline of 18F-FP-CIT binding ratio would be consistent with rates of progression from Nurmi’s finding [19]. Nevertheless, it is difficult to compare the exact value of DAT binding ratio in different studies on the nigrostriatal hypofunction in PD, since the use of tracers, cameras, analytical approaches, and patient populations vary between the studies.
SPM analysis was also performed in our study, it can overcome the difficulty of ROI method in distinguishing the striatal boundaries on putamen simply on the basis of radioactivity distribution. By means of voxel-based SPM we delineated the uneven loss of DAT binding in the striatum, confirming that the posterior putamen was the most severely affected striatal subregion showing the highest correlation with clinical assessment, followed by the anterior putamen and caudate nucleus. These results were compatible with many functional neruoimaging studies with several radiotracers for DAT [3, 16]. Furthermore, postmortem studies showed that the degeneration of dopamine neurons occurs first in the pars compacta of ventrolateral substantia nigra projecting to the posterior putamen. When the disease advances, the dysfunction of nigrostriatal projections spreads toward more anterior and ventral putamenal areas, while the projections to caudate nucleus are relatively spared.
We also demonstrated significant correlation between motor UPDRS score and striatal DAT binding, in agreement with previous cross-sectional PET [3, 5, 17] and SPECT neuroimaging studies [21, 27, 28]. In other PET studies, however, Ma [16] and Antonini [29] did not find significant correlation between 18F-FP-CIT or [11C]FE-CIT putamen uptake and UPDRS motor scores, as most of their patients were at an early clinical stage and little variability was present in the motor scores. The outcome of the present study may be more convincing since the HY stages of our parkinsonian patients have overcome the drawback of narrow range of disease severity in these previous cross-sectional reports [3, 16, 29]. If few apparent outliers in Fig. 2, assumed to be due to early onset before 45 years old, were removed from the analysis, the correlation between the motor UPDRS and 18F-FP-CIT binding in striatal subregions seems to be more significant, implying a better value of 18F-FP-CIT served as a biomarker for PD.
Although the results of our cross-sectional study support 18F-FP-CIT as a biomarker to describe the severity of PD, whether it can document the longitudinal progression of PD remains to be seen and some criticism has even been put forward. In Nurmi’s longitudinal study, there was no significant correlation between the change in the UPDRS total motor or bradykinesia score and the change in the [18F]CFT uptake ratio in any striatal subregion studied [19]. It has also been suggested by Morrish [30] and Ravina [31] that DAT imaging could not be used as a surrogate endpoint in clinical trials of PD. The disparity between the cross-sectional and longitudinal studies seems to be due to the fact that the decrease in DAT binding in follow-up of PD could be influenced by the confounding effect of anti-parkinsonian medications, age on onset, non-linear progression rate of the disease, or by the length of the imaging interval. Analyzing the longitudinal data of these patients in this paper will be the subject of further studies, and multiple endpoints of progression will be required in the future research to fully clarify the clinical course of PD.
References
Booij J, Habraken JB, Bergmans P, et al. (1998) Imaging of dopamine transporters with iodine-123-FP-CIT SPECT in healthy controls and patients with Parkinson’s disease. J Nucl Med 39:1879–1884
Ribeiro MJ, Vidailhet M, Loc’h C, et al. (2002) Dopaminergic function and dopamine transporter binding assessed with positron emission tomography in Parkinson disease. Arch Neurol 59:580–586
Rinne JO, Ruottinen H, Bergman J, et al. (1999) Usefulness of a dopamine transporter PET ligand [(18)F]beta-CFT in assessing disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry 67:737–741
Morrish PK, Rakshi JS, Bailey DL, et al. (1998) Measuring the rate of progression and estimating the preclinical period of Parkinson’s disease with [18F]dopa PET. J Neurol Neurosurg Psychiatry 64:314–319
Kazumata K, Dhawan V, Chaly T, et al. (1998) Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med 39:1521–1530
Kung HF, Kim HJ, Kung MP, et al. (1996) Imaging of dopamine transporters in humans with technetium-99 m TRODAT-1. Eur J Nucl Med 23:1527–1530
Huang WS, Chiang YH, Lin JC, et al. (2003) Crossover study of (99 m)Tc-TRODAT-1 SPECT and (18)F-FDOPA PET in Parkinson’s disease patients. J Nucl Med 44:999–1005
Rinne OJ, Nurmi E, Ruottinen HM, et al. (2001) [(18)F]FDOPA and [(18)F]CFT are both sensitive PET markers to detect presynaptic dopaminergic hypofunction in early Parkinson’s disease. Synapse 40:193–200
Tedroff J, Ekesbo A, Rydin E, et al. (1999) Regulation of dopaminergic activity in early Parkinson’s disease. Ann Neurol 46:359–365
Ishikawa T, Dhawan V, Kazumata K, et al. (1996) Comparative nigrostriatal dopaminergic imaging with iodine-123-beta CIT-FP/SPECT and fluorine-18-FDOPA/PET. J Nucl Med 37:1760–1765
Wang GJ, Volkow ND, Fowler JS, et al. (1995) Comparison of two pet radioligands for imaging extrastriatal dopamine transporters in human brain. Life Sci 57:L187-L191
Guttman M, Burkholder J, Kish SJ, et al. (1997) [11C]RTI-32 PET studies of the dopamine transporter in early dopa-naive Parkinson’s disease: implications for the symptomatic threshold. Neurology 48:1578–1583
Ilgin N, Zubieta J, Reich SG, et al. (1999) PET imaging of the dopamine transporter in progressive supranuclear palsy and Parkinson’s disease. Neurology 52:1221–1226
Laakso A, Bergman J, Haaparanta M, et al. (1998) [18F]CFT [(18F)WIN 35,428], a radioligand to study the dopamine transporter with PET: characterization in human subjects. Synapse 28:244–250
Robeson W, Dhawan V, Belakhlef A, et al. (2003) Dosimetry of the dopamine transporter radioligand 18F-FPCIT in human subjects. J Nucl Med 44:961–966
Ma Y, Dhawan V, Mentis M, et al. (2002) Parametric mapping of [18F]FPCIT binding in early stage Parkinson’s disease: a PET study. Synapse 45:125–133
Eshuis SA, Maguire RP, Leenders KL, et al. (2006) Comparison of FP-CIT SPECT with F-DOPA PET in patients with de novo and advanced Parkinson’s disease. Eur J Nucl Med Mol Imaging 33:200–209
Nurmi E, Ruottinen HM, Kaasinen V, et al. (2000) Progression in Parkinson’s disease: a positron emission tomography study with a dopamine transporter ligand [18F]CFT. Ann Neurol 47:804–808
Nurmi E, Bergman J, Eskola O, et al. (2003) Progression of dopaminergic hypofunction in striatal subregions in Parkinson’s disease using [18F]CFT PET. Synapse 48:109–115
Marek K, Innis R, van Dyck C, et al. (2001) [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson’s disease progression. Neurology 57:2089–2094
Benamer HT, Patterson J, Wyper DJ, et al. (2000) Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 15:692–698
Hughes AJ, Daniel SE, Kilford L, et al. (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Pellizari CA, Chen GTY, Spelbring DR (1989) Accurate three-dimensional registration of CT, PET and/or MR images of the brain. J Comput Assist Tomogr 13:20–26
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. Thieme, Stuttgart
Ma Y, Dhawan V, Spetsieris P, et al. (2002) Comparison of Mapping Parameters in 18F-FPCIT imaging of early stage Parkinson’s disease. In: Michio Senda, Yuichi Kimura, Peter Herscovitch (eds) Brain imaging using PET. Academic Press, San Diego, pp 277–283
Carbon M, Ma Y, Barnes A, et al. (2004) Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neuroimage 21:1497–1507
Asenbaum S, Brucke T, Pirker W, et al. (1997) Imaging of dopamine transporters with iodine-123-beta-CIT and SPECT in Parkinson’s disease. J Nucl Med 38:1–6
Seibyl JP, Marek KL, Quinlan D, et al. (1995) Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann Neurol 38:589–598
Antonini A, Moresco RM, Gobbo C, et al. (2001) The status of dopamine nerve terminals in Parkinson’s disease and essential tremor: a PET study with the tracer [11-C]FE-CIT. Neurol Sci 22:47–48
Morrish PK (2003) How valid is dopamine transporter imaging as a surrogate marker in research trials in Parkinson’s disease? Mov Disord 18(Suppl 7):S63-S70
Ravina B, Eidelberg D, Ahlskog JE (2005) The role of radiotracer imaging in Parkinson disease. Neurology 64:208–215
Acknowledgement
We thank Dr. Yilong Ma at North Shore University Hospital in New York for his critical review of this manuscript and for gracefully providing a 3D stereotactic PET brain template for 18F-FP-CIT binding. We also gratefully acknowledge the financial supports from China’s National Key Technologies R&D Program in the 10th five-year plan (2001BA702B02) and Key Research Grants of Health Department in Jiangsu Province (H2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Received in revised form: 11 June 2006
Rights and permissions
About this article
Cite this article
Wang, J., Zuo, CT., Jiang, YP. et al. 18F-FP-CIT PET imaging and SPM analysis of dopamine transporters in Parkinson’s disease in various Hoehn & Yahr stages. J Neurol 254, 185–190 (2007). https://doi.org/10.1007/s00415-006-0322-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0322-9