Abstract
In the Australian state of Victoria, all fatalities that were recorded from 2002 through to 2008 involving the use of certain serotonin active drugs (tramadol, venlafaxine, fluoxetine, sertraline, citalopram and paroxetine), were reviewed to assess the incidence of contraindicated or ill advised drug combinations. More than 1,000 were identified of which 326 cases formed the basis of this study. These cases involved contraindicated or inappropriate drug combinations that can lead to adverse drug reactions (ADRs) and subsequent fatal toxicity. Of these, 46% were drug-related, 35% were a result of natural disease and 13% were classified as external injury cases. The remaining cases were those where the cause of death (COD) was unascertained. Tramadol was the most common drug, usually detected alongside a serotonergic antidepressant (in 20% of cases). Twenty-five (8%) cases involved contraindicated drug combinations while the remainder (301 cases, 92%) involved drug combinations that are associated with adverse interactions ranging from minor to major severity. Of these 326 cases, the Coroner determined 166 cases (51%) to be acts of intentional self-harm or drug misuse, with the remainder unascertained or attributed to natural disease. Very few post-mortem reports and Coroners’ findings made mention of possible ADRs when such combinations were actually present. The majority of cases comprising contraindicated drug combinations involved the combined use of five drugs (24%) at the time of death. A combination of three to five drugs was most common in cases involving inadvisable drug combinations. Combined drug toxicity was the most common COD, with heart disease the most common co-morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Each year, at least 1.5 million Australians suffer an adverse drug reaction (ADR) following the use of the wrong medicine or the incorrect drug dosage, resulting in at least 400,000 visits to general practitioners and 140,000 hospital admissions [1]. This ultimately increases morbidity and mortality and community healthcare costs [2].
There are a wide range of over-the-counter (OTC) and prescription medications, herbal products and drugs of abuse that are serotonin-active and commonly reported in ADRs. When co-administered, many of these are capable of producing adverse events, most notably potentially fatal serotonin toxicity, which is caused by the accumulation of serotonin in the central nervous system (CNS) [3–6]. Serotonin-active drugs are therefore of considerable interest with regards to inappropriate drug combinations.
Compendial lists of available drugs in Australia include advice concerning contraindicated agents where use could result in a life-threatening situation [7, 8]. References of this nature also document where co-administration is inadvisable because of a known interaction, indicating that caution should be used with concomitant use. Despite these warnings, patients are often co-prescribed medications which are associated with the potential for severe drug interactions [2].
Numerous studies have investigated the impact of co-prescribing contraindicated pharmaceuticals, however most focus purely on higher risk populations such as the elderly [9, 10], or interactions involving a specific drug such as cisapride or the statins [11, 12]. More recently, inappropriate prescribing of serotonergic drugs in Australian veterans has been examined, with results indicating potentially toxic co-prescriptions in up to 21% of veterans [13]. In another study, it was found that 8% of the veterans (20,658 individuals) had been prescribed multiple serotonergic drugs on at least one occasion [14], with many contraindicated prescriptions dispensed on the same day. Few studies have specifically examined the involvement of serotonergic drugs in a wide scale study of forensic investigation. The aim of this study was to therefore review cases reported to the Victorian State Coroner over a seven-year period, in order to examine the incidence of inappropriate prescribing patterns involving serotonergic drugs.
Methods
Cohort selection
The cohort of deceased cases used in this research was obtained from a wider study examining 1,123 deaths involving the serotonergic drugs fluoxetine, sertraline, citalopram, paroxetine, venlafaxine, tramadol and 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) [15]. Cases involving the use of MDMA and no other drugs of interest (n = 87) were excluded from the cohort since this research focused upon prescription drugs only. The drugs of interest (fluoxetine, sertraline, citalopram, paroxetine, venlafaxine and tramadol) are hereinafter referred to as the ‘target drugs’.
The National Coroners Information System (NCIS) is a national Internet-based data storage and retrieval system containing information including pathology, toxicology and Coroners’ findings for every death reported to an Australian Coroner since July 2000 (January 2001 for the state of Queensland). The NCIS was used to retrieve all completed Victorian cases investigated between January 2002 and December 2008, where at least one of the target drugs was detected in post-mortem blood.
Using information from Drugdex® Evaluations in the Micromedex® Internet database, a list was compiled of drugs which interact with the target drugs. These drug interactions were classified as ‘contraindicated’, ‘major’, ‘moderate’ or ‘minor’ drug interactions, as specified by the severity rating and interaction effect listed on the database (Table 1) [8]. ‘Major’ interactions were those where combination of the drugs was likely to cause a potentially fatal toxic reaction, such as tramadol and fluoxetine induced serotonin toxicity. ‘Moderate’ interactions were those where a potentially toxic drug interaction was possible, such as sertraline and lithium, leading to increased lithium concentrations and/or an increased risk of serotonin toxicity. ‘Minor’ interactions were those where combination of the drugs may have led to a non-life-threatening reaction, such as fluoxetine with diazepam, leading to higher serum concentrations of diazepam. Although a number of factors can increase or decrease the risk of an ADR when using these drugs (e.g., genetic predisposition, diet and disease), the presence of the drug combinations was considered the foremost risk and these other factors were taken into consideration as contributive factors.
There were some drug combinations that have been associated with an ADR (such as serotonin toxicity) and were not listed on Micromedex®, such as pethidine with tramadol [16], or mirtazapine with sertraline [17, 18]. Despite controversy regarding the serotonergic potential of mirtazapine [19], it is listed in Micromedex® as a major drug interaction when combined with fluoxetine and venlafaxine due to the increased risk of serotonin toxicity. Considering the potent serotonergic activity of sertraline even when compared with fluoxetine, and the pro-serotonergic activity of mirtazapine with fluoxetine or venlafaxine, this drug combination was listed as a moderate drug interaction [20]. Similarly, the combination of methadone with selective serotonin reuptake inhibitors (SSRI) can increase methadone concentrations as a result of the CYP450 enzyme inhibitory action of these antidepressants, in particular fluoxetine and paroxetine [21, 22]. These drug interactions were therefore included in the table as possible drug interactions. If there was a combination of two SSRIs or serotonin noradrenaline reuptake inhibitors (SNRI), this was considered to be a moderate drug interaction risk, and if there were three, or a tricyclic antidepressant (TCA) was additionally involved, it was deemed a major risk. Using this drug interaction classification system, the cases could be ranked according to the risk of an ADR.
Drugs administered during emergency treatment were excluded from the analysis since these were unlikely to play a role in the cause of death (COD) or be involved with the underlying reason of admission for treatment.
Toxicological analysis
In Victoria, all deaths reported to the Coroner receive a full medico-legal death investigation which includes a comprehensive review of all available medical records, an autopsy and toxicological analysis (in most cases) in order to screen for the presence of alcohol, drugs of abuse, common prescription drugs and OTC drugs. Preliminary drug screens used a semi-quantitative gas-chromatographic method [23] for a variety of common drugs and a conventional immunoassay on urine (CEDIA) and/or blood (ELISA) for drugs of abuse such as amphetamines, benzodiazepines, cannabis, cocaine and opiates (Thermofisher®). In most cases, post-mortem blood was collected from the femoral vein in order to minimize redistribution [24]. Identified drugs were subsequently confirmed using quantitative gas chromatography–mass spectrometry with lower limits of quantitation at the low end of their respective blood concentrations when used therapeutically.
Case analysis
The cases of interest, where a drug combination was detected that is associated with a known interaction, were categorized into four main groups. Group A were cases where the target drugs were detected in combination with contraindicated drugs. Groups B, C and D were cases where the target drugs were detected in combination with other drugs that can cause a major, moderate or minor adverse drug interaction, respectively, and thus should only be co-prescribed with caution (Table 1).
The groups were then sub-divided (Groups A1–A5, B1–B5, etc.) depending on the case circumstances, COD, associated drugs and probability of ADR (according to the Micromedex® classifications), so that the prevalence of contraindicated and inadvisable drug combinations and the likelihood of an ADR having contributed to the COD could be evaluated (Table 2). For example, Group A1 classification (accidental drug-related) was denoted to cases where a combination of a target drug with one or more contraindicated drugs was detected and circumstances suggested full medication compliance and no drug misuse. If an illicit drug was also detected or the circumstances suggested suicide (e.g., exceptionally high drug concentrations or where a suicide note was found), a Group A2 classification was assigned. Deaths that were not directly caused by drug use (i.e., natural disease or external injury), were included in the study since the focus of investigation was the incidence and combinations (and concentrations) of drugs present.
Many cases involved incidental concentrations of drugs that were not regarded as a significant contribution to the COD. However, in cases involving contraindicated or inadvisable combinations of drugs, their contributive role in causing an ADR, regardless of the concentration, was considered.
The concentrations of all drugs of abuse in post-mortem cases are potentially affected by redistributive processes following post-mortem disruption of cellular membranes. This post-mortem redistribution causes the greatest variations in post-mortem drug concentrations for drugs with high lipid solubility or blood taken from non-peripheral regions such as the heart. Methamphetamine, for example, shows two-fold increases in heart blood specimens compared with femoral blood, whilst methadone shows increases of up to four-fold. Adversely, drugs with low volumes of distribution, such as benzodiazepines, show relatively minor changes in the post-mortem period [23]. The sample site, post-mortem interval and drug(s) involved were therefore carefully considered in each case to assess the likely extent of redistribution and its influence on drug concentrations and effects.
Statistical analysis
Statistical comparisons between the cases of interest were determined using one-way ANOVA tests and Games–Howell post-hoc tests with the SPSS package (software release Version 17.0), where p < 0.05 was considered statistically significant. The F ratio, degrees of freedom, outcome and significance values are reported.
Ethical review
The research study was approved by the Victorian Institute of Forensic Medicine Ethics Committee and the Department of Justice Human Research Ethics Committee.
Results
Basic characteristics
Out of 14,682 reported Coroners cases that underwent full toxicological testing during 2002 to 2008, a total of 1,036 relevant cases involving the six target drugs were identified. Twenty-five cases involved contraindicated pharmaceuticals (Group A) whilst 301 cases involved drug combinations that were inadvisable with known adverse drug interactions (Groups B, C and D) (Table 3). These cases were subsequently sub-grouped according to COD as classified by the Coroner. The remaining 710 cases were excluded from further investigation due to involvement of drug combinations not known to cause adverse events (thus considered inherently safe when co-prescribed) or illicit drug use.
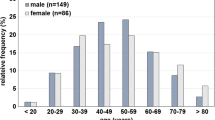
Ages ranged from 15 to 94 years (median 46 years), with a higher incidence of older individuals in the natural disease deaths and younger individuals in the accidental drug-caused deaths, F (9,316) = 4.706, p < 0.05. There were no statistically significant differences in the number of males across groups, F (9,316) = 0.981, p > 0.05.
Toxicology
Sertraline and fluoxetine were the most common drugs identified amongst cases in Group A (contraindicated drug combinations) and were detected in combination with moclobemide or metoclopramide. Tramadol was detected most commonly in Groups B and D, usually co-administered with a serotonin reuptake inhibitor (SRI), whilst citalopram was detected most commonly in Group C (Table 4). Apart from the target drugs, the majority of prescription drugs detected were associated with treatment of psychiatric conditions, chronic pain and cancer.
Some cases of non-accidental drug related deaths involved the use of illicit drugs such as heroin or amphetamines. In Groups B, C and D, there were six, four and one deaths, respectively, where death had occurred subsequent to heroin use. Amphetamines were detected in five Group B cases, five Group C cases and two Group D cases. There were no contraindicated drug deaths involving the use of illicit drugs.
There were many drug combinations detected in the sample (Table 3). The majority of Group A cases (18 cases) involved the combined use of between two to six drugs at the time of death. A combination of five drugs was most common in 20% of Group B cases. Analgesics such as paracetamol, naproxen, ibuprofen and codeine were among the most frequently detected drugs in two-thirds of Group A cases.
Although ADRs are associated with a wide range of drugs, the most common combinations identified within this cohort were serotonergic antidepressants with tramadol (amongst Group B cases) and multiple serotonergic medications such as SRIs with other SRIs or TCAs (amongst Group B and C cases) (Table 4).
Of the 160 cases attributed to natural, unascertained or accidental causes (i.e., no drug misuse or intentional self-harm), there were 24 cases (15%) involving prescription drug concentrations above the therapeutic range (following careful consideration of post-mortem redistribution). SSRIs, tramadol and venlafaxine were frequently involved in these 24 cases (five, 20 and three cases, respectively, with multiple drugs detected in some cases).
Pathology
The most common CODs are listed in Table 3. Combined drug toxicity was the most common COD type across all four groups. Of the 12 cases in Table 5 (accidental deaths) and 19 cases in Table 6 (unascertained CODs), 21 cases received a full autopsy. The most common co-morbidities in the accidental deaths (Table 5) were cardiovascular in nature (seven out of eight natural disease cases), predominantly coronary artery disease (four cases) and cardiomegaly (two cases). It must be noted that many cardiovascular drugs are not detected in routine toxicology testing (e.g., antihypertensive agents, antiarrhythmic drugs and medications for congestive heart disease). Liver pathology was also noted in the form of fatty liver (two accidental cases and two unascertained cases), hepatitis C and cirrhosis (case 31). Nephrosclerosis was also found in case 5.
Overall, 166 cases from 326 cases in Groups A through to D involved intentional self harm and/or the misuse of illicit or licit drugs. The remaining 160 cases (49%) demonstrated no suicidal intent or misuse of drugs, with causes of death remaining unknown in 19 cases and attributed to natural disease in 116 cases. Fifteen cases involved a contribution of drugs with natural disease (seven Group B, one Group C and seven Group D cases).
Cases of most interest
There were five Group B1 cases and seven Group D1 cases where circumstances did not suggest drug overdose but where drug toxicity was regarded as the most likely COD (Table 5). In these cases, the coroner determined that external injury, natural disease and drug misuse were not involved, but inappropriate drug combinations were present which could have caused death.
Deaths with an unascertained COD within groups A5–D5 involved contraindicated or inadvisable combinations of drugs, which were at concentrations not usually associated with misuse. The case circumstances supported compliance to medication with no indication of suicidal intent following a comprehensive medico-legal death investigation, including autopsy in most cases (12 of 19 cases) (Table 6).
Certain drug combinations in the drug related deaths (Table 5) were also detected amongst unascertained deaths (Table 6). However, the involvement of these particular drugs was not reported. For example, detection of tramadol alongside an antidepressive agent acting upon serotonin (such as an SSRI, mirtazapine or tricyclic antidepressant [TCA]) was reported as a death by multiple drug toxicity in cases 1 through to 4, but not amongst cases involving detection of this same combination (cases 13 through to 20). Similarly, cases 6 through to 12 reported drug toxicity with the combination of a SRI with methadone, along with incidental concentrations of other drugs, but drug toxicity was not reported with cases concerning the same drug combination (cases 27 through to 30).
Discussion
Drug prescribing involves the consideration of many factors, including appropriate choice of drug, accurate dosage, the correct diagnosis by taking a comprehensive patient history and determining any co-morbidities, allergies and current drug use (including prescription medicines as well as non-prescription medicines and recreational drugs). The patient must then be monitored to achieve the desired outcome with minimal side effects [25]. Accidental or intentional misuse of medication by patients and the erroneous substitution of drugs due to similar sounding drug names (i.e., Celebrex® [celecoxib] and Cerebyx® [fosphenytoin]) can occur. Prescription errors are therefore not uncommon [26].
In the present study, cases were categorized according to whether the combinations of drugs detected were either contraindicated or inadvisable because of associated adverse reactions. Three hundred and twenty-six (31%) of the 1,036 retrieved cases (Groups A through to D) involved potentially inappropriate administration or use of the detected drug(s) and thus many of these deaths may have been avoidable. All cases involved the use of two or more prescribed drugs that are associated with various degrees of drug interactions when administered concomitantly (including 20 which also involved the use of one or more illicit drugs). Although a considerable number of cases involved intentional self harm and/or clear misuse of drugs, the majority of cases revealed no suicidal intent or use of illicit drugs. Indeed, the COD in most of these cases was either natural disease or unascertained. The possibility exists that in cases of natural disease, the involvement of drugs was masked or even overlooked once another likely COD had been established. Furthermore, in five of the cases (cases 5, 6, 19, 25, 31), liver disease in the form of Hepatitis C, cirrhosis or fatty liver, was reported, in addition to one case of nephrosclerosis (case 5), which may have impaired drug metabolism and consequently lead to changes in blood drug concentrations and subsequent toxicity [27, 28]. Research indicates that particularly in the older demographic, multiple co-morbidities are strong predictive factors for ADRs, especially cardiac and liver disease, diabetes and tumours [29]. It is therefore possible that cases in this cohort that involved co-morbidities such as cardiac, kidney or liver disease were predisposed to an increased risk of ADR and ensuing fatal outcome. Considering the drug combinations and concentrations noted amongst the unascertained cases, it is likely that a number of these deaths were in fact drug related and caused by synergistic adverse drug effects.
Although the mean age across groups ranged from 40 to 53 years, many cases involving contraindicated drugs or inadvisable combinations were elderly individuals, with 23% of the 326 cases above 60 years of age. Generally, these findings are consistent with the literature, which report that suboptimal prescribing and consequential ADRs are more common in the elderly [9, 10]. Indeed, up to 30% of all Australian hospital admissions for patients aged 75 and above are medication related, of which nearly 75% are potentially preventable [2]. Reasons for this increased risk include age-related changes in physiology which ultimately influence drug response [30], multiple health problems which may require numerous medications and high-risk combinations of drugs for effective control [10, 31], and accidental misuse of medication because of impaired visual or functional capacity [9, 32]. However, comparison with 2001 Australian data shows that rates of accidental drug-induced deaths are highest among young adults, peaking at 8 deaths per 100,000 persons. Between 60 and 84 years of age, the rates reduce to between 1 and 3 deaths per 100,000 persons, then sharply increase amongst the elderly aged 85 years and over, with 5 deaths per 100,000 persons reported [33]. This suggests that although ADRs are relatively common in the elderly when compared to other age groups, they are not usually the most common COD, with most elderly individuals dying from natural disease processes.
In addition to the inappropriate combinations observed, 15% of the apparently accidental deaths involved prescription drug concentrations above those normally consistent with therapeutic use. This may have resulted from erroneous prescribing by the treating physician or incorrect administration by the patients themselves. Considering the number of cases involving significant natural disease, it is also possible that these cases involving relatively high drug concentrations and inadvisable combinations were a result of overlooking drug–drug or drug–disease interactions, since inappropriate prescribing and subsequent ADRs are more common in the critically ill [25]. However, in cases involving drugs susceptible to the development of tolerance and neuroadaptation, increased administration to obtain the same therapeutic response may have resulted in higher drug concentrations [23]. This may have been the case for the many cases involving tramadol levels exceeding the range that is usually considered therapeutic. Furthermore, issues associated with toxicological analysis of post-mortem samples, such as redistribution, bacterial degradation and residual tissue enzyme activity, make the interpretation of analytical data difficult, particularly in relation to drugs such as tramadol that show differences in post-mortem concentrations depending on sample site and post-mortem interval [23, 34–36].
Simultaneous use of multiple pharmaceuticals, also referred to as polypharmacy, has been shown to drastically increase the risk of drug–drug and drug–disease interactions. In a hospital study, Goldberg and colleagues [37] found that the risk of ADRs rose exponentially from 13% for patients taking two medications up to 82% for patients taking seven or more medications simultaneously. Drug–disease interactions are related to differences in pharmacokinetics and pharmacodynamics in particular disease states, often in addition to physiological age related differences in the elderly. In this investigation, polypharmacy was very common at the time of death, with most cases involving four or more drugs. This raises issues of whether treating physicians were fully aware of each individual’s complete medication profile and whether all relevant (and potential) interactions were considered, particularly in elderly and critically ill patients.
Cases in our study involved a wide range of drugs, complicating the identification of increased risk patterns in cases of potential ADRs. However there was a dominance of certain combinations, particularly tramadol combined with a serotonergic antidepressant drug. The combination of tramadol with a CYP450 inhibiting serotonergic antidepressant, such as fluoxetine, can lead to reduced efficacy of tramadol and an increased risk of seizures, manic symptoms and potentially fatal serotonin toxicity [16, 17, 38–40]. Multiple serotonergic antidepressants (including SSRIs, SNRIs and TCAs) were also detected in a number of cases which, when used concomitantly, can increase the risk of fatal serotonin toxicity [3].
Numerous other cases involved the concomitant use of serotonergic antidepressants with methadone. Whilst ADRs involving multiple serotonergic antidepressants are well known [41–44], interactions between methadone therapy and serotonergic antidepressants are less documented. However, research indicates that the CYP450 inhibition of many serotonergic antidepressants (in particular fluoxetine and paroxetine) inhibits methadone metabolism, increasing plasma concentration and potentially leading to toxicity, especially in genetically or physiologically susceptible individuals [22, 45].
Increasingly, genetic variation in drug metabolising enzymes (particularly the CYP450 family) is being seen as a major contributor to differences in drug response and treatment outcome [46–48]. Studies indicate that a proportion of society is unable to effectively metabolise certain drugs (including a number of opioids, SSRIs and TCAs) because of genetic variations in CYP450 gene expression. This can lead to acute increases in drug concentrations and consequential drug toxicity with drugs such as fluoxetine [49], or alternatively a reduction in efficacy of prodrugs such as tramadol, which require metabolism to elicit therapeutic effects [50]. Similarly, ultra-rapid metabolizers may experience drug toxicity or inadequate therapeutic response, depending on the drug consumed [46, 51]. The clinical significance of these interactions however is widely disputed because of the potential contribution of other factors to drug outcome, such as sex, age, disease, diet and lifestyle [48]. These contributive factors mean that it is impossible to predict an outcome of drug treatment based purely on gene expression. However it may provide some explanation in cases where the death remains unascertained and the deceased was compliant to their medication, such as cases in Table 6 where many of the drugs involved are susceptible to variations in metabolic capability.
It is interesting to note that in numerous cases where the COD involved significant natural disease or external injury, drug involvement was not mentioned by the pathologist or Coroner, even though drug concentrations and combinations suggested at least some level of contribution to the COD. Furthermore, in other cases where similar drug concentrations or combinations are detected in the absence of natural disease or external injury, the COD is reported as drug-related. This raises the issue of whether drug related deaths are under-recognized and under-reported because other more obvious conditions are masking the contributory effects of drugs, or if these drug effects are neglected in the post-mortem report because a likely COD has already been established.
The primary limitation of this study is the presence of multiple drugs complicating interpretation of the influence, as well as possible interactions, of each individual drug, particularly when the degree of tolerance and an accurate history of drug intake prior to death are not certain [23, 52]. Interpretation of drug involvement in the non-accidental drug related deaths was complicated by certain cases which involved intentional self harm and/or the use of illicit drugs such as heroin or amphetamines, implying that the prescription drugs may have been deliberately misused. These factors were carefully considered as much as possible in the analysis, in addition to the influence of post-mortem redistribution in altering drug concentrations [23, 52–56]. The sample site and post-mortem interval were contemplated in each case when interpreting the toxicological results to account for higher drug concentrations in centrally or cardiac-derived blood compared with peripheral specimens and the potential for bacterial degradation altering drug concentrations in cases involving a longer post-mortem interval [23, 35]. For example, drug concentrations detected from centrally derived blood were considered to be elevated in cases 4, 23 and 30, compared with peripheral blood samples. A further limitation was inaccessible information such as clinical diagnoses and ante-mortem data (e.g., electrolyte balance and other chemistries) which would have been helpful in the interpretation of the drug-associated deaths.
Since mortality data was utilized in this investigation, the reported incidence of inappropriate prescribing patterns involving serotonergic agents should be interpreted with caution. There is a real possibility that a higher incidence of these prescribing events occurs in the general population since the individual patient may exhibit symptoms that do not result in death.
Conclusion
The present study demonstrates that contraindicated and inadvisable drug combinations involving serotonergic agents are detected relatively frequently in Victorian cases reported to the Coroner. Our data shows that adverse events involving serotonergic drugs may be associated with co-morbidity and polypharmacy with a variety of different drugs and combinations (particularly tramadol interacting with SSRIs and SNRIs, and the concomitant use of multiple serotonergic antidepressants). These results warrant a review of training and education within the medical profession in relation to encouraging safer prescribing and recognizing potential drug interactions involving serotonergic agents.
References
Roughead EE, Lexchin J (2006) Adverse drug events: counting is not enough, action is needed. Med J Aust 184(7):315–316
Runciman WB, Roughead EE, Semple SJ, Adams RJ (2003) Adverse drug events and medication errors in Australia. Int J Qual Health Care 15(Suppl 1):i49–i59
Bijl D (2004) The serotonin syndrome. Neth J Med 62:309–314
Birmes P, Coppin D, Schmitt L, Lauque D (2003) Serotonin syndrome: a brief review. CMAJ 168(11):1439–1442
Boyer EW, Shannon M (2005) The serotonin syndrome. N Engl J Med 352(11):1112–1120
Sternbach H (1991) The serotonin syndrome. Am J Psychiatry 148:705–713
Emims (1996–2010) www.mims.com.au (Accessed 2009–2010)
Micromedex® healthcare series [internet database] (1974–2010) http://www.thomsonhc.com.ezproxy.lib.monash.edu.au (Accessed 16 July 2010)
Tamblyn RM, McLeod PJ, Abrahamowicz M, Monette J, Gayton DC, Berkson L, Dauphinee WD, Grad RM, Huang AR, Isaac LM, Schnarch BS, Snell LS (1994) Questionable prescribing for elderly patients in Quebec. CMAJ 150(11):1801–1809
Hamilton H, Gallagher P, O’Mahony D (2009) Inappropriate prescribing and adverse drug events in older people. BMC Geriatr 9(1):5
Jones JK, Fife D, Curkendall S, Goehring E Jr, Guo JJ, Shannon M (2001) Coprescribing and codispensing of cisapride and contraindicated drugs. JAMA 286(13):1607–1609
Stang P, Morris L, Kempf J, Henderson S, Yood MU, Oliveria S (2007) The coprescription of contraindicated drugs with statins: continuing potential for increased risk of adverse events. Am J Ther 14(1):30–40
Roughead EE, Anderson B, Gilbert AL (2007) Potentially inappropriate prescribing among Australian veterans and war widows/widowers. Intern Med J 37(6):402–405
Ringland C, Mant A, McGettigan P, Mitchell P, Kelman C, Buckley N, Pearson SA (2008) Uncovering the potential risk of serotonin toxicity in Australian veterans using pharmaceutical claims data. Br J Clin Pharmacol 66(5):682–688
Pilgrim JL, Gerostamoulos D, Drummer OH (2010) Deaths involving serotonergic drugs. Forensic Sci Int 198(1–3):110–117
Gillman PK (2005) Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth 95(4):434–441
Houlihan DJ (2004) Serotonin syndrome resulting from coadministration of tramadol, venlafaxine, and mirtazapine. Ann Pharmacother 38(3):411–413
Hernandez JL, Ramos FJ, Infante J, Rebollo M, Gonzalez-Macias J (2002) Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother 36(4):641–643
Gillman PK (2006) A review of serotonin toxicity data: implications for the mechanisms of antidepressant drug action. Biol Psychiatry 59(11):1046–1051
Rahola JG (2001) Antidepressants: pharmacological profile and clinical consequences. Int J Psychiatry Clin Pract 5(1):19–28
Eap CB, Bertschy G, Powell K, Baumann P (1997) Fluvoxamine and fluoxetine do not interact in the same way with the metabolism of the enantiomers of methadone. J Clin Psychopharmacol 17(2):113–117
Begre S, von Bardeleben U, Ladewig D, Jaquet-Rochat S, Cosendai-Savary L, Golay KP, Kosel M, Baumann P, Eap CB (2002) Paroxetine increases steady-state concentrations of (r)-methadone in CYP2D6 extensive but not poor metabolizers. J Clin Psychopharmacol 22(2):211–215
Drummer OH (2004) Postmortem toxicology of drugs of abuse. Forensic Sci Int 142(2–3):101–113
Drummer OH (2007) Post-mortem toxicology. Forensic Sci Int 165(2–3):199–203
Buajordet I, Ebbesen J, Erikssen J, Brors O, Hilberg T (2001) Fatal adverse drug events: the paradox of drug treatment. J Intern Med 250(4):327–341
Shah S, Aslam M, Avery A (2001) A survey of prescription errors in general practice. Pharm J 267:860–863
Gleason OC, Yates WR, Philipsen MA, Isbell MD, Pollock BG (2004) Plasma levels of citalopram in depressed patients with hepatitis C. Psychosomatics 45(1):29–33
Henz S, Maeder MT, Huber S, Schmid M, Loher M, Fehr T (2008) Influence of drugs and comorbidity on serum potassium in 15 000 consecutive hospital admissions. Nephrol Dial Transplant 23(12):3939–3945
Zhang M, Holman CD, Price SD, Sanfilippo FM, Preen DB, Bulsara MK (2009) Comorbidity and repeat admission to hospital for adverse drug reactions in older adults: retrospective cohort study. BMJ 338:a2752
Mangoni A, Jackson S (2004) Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 57:6–14
Spinewine A, Schmader K, Barber N, Hughes C, Lapane K, Swine C, Hanlon J (2007) Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet 370:173–184
Woodhouse K, O’Mahony M (1997) Frailty and ageing. Age Ageing 26:245–246
Australian Bureau of Statistics (2003) Drug-induced death, Australia, 1991–2001. Canberra, Australia
Butzbach DM (2009) The influence of putrefaction and sample storage on post-mortem toxicology results. Forensic Sci Med Pathol 6(1):35–45
Barnhart FE, Bonnell HJ, Rossum KM (2001) Post-mortem drug redistribution. Forensic Sci Rev 13(2):101–129
Clarot F, Goulle JP, Vaz E, Proust B (2003) Fatal overdoses of tramadol: is benzodiazepine a risk factor of lethality? Forensic Sci Int 134(1):57–61
Goldberg RM, Mabee J, Chan L, Wong S (1996) Drug–drug and drug–disease interactions in the ED: analysis of a high-risk population. Am J Emerg Med 14(5):447–450
Gnanadesigan N, Espinoza RT, Smith R, Israel M, Reuben DB (2005) Interaction of serotonergic antidepressants and opioid analgesics: is serotonin syndrome going undetected? J Am Med Dir Assoc 6(4):265–269
Mahlberg R, Kunz D, Sasse J, Kirchheiner J (2004) Serotonin syndrome with tramadol and citalopram. Am J Psychiatry 161(6):1129
Launiainen T, Rasanen I, Vuori E, Ojanpera I (2010) Fatal venlafaxine poisonings are associated with a high prevalence of drug interactions. Int J Legal Med. doi:10.1007/s00414-010-0461-5
Dams R, Benijts TH, Lambert WE, Van Bocxlaer JF, Van Varenbergh D, Van Peteghem C, De Leenheer AP (2001) A fatal case of serotonin syndrome after combined moclobemide-citalopram intoxication. J Anal Toxicol 25(2):147–151
Marino MR, Langenbacher M, Ulderman HD (1996) Interaction of nefazodone and fluoxetine. Clin Pharmacol Ther 59:180
Martinelli V, Bocchetta A, Palmas AM, Del Zompo M (1993) An interaction between carbamazepine and fluvoxamine. Br J Clin Pharmacol 36(6):615–616
Bertschy G, Vandel S, Vandel B, Allers G, Volmat R (1991) Fluvoxamine-tricyclic antidepressant interaction. An accidental finding. Eur J Clin Pharmacol 40(1):119–120
Weschules DJ, Bain KT, Richeimer S (2008) Actual and potential drug interactions associated with methadone. Pain Med 9(3):315–344
Cascorbi I (2003) Pharmacogenetics of cytochrome p4502d6: genetic background and clinical implication. Eur J Clin Investig 33(Suppl 2):17–22
Gardiner SJ, Begg EJ (2006) Pharmacogenetics, drug-metabolizing enzymes, and clinical practice. Pharmacol Rev 58(3):521–590
Kollek R, van Aken J, Feuerstein G, Schmedders M (2006) Pharmacogenetics, adverse drug reactions and public health. Community Genet 9(1):50–54
Stipp D (2000) A DNA tragedy. Fortune 142(10):170–174, 178, 180 passim
Stamer UM, Musshoff F, Kobilay M, Madea B, Hoeft A, Stuber F (2007) Concentrations of tramadol and o-desmethyltramadol enantiomers in different cyp2d6 genotypes. Clin Pharmacol Ther 82(1):41–47
Gasche Y, Daali Y, Fathi M, Chiappe A, Cottini S, Dayer P, Desmeules J (2004) Codeine intoxication associated with ultrarapid cyp2d6 metabolism. N Engl J Med 351(27):2827–2831
Koski A (2005) Interpretation of postmortem toxicology results: pharmacogenetics and drug–alcohol interaction. PhD thesis, University of Helsinki, Helsinki, Finland
Skopp G (2004) Preanalytic aspects in postmortem toxicology. Forensic Sci Int 142(2–3):75–100
Levisky JA, Bowerman DL, Jenkins WW, Johnson DG, Karch SB (2001) Drugs in postmortem adipose tissues: evidence of antemortem deposition. Forensic Sci Int 121(3):157–160
Madea B, Musshoff F (2004) Postmortem toxicology. Forensic Sci Int 142(2–3):71–73
Wagner M (2003) Stability of drugs of abuse in biological specimens. MSc thesis, Columbia Pacific University
Sporer KA (1995) The serotonin syndrome. Implicated drugs, pathophysiology and management. Drug Saf 13(2):94–104
Gillman PK (2007) Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol 151(6):737–748
Gillman PK (1999) The serotonin syndrome and its treatment. J Psychopharmacol 13(1):100–109
Fisher AA, Davis MW (2002) Serotonin syndrome caused by selective serotonin reuptake-inhibitors–metoclopramide interaction. Ann Pharmacother 36(1):67–71
Bush E, Miller C, Friedman I (2006) A case of serotonin syndrome and mutism associated with methadone. J Palliat Med 9(6):1257–1259
Acknowledgements
We thank the National Coroners Information System and the State Coroner’s Office for their help in obtaining mortality data. We also acknowledge the assistance of pathologists, forensic technicians and toxicologists, at the Victorian Institute of Forensic Medicine. Thanks also to Dr. Steven Haas for his assistance in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pilgrim, J.L., Gerostamoulos, D. & Drummer, O.H. Deaths involving contraindicated and inappropriate combinations of serotonergic drugs. Int J Legal Med 125, 803–815 (2011). https://doi.org/10.1007/s00414-010-0536-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-010-0536-3