Abstract
The successful identification of the deceased is vital to the progress of any forensic investigation. One of the principal biological traits to be established from skeletal remains is the sex of the individual. This becomes more difficult if only parts of a skeleton are found or if the bones are compromised by physical insults such as fire, explosions or violence. The basal region of the occipital bone is covered by a large volume of soft tissue and is therefore in a relatively well-protected anatomical position, and as such, classification of sex using the occipital bone may prove useful in cases of significantly disrupted remains. The aim of this paper is to evaluate manually recorded morphometric variables of the region of the foramen magnum using both discriminant function analysis and linear regression. The skulls utilised in this study were selected from the eighteenth to nineteenth century documented skeletal collection of St. Bride’s Church, Fleet Street, London. Adult human skulls n = 158 (♂82/♀76) were measured to derive statistical functions. The results demonstrated that significant sexual dimorphism is present in the cranial base of the St. Bride’s material. The correctly classified crania within this population ranged from 65.8% for univariate functions to 70.3% for multivariate functions within the cranial sample. Males were correctly classified at 70.7% and females at 69.7% using multivariate functions. The linear regression equations predicted sex in the cranial sample correctly for 76% of the males and 70% for the females using different variables; however, overall highest correct prediction percentage was only 68%. Cross-validation brought the percentage down in some cases, but it was concluded that, overall, the expression of sexual dimorphism in the foramen magnum region within the St. Bride’s population is significantly demonstrable, and therefore, this area of the skull should be considered useful in the identification of sex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Identification of human skeletal remains is of major importance in medico-legal situations such as criminal cases, mass disasters and human rights abuse investigations. One of the principal biological indicators of identity is the sex of the individual. This is relatively uncomplicated to achieve when the remains are complete, but fragmented or dispersed remains result in an incomplete assessment base, which may result in some aspects of identity being inconclusive, including sex evaluation. The most sexually dimorphic areas of the human skeleton are the os coxae and the skull, with the most dimorphic regions of the former tending not to survive inhumation particularly well [27]. Furthermore, fragmentary crania are relatively common due to intentional destruction, perhaps in an attempt to obscure facial identity or as a result of physical injury due to weapons, fire, explosives or simply the effects of inhumation. It is therefore essential that every part of the human skeleton be assessed on its own merit to determine its value for identification purposes as the investigator has no influence over which elements may be presented for examination.
Due to the thickness of the cranial base and its relatively protected anatomical position, this area of the skull tends to withstand both physical insults and inhumation somewhat more successfully than many other areas of the cranium [8]. Therefore, there is considerable merit in investigating the value of this particular region in the process of sex determination. Previous publications on sexual dimorphism of the cranial base included analysis of the foramen magnum and condylar region [9, 14, 26, 31, 36]. The region consists of the foramen magnum (FM) and the laterally placed condyles for articulation with the superior facets of the first cervical vertebra. The traditional methods of recording measurements of the cranial base include the use of spreading calipers and calibrated paper strips [26]. Some of the problems associated with these methods have been outlined in past publications [5, 36].
The skull, and particularly the skull base, has been analysed with varying results and levels of success. Measurements including the length of the foramen magnum achieved an accuracy of almost 85% correct prediction utilising a ‘Cape coloured’ population [16]. In 1963, Giles and Elliot examined sex determination of the skull by discriminant function analysis using Fisher’s [3] method. Their accuracy for Negroid and Caucasoid material ranged between 82% and 89% [7].
Again, using discriminant function analysis, Kajanoja [15] achieved correct determination of male sex in 79.4% and female sex in 79.1% of the cases in a Finnish cranial sample. He found that an overall accuracy of 79.5% was similar to results obtained by others. Interestingly, he also tested Giles and Elliot’s statement that their functions were independent of racial variations but found that this was not the case [7, 15].
Henke [12] achieved 88% accurate determination by using discriminant functions utilising between two and eight variables of the human skull.
Routal et al. [26] claimed up to 100% accuracy in predicting sex from the foramen magnum region, whilst Holland [15] scored between 71% and 90% in the main sample and 70–85% in the control group. It should be noted that he used regression equations and not discriminant function analysis. Westcott and Moore-Jansen [36] classified 76% of skulls correctly by using Holland’s [15] method; however, it has to be pointed out that they used discriminant function analysis for their calculations.
In this preliminary study, the use of only the foramen magnum for sex determination is re-evaluated for a specific reason: to assess the levels of sexual dimorphism in a historical documented skeletal collection that has not previously been examined for this feature.
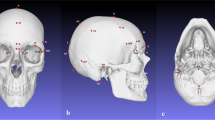
The foramen magnum is a three-dimensional aperture within the basal central region of the occipital bone (see Fig. 1). It is inclined slightly anteriorly and permits passage of a variety of structures including the medulla oblongata, vertebral arteries and spinal part of the accessory cranial nerve. The anterior border of the foramen is formed by the basilar process of the occipital bone, the lateral borders by the left and right ex-occipitals, and the posterior border is formed by the supra-occipital part of the occipital bone [28]. Two convex kidney-shaped condylar facets are found on either side of the foramen for articulation with the first cervical vertebra at the synovial atlanto-occipital joint. The hypoglossal canals are located internally and superior to the condylar processes, connecting the internal posterior cranial fossa to the external cranial base for the passage of cranial nerve XII.
Materials and methods
The St. Bride’s documented skeletal collection
The eighteenth and nineteenth century documented skeletal collection housed in St. Bride’s Church, Fleet Street, London, consists of 192 adult human skeletons (♂ = 100, ♀ = 92). The individuals were accepted as “adult” at an age of 18 years and older. Of the 192 adult remains, 158 (♂ = 82, ♀ = 76) were of use for the evaluation of sex differences in the foramen magnum. The reduced number of crania was mainly due to missing, badly damaged and/or pathological skull bases.
The average age of the crania in the St. Bride’s collection is demonstrated in Table 1.
Measurements
For the St. Bride’s crania, manual measurements were chosen from past publications [5, 14, 26, 36].
The variables recorded for this publication were:
-
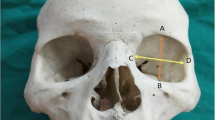
Maximum length of the foramen magnum (LFM) measured in an anteroposterior direction along the principal axis of the foramen
-
Maximum width of the foramen magnum (WFM) measured approximately perpendicular to the LFM and recorded at the widest transverse diameter of the foramen
-
Circumference of the foramen magnum (FMC) measured by pressing a narrow strip of calibrated paper along the inner margin of the foramen magnum. The paper strip was then unrolled and measured with digital sliding calipers (Fig. 2).
All measurements were recorded to two decimal places. Intraobserver and interobserver errors for these measurements were analysed by two of the authors in a previous publication [5]. The St. Bride’s measurements were recorded by a single observer (RG) using the same equipment.
Formulae
The LFM, WFM and FMC can be inserted into one of three different formulae to estimate the area of the foramen magnum.
The first method used a formula published by Routal et al. [26], which is based on the height (h) and width (W) of the foramen magnum. The LFM in this publication is the same as Routal’s and Teixeira’s height measurements [26, 31].
The second method used a formula derived by Teixeira [31], which also uses the width and height of the foramen magnum.
The third method consists of a formula suggested by the authors of this publication and uses the circumference (C) to estimate the radius (r) of the foramen magnum, assuming it to be circular. This radius is then applied to the formula for the area of a circle.
Throughout this communication, the different area formulae are referred to as (1) Routal, (2) Teixeira and 3) area circumference (AreaCirc.).
Statistical methods
The general descriptive statistics for the 158 skulls can be found in Table 2. Univariate and multivariate discriminant function analysis was used to analyse sex differences within skulls, and these results are to be found in Table 3.
The discriminant function is constructed by assigning a discriminant score to each case. Depending on the variable and combination of variables for a function, the score changes from case to case. A sectioning point (SP) is created by using the mean male and female discriminant scores, which are also known as the group centroids. Therefore, each function has a different sectioning point, which is based on the variables entered in the function. Unstandardised discriminant coefficients are used for building the formula. The standardised (Fisher’s) coefficients are used to compare the relative importance of the independent variables.
A discriminant function is built as follows: P = a 1 × x 1 + a2 × x 2 + … + a n × x n + b, where ‘a 1’ through ‘a n ’ are the discriminant coefficients, ‘x 1’ through ‘x n ’ are the discriminating variables and ‘b’ is the constant. To assign the case to either male or female sex, the product P is compared to the sectioning point derived by the discriminant function. A value higher than the sectioning point was deemed to be male and a value below it deemed to be female.
The “leave-one-out method” was used at the end of the analysis. In this form of cross-validation, each case is classified using a discriminant function based on the rest of the sample. Table 4 shows the results of this cross validation.
Linear regression was also used to analyse the 158 skulls (Table 5). Past publications advocated that it may be of advantage to use both discriminant functions and regression equations to predict sex [15]. Linear regression applies maximum likelihood estimation and is similar to discriminant analysis in that it assigns a score to each case. In the St. Bride’s sample, the sectioning point was 0.5, whilst male sex was assigned 0 and female sex 1. The regression equation is similar to the discriminant function in structure using coefficients and a constant.
All statistical analysis was carried out using SPSS 12.0.1 for Windows.
Results
The results of the descriptive statistics for the 158 crania (Table 2) show that the differences between all male and female variables investigated display statistically significant differences (p < 0.001).
The discriminant functions for each single variable and the five most reliable combinations of variables for the 158 crania are shown in Table 3. For example, using the function from Table 3 that incorporates foramen magnum width (WFM), circumference (FMC) and area circumference (AreaCirc.):
An unknown adult skull has the following measurements: WFM = 34.16 mm, FMC = 96.90 mm and AreaCirc. = 747.20 mm2.
The function would be 0.32(34.16) − 0.142(96.90) + 0.014(747.20) − 6.626 = 10.93 − 13.76 + 10.46 − 6.626 = 1.004.
The sectioning point is −0.012, and the male centroid is 0.313. Therefore, this is most likely to be a male skull. The expected correct classification for this function is (without cross-validation) 68.3% for males.
The most reliable variable for sex determination was the WFM (65.8% overall accuracy) followed by Teixeira’s area (65.2%) and the FMC (64.6%). The best combined variables proved to be WFM + FMC (70.3%) followed by WFM + AreaCirc. (69.6) and then by WFM + FMC + AreaCirc. (69%). Using the formula sex = 0.317(WFM) + 0.083(FMC) − 17.562 will result in the highest possible prediction percentage for this population.
The cross-validation method demonstrated reduced classification percentages (Table 4). In the cranial sample, discriminant functions classify males and females almost equally well when using a combination of variables. Only single variables showed greater correct classification rates for males than females.
The regression equations for the 158 skulls demonstrated in Table 5 show a reduced capability to correctly classify sex within the sample compared to the results achieved by discriminant function. However, when combined, the WFM and FMC are better at classifying females (70%) than was achieved for the discriminant function approach.
Discussion
Analytical methodologies developed on skeletal data obtained from European documented skeletal collections have increased with the growing expertise of forensic anthropology in Europe, which is evident by the setup of academic association bodies such as the Forensic Anthropology Society of Europe or FASE [1]. There has been no previous evaluation of sexual dimorphism of the foramen magnum region within a documented British population. Sex determination in the human cranium is generally based on size differences and robusticity [13, 17, 18, 24, 30]. These differences are unique to each population and thought to be influenced by genetic, environmental and socio-economic factors [10, 20, 24, 27]. Sexual dimorphism is population specific [10], and discriminant functions applied to cases other than the source population used to develop these functions have shown incorrect classification percentages between 32% and 48% [11, 15, 37]. It is therefore necessary to study its expression in as many geographically and temporally diverse populations as possible.
Utilising measurements for sex identification rather than morphognostic observation permits the representation of results in an objective manner. Morphometric analysis should be used as part of the investigative process prior to any destructive analysis, e.g. stable isotope analysis, dating or DNA extraction. Past researchers used measurements of the whole cranium [4, 7, 12, 15], the mandible [6, 22] as well as the dentition [25] to determine sex by applying discriminant function analysis and regression equations. These past studies have demonstrated that statistically significant differences exist between male and female skulls. These differences can be used to predict sex in an unknown skull. However, the need for methods to identify sex from cranial fragments becomes apparent when considering the fragile nature of the splanchno-cranium (viscero-cranium). The cranial base has been noted for its ability to remain intact in cases where the rest of the cranium has been compromised [8], and researchers have made use of that fact by analysing sexually dimorphic traits of this anatomical region [2, 8, 9, 14, 21, 26, 31, 33, 34, 36, 37].
In this study, the foramen magnum of a British eighteenth and nineteenth century London crypt sample was analysed for sex differences by using manual craniometry. The results demonstrated statistically significant differences between male and female skulls within this population.
The St. Bride’s sample of crania allowed for the development of discriminant functions that predicted the correct sex in 70.3% of all cases. Although the method of obtaining the FMC manually using a paper strip may not seem to be as robust as other measurements, the results obtained proved to be highly predictive of sex.
The degree of expression of sexual dimorphism within the foramen magnum dimensions may be explained by its development. Compared to many other skeletal elements, the foramen magnum reaches its adult size rather early in childhood [29] and is therefore unlikely to respond to significant secondary sexual changes. From a biomechanical viewpoint, no muscles act upon the shape and size of the foramen magnum, and its prime function is to accommodate the passage of structures into and out of the cranial base region, and in particular, the medulla oblongata, which occupies the greatest proportion of the foraminal space. As the nervous system is the most precocious of all body systems, it reaches maturity at a very young age and therefore has no requirement to increase in size. This is evidenced by the completion of fusion of the different elements of the occipital bone by 5–7 years of age and completion of fusion in the C1 centres by 5–6 years of age [28]. As the weight of the head is transmitted through the atlanto-occipital joint, it is unlikely that there is a significant influence of weight transfer in the region of the foramen magnum. Any expression of sexual dimorphism in the foramen magnum region must therefore be laid down during an early period of development at a time when other skeletal elements have also not fully developed sex-specific traits.
Population differences are also important in defining sexual differences in the cranium. In Table 6, the general length and width of the foramen magnum in Turkish [21] as well as Spanish [2] populations are comparable to the St. Bride’s population, with only the length of the foramen magnum being slightly smaller in the latter. This differs from the mean foramen magnum dimensions of the Indian population analysed by Routal et al. [26] with generally smaller variables. Therefore, it is necessary to know the source population of any unidentified skull and choose a method based on data from that population or a population with similar expression of sexual dimorphism. Interestingly, the prediction percentages for a combination of foramen magnum variables from the St. Bride’s collection show similar levels of prediction when compared to studies of other populations [14, 31]. This could indicate that overall expression of sexual dimorphism within the foramen magnum area in relatively modern populations is limited to a level of approximately 70%.
Regression equations developed on the 158 crania sample demonstrated a lower level of correct predictions (Table 5) when compared to the discriminant functions (Table 3). The highest mean percentages for the discriminant function analysis and the regression equations seem to be very similar. However, males and females are almost equally well predicted by discriminant function analysis, whilst the single variables in the regression equations favour the male sex, the combined variables, particularly when using the WFM and FMC, favour the female sex. The discriminant function analysis is robust, as it predicts both sexes equally well.
Other researchers have used image analysis techniques in extraction of information about the foramen magnum before, such as Murshed et al. [21] analysing the sagittal and transverse diameters and the area of the foramen magnum in computerised tomographic images, and Catalina-Herrera [2] who mentioned the use of a graphic image analyser but unfortunately failed to demonstrate its use. The foramen magnum area in the St. Bride’s cranial sample is a useful indicator of sex, and comparisons to the variables and area of other populations demonstrate similar mean results between some populations (Table 6). It can be argued that due to the limited expression of sexually dimorphic features within the foramen magnum region, the use of methods involving this anatomical landmark should not be recommended in cases of complete crania. However, if faced with an incomplete human skull or a fragment of the cranial base, the correct prediction percentage demonstrated in this study give a statistically useful guide to whether the skull belonged to a male or female when using the correct formulae based on the appropriate population. Furthermore, populations with a similar expression of sexual dimorphism within the foramen magnum region could make use of the same formula or a slightly altered function by artificially changing the sectioning point [11, 12]. Further research will concentrate on the use of the occipital condyles alone as well as in conjunction with the foramen magnum for the St. Bride’s population in analysing the manifestation of sexual dimorphism. Expression of sexual dimorphism within these structures (condyles) may prove useful particularly when considering their biomechanical properties. Studies evaluating sex differences in the cervical vertebrae region may also yield promising results [19, 35].
In conclusion, this study demonstrates that there is limited, albeit statistically significant, expression of sexual differences in the foramen magnum region of the St. Bride’s cranial population, which may prove useful in predicting sex in severely fragmented cranial bases by discriminant function analysis. Due to the low percentage of correct classifications, however, this method cannot be recommended for sexing complete skulls. It may only serve as an added confirmation of sex when other, more traditional classification methods for the human skull have already been used. However, analysing a skull by traditional non-metric observation and then using the discriminant functions and regression equations may result in a higher percentage of correct classification. It is also recommended in cases where statistical backup of an initial subjective observation is needed.
Another caveat must be to use discriminant functions only on cases that are known to come from the same population from which the functions were derived [11, 23, 32], except for a population with similar mean values of the variables and expression of sexual dimorphism in the foramen magnum area. Particularly, in forensic cases, only methods developed on documented skeletal samples of known age, sex and ethnicity should be used. The social bias of the St. Bride’s population should also be considered when applying the functions to other British populations in cemetery studies. As a sample of crypt burials within London, a social and health bias must certainly be accepted.
References
Brinkmann B (2007) Forensic anthropology. Int J Legal Med 121:431–432
Catalina-Herrera CJ (1987) Study of the anatomic metric values of the foramen magnum and its relation to sex. Acta Anat 130:344–347
Fisher RA (1936) The use of multiple measurements in taxonomic problems. Ann Eugen 7:179–188
Franklin D, Freedman L, Milne N (2005) Sexual dimorphism and discriminant function sexing in indigenous South African crania. Homo 55:213–228
Gapert R, Last J (2005) The adult human occipital bone: measurement variance and observer error. In: Zakrzewski SR, Clegg M (eds) Proceedings of the Fifth Annual Conference of the British Association for Biological Anthropology and Osteoarchaeology. BAR International Series. Archaeopress, Oxford, pp 119–122
Giles E (1964) Sex determination by discriminant function analysis of the mandible. Am J Phys Anthropol 22:129–136
Giles E, Elliot O (1963) Sex determination by discriminant function analysis of crania. Am J Phys Anthropol 21:53–68
Graw M (2001) Morphometrische und morphognostische Geschlechtsdiagnostik an der menschlichen Schädelbasis. In: Oehmichen M, Geserick G (eds) Osteologische Identifikation und Altersschätzung. Schmidt-Römhild, Lübeck, pp 103–121
Günay Y, Altinkök M (2000) The value of the size of foramen magnum in sex determination. J Clin Forensic Med 7:147–149
Hamilton ME (1982) Sexual dimorphism in skeletal samples. In: Hall RL (ed) Sexual dimorphism in homo sapiens—a question of size. Praeger Publishers, New York, pp 107–163
Henke W (1972) Zur Methode der diskriminanzanalytischen Geschlechtsbestimmung am Schädel. Homo 24:99–117
Henke W (1977) On the method of discriminant function analysis for sex determination of the skull. J Hum Evol 6:95–100
Hermann B, Grupe G, Hummel S, Piepenbrink H, Schutkowski H (1990) Prähistorische Anthropologie—Leitfaden der Feld- und Labormethoden. Springer, Berlin
Holland TD (1986) Sex determination of fragmentary crania by analysis of the cranial base. Am J Phys Anthropol 70:203–208
Kajanoja P (1969) Sex determination of Finnish crania by discriminant function analysis. Am J Phys Anthropol 24:29–34
Keen JA (1950) A Study of the differences between male and female skulls. Am J Phys Anthropol 8:65–78
Knussmann R (1996) Vergleichende Biologie des Menschen—Lehrbuch der Anthropologie und Humangenetik, 2. Auflage. Gustav Fischer Verlag, Stuttgart
Krogman WM (1978) The human skeleton in forensic medicine, 3rd Printing. Charles C. Thomas, Springfield, IL
Marino EA (1995) Sex estimation using the first cervical vertebra. Am J Phys Anthropol 97:127–133
Mays S, Cox M (2002) Sex determination in skeletal remains. In: Cox M, Mays S (eds) Human osteology in archaeology and forensic science. Greenwich Medical Media, London, pp 117–130
Murshed KA, Cicekcibasi AE, Tuncer I (2003) Morphometric evaluation of the foramen magnum and variations in its shape: a study on computerized tomographic images of normal adults. Turk J Med Sci 33:301–306
Pötsch-Schneider L, Endris R, Schmidt H (1985) Diskriminanzanalyse zur Geschlechtsbestimmung an Unterkieferknochen. Z Rechtsmed 94:21–30
Ramsthaler F, Kreutz K, Verhoff MA (2007) Accuracy of metric sex analysis of skeletal remains using Fordisc® based on a recent skull collection. Int J Legal Med 121:477–482
Rösing FW, Graw M, Marré B, Ritz-Timme S, Rothschild MA, Rötzscher K, Schmeling A, Schröder I, Geserick G (2007) Recommendations for the forensic diagnosis of sex and age from skeletons. Homo 58:75–89
Rösing FW, Paul G, Schnutenhaus S (1995) Sexing skeletons by tooth size. In: Radlanski RJ, Renz RJ (eds) Proceedings of the 10th International Symposium on Dental Morphology, Berlin. M-Marketing Services, Berlin, pp 373–376
Routal RR, Pal GP, Bhagwat SS, Tamankar BP (1984) Metrical studies with sexual dimorphism in foramen magnum of human crania. J Anat Soc India 2(33):85–89
Saunders SR, Yang D (1999) Sex determination: XX or XY from the human skeleton. In: Fairgrieve SI (ed) Forensic osteological analysis. Charles C. Thomas, Springfield, IL, pp 36–59
Scheuer L, Black S (2000) Developmental juvenile osteology. Academic, London
Scheuer L, Black S (2004) The juvenile skeleton. Elsevier, London
Stewart TD (1979) Essentials of forensic anthropology—especially as developed in the United States. Charles C. Thomas, Springfield, IL
Teixeira WRG (1982) Sex identification utilizing the size of the foramen magnum. Am J Forensic Med Pathol 3(3):203–206
Ubelaker DH, Ross AH, Graver SM (2002) Application of forensic discriminant functions to a spanish cranial sample. Forensic Sci Commun 4. http://www.fbi.gov/hq/lab/fsc/backissu/july2002/ubelaker1.htm. Cited 28 January 2008
Uysal SRM, Gokharman D, Kacar M, Tuncbilek I, Kosar U (2005) Estimation of sex by 3D CT measurements of the foramen magnum. J Forensic Sci 50:1310–1314
Wahl J, Graw M (2001) Metric sex differentiation of the pars petrosa ossis temporalis. Int J Legal Med 114:215–223
Westcott DJ (2000) Sex variation in the second cervical vertebra. J Forensic Sci 45:462–466
Westcott D, Moore-Jansen P (2001) Metric variation in the human occipital bone: forensic anthropological applications. J Forensic Sci 5(46):1159–1163
Williams MM (1987) Sex determination of fragmentary crania by analysis of the cranial base: applications for the study of an Arikara skeletal sample. Thesis, University of Tennessee
Acknowledgement
We would like to thank the staff at St. Bride’s Church for their gracious hospitality and immense help in obtaining the results presented in this paper—Canon David Meara, James Irving, Matthew Busby, Claire Seaton, David Smith and Bob Drakes. Thanks are also expressed to Professor John Bannigan, University College Dublin, Ireland, for encouraging this research; Professor Louise Scheuer, University of Dundee, UK, for her very helpful comments and a catalogue of the St. Bride’s skeletal remains; and Professor Friedrich W. Rösing, University of Ulm, Germany, for his comments on the occipital bone. Many thanks also to the staff at University College Dublin, Ireland, for their help and patience. Special thanks to the two anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gapert, R., Black, S. & Last, J. Sex determination from the foramen magnum: discriminant function analysis in an eighteenth and nineteenth century British sample. Int J Legal Med 123, 25–33 (2009). https://doi.org/10.1007/s00414-008-0256-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-008-0256-0