Abstract
We performed quantification of IL 2, IL 4, IL 6, IL 8, IL 10, GM-CSF, IFN γ, and TNF α in human dermal wounds for wound age estimation. The proliferation of dermal cells and infiltration of inflammatory cells were also analyzed. Neutrophils and macrophages were detected from 2 h post-injury, and strong infiltrations were seen at 33–49 h. T and B lymphocytes also infiltrated simultaneously from 71 h. Strong proliferation of fibroblasts were shown from 246 h, and thickening of the epidermis from 71 h. IL 10, GM-CSF, IFNγ, and TNF α increased from the early phase of dermal wound healing, IL 6 exclusively in the middle phase, IL 2, IL 4, and IL 8 from the middle phase to the late phase. Among the cytokines analyzed in the present study, IL 6, IL 8, IFNγ, and TNF α were strongly expressed. Results of the present study suggest that multiplex cytokine analysis at the wound site can be useful for wound age estimation. In addition, multiplex data obtained from the same sample with a single method would demonstrate more accurate interactions of cytokines during dermal wound healing. Although the present study was oriented to practical forensic pathology, the data obtained would be informative for various fields of medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To forensic pathologists, wound age provides valuable information for the reconstruction of crime scenes and determination of the cause of death. As skin covers the outer surface of the body, it is the most vulnerable part, and dermal wound age is a critical issue in routine forensic autopsies. Inflammatory cell dynamics [1] and enzyme activities, such as ATPase, acid phosphatase, and alkaline phosphatase [10] are still applied to wound age estimation. Analyses of cytokine expressions in murine skin and immunohistochemical detection in human skin have also been reported, and their usefulness for dermal wound age estimation has been suggested [9, 16, 22, 31, 32].
Although cytokine mRNA can be detected by real-time polymerase chain reaction (PCR), and secreted cytokines can be quantified with the enzyme-linked immunosorbent assay, these methods have some limitations, for example, the need for a large sample volume. In addition, these techniques are laborious and time-consuming. Therefore, multiplexed examination of tissue proteins has been extremely difficult. These problems have been removed by the commercial availability of a bead-based immunoassay (Bio-Plex Suspension Array System, Bio-Rad, Hercules, CA). Although the ability to simultaneously quantify multiple signaling proteins would be beneficial to deciphering how they affect dermal wound healing, this technology has not been applied to the study of cytokine expression during dermal wound healing.
In this study, quantitative analyses of the time-dependent expressions of eight cytokines, including interleukin 2 (IL 2), interleukin 4 (IL 4), interleukin 6 (IL 6), interleukin 8 (IL 8), interleukin 10 (IL 10), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon γ (IFNγ), and tumor necrosis factor α (TNF α), in human dermal penetrating wounds, were performed to evaluate their utility for wound age estimation using bead-based immunoassay.
Materials and methods
Samples
We collected samples of human skin from skin penetrating injuries including incised wounds, stab wounds, laceration, and contusions, that could be dated chronologically from depositions taken at the scene of injuries. The postmortem interval was within 36 h. Skin samples 0.5 × 0.5 cm were taken parallel along the wound margin. The subcutis was then removed. Tissue samples from 104 dermal wounds taken from 29 males and 30 females were investigated. A total of 16 control samples from the left clavicular area taken from eight males and eight females were analyzed simultaneously (Table 1). The specimens were categorized into the following 10 groups according to wound age: 0.1–0.5 h (n = 17), 1–1.92 h (n = 15), 2–2.75 h (n = 13), 3–4.5 h (n = 14), 6–7 h (n = 11), 9 h (n = 8), 12–14.75 h (n = 10), 33–49 h (n = 5), 71–116.5 h (n = 6), 246–744 h (n = 5), and intact control tissue (n = 16).
Individuals with a known history of immunodeficiency or immunotherapy were excluded from the study.
Immunohistochemical studies
To identify cells infiltrated into the skin, immunostaining was performed using mouse anti-neutrophil elastase (Spring Bioscience, Fremont, CA) for neutrophils, mouse anti-CD68 (Zymed Laboratories, South San Francisco, CA) for macrophages, mouse anti-CD 3 (Nichirei, Tokyo, Japan) for T lymphocytes, mouse anti-L 26 (Nichirei, Tokyo, Japan) for B lymphocytes, and rabbit anti-vimentin (Abcam, Cambridge, UK) for fibroblasts as the primary antibodies.
Extraction of total protein
The skin samples were washed with Bio-Plex Cell Wash Buffer (Bio-Rad Laboratories), and pulverized mechanically in an oscillating mill with continuous addition of liquid nitrogen (Cryo-Press, Microtec, Funabashi, Japan). The resulting product was homogenized in a tube (MAZERA Z, EYALA, Tokyo, Japan) containing 500 μl of lysing solution (Bio-Plex Cell Lysis Kit, Bio-Rad Laboratories). Samples were placed in a 1.5-ml tube and frozen at −80°C. After thawing, the samples were sonicated on ice using Bioruptor (Cosmo Bio, Tokyo, Japan), and centrifuged at 6,000 rpm for 6 min at 4°C. The supernatant was filtered with cell strainer (BD, Franklin lakes, NJ), and transferred to a tube. The protein concentration was determined by the Bradford method [2] using a commercial assay kit (Bio-Rad Laboratories). The protein concentration of each sample was adjusted to 500 μg/ml with lysing solution.
Multiplex analysis
A Bio-Plex assay for eight cytokines (IL 2, IL 4, IL 6, IL 8, IL 10, GM-CSF, IFNγ, TNF α) was run following the manufacturer’s instruction (Bio-Rad Laboratories). Briefly, a standard curve was created from fourfold dilution series of premixed standards ranging from 3,200 to 0.2 pg/ml. The assay was performed in a 96-well filtration plate supplied with the Bio-Plex kit. Premixed beads coated with the target antibodies were added to each well, then washed twice with Bio-Plex Wash Buffer. Premixed standards and samples (50 μl) were then added to the wells, before shaking at 1,100 rpm for 30 s and incubation for 30 min together with shaking at 300 rpm at room temperature. Subsequently, wells were washed three times with Bio-Plex Wash Buffer, and 25 μl of the premixed detection antibodies was added, before shaking at 1,100 rpm for 30 s and at 300 rpm for 30 min at room temperature. Wells were again washed three times with Bio-Plex Wash Buffer, and 50 μl streptoavidin-phycoerythrin was added to the wells. After incubation for 10 min with shaking at 300 rpm, the wells were washed three times with Bio-Plex Wash Buffer, and the beads were resuspended in 125 μl Bio-Plex Assay Buffer. Using the Bio-Plex Suspension Array System, the factor was detected by the fluorescence of the beads, and simultaneously quantified by that of phycoerythrin. All samples were analyzed in duplicate.
Evaluation
The ratios of cytokine expression to total protein and cytokine concentration in dermal tissue were analyzed. Furthermore, differences of cytokine expressions between control and each injured tissue were statistically analyzed using the Dunnet test, and P values of 0.05 or less were considered statistically significant.
The research described in this report was conducted in accordance with the guidelines for forensic experimentation of Japanese Society of Legal Medicine.
Results
Immunohistochemical analysis within 5 mm from the edge
Neutrophils and macrophages were detected from 2 h post-injury, and strong infiltration was seen at 33–49 h. T and B lymphocytes also infiltrated simultaneously from 71 h. Although fibroblasts appeared in the intact skin, strong proliferation was shown from 246 h in injured skins. Thickening of the epidermis was seen from 71 h (Table 2).
Cytokine expressions per total protein
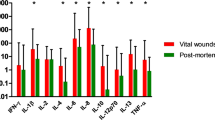
Time-dependent cytokine expressions per total protein are shown in Fig. 1. IL 10, GM-CSF, IFN γ, and TNF α increased from the early phase of dermal wound healing. TNF α showed a significant increase with very short survival times of less than 0.5 h, whereas rises of IL 10, GM-CSF, and IFN γ were seen until 1.92 h. These cytokines were active throughout the healing process, and peaks of TNF α at 9 h, IFN γ at 12–14.75 h, GM-CSF at 33–49 h, and IL 10 at 71 to 116.5 h were observed in the middle and late phases. IL 6 showed exclusive expressions in the middle phase, and peaked at 9 h. IL 2, IL 4, and IL 8 were mainly seen from the middle phase to the late phase. Peak expressions of IL 2 and IL 8 at 9 h, and IL 4 at 33–49 h were seen. On the whole, IL 6, IL 8, IFN γ, and TNF α were strongly expressed. Among the cytokines analyzed in the present study, IL 8 was the most strongly expressed.
Cytokine expressions per tissue weight
Cytokine expressions per tissue weight were similar to those per total protein (Fig. 1). Increases of IL 10, GM-CSF, and IFN γ until 1.92 h were detected. Peaks of TNF α at 9 h, IFN γ at 12–14.75 h, and GM-CSF and IL 10 at 71 to 116.5 h were observed in the middle and late phases. IL 6 also peaked at 9 h. IL 2, IL 4, and IL 8 were mainly seen from the medium phase to the late phase, and peaked at 71–116.5 h. Overall, strong expressions of IL 6, IL 8, IFN γ, and TNF α were seen. In addition, the most abundant factor was IL 8.
Discussion
Dermal wound healing is a sequential biological process that involves the integration of chemotaxis of inflammatory cells, migration of keratinocytes, and remodeling of the scar tissue [26], and cellular infiltration has been considered to be one of the important tools for wound age estimation. Previous morphological analyses demonstrated that neutrophils and macrophages were detected during very early phase of dermal wound healing from 30 min and 3 h post-injury, respectively, whereas, lymphocytes were detected from 192 h [1]. In the present immnohistochemical analyses, neutrophils and macrophages simultaneously appeared from 2 h and strongly infiltrated at 33–49 h, whereas T and B lymphocytes infiltrated simultaneously from 71 h. These findings suggest few differences in behavior between nentrophils and macrophages, and remarkable changes of inflammatory cell dynamics were seen chiefly in the late phase. Neutrophils senescent within viable tissue are phogocytosed by macrophages [21], and the present study demonstrated that these phenomena would start from the early phase of dermal wound healing. Thus, it was considered that morphological analyses of cellular infiltration and proliferation were unsuitable for the markers of the early phase, in spite of many cases of a short survival time in forensic cases. On the other hand, reproducible time-dependent changes of cytokine expressions, which precede those of inflammatory cells, were observed in the present study. This indicates that quantitative analyses of these cytokines in tissue extracts can contribute to wound age estimation. Compared with cytokine expressions per tissue weight, significant changes of those per total protein were obvious, indicating that the latter would be useful as parameters. This dissociation between total protein and tissue weight may have resulted from postmortem water loss in organs and tissues. The potential parameters would be IL 10, GM-CSF, IFN γ, and TNF α in the early phase of dermal wound healing, IL 6 in the middle phase, and IL 2, IL 4, and IL 8 in the middle and late phases. The estimated ranges of wound age were rather wide in the immunohistochemical evaluations [24], whereas quantitative analyses of tissue extracts could indicate narrower intervals. Compared with the data obtained from animal experimental studies and previous histochemical analyses of human samples [11, 14, 15, 23], the early expression of IFN γ in the present study would appear significant. On the other hand, the expression patterns of IL 6, IL 8, IL 10, and TNF α resembled those reported in previous studies.
Cytokines can alter the behavior and properties of immune and other cells, and their combined effects are often more important than the function of one isolated component. Therefore, elucidating the interactive behavior of cytokines during dermal wound healing is essential for understanding dermal immune responses. Among the cytokines appearing in the early post-traumatic period, TNF α originates in keratinocytes [13], and GM-CSF in keratinocytes [3], endothelial cells [4], and fibroblasts [6]. Because TNF α promotes IL 10 production in macrophages [33], its expression would increase successively as shown in the present study. As these cytokines were seen throughout the posttraumatic period, they would play an important role in wound healing.
On the other hand, IFN γ was reported to have a chemotactic effect on neutrophils [17] and macrophages [20] during the early phase of wound healing. In the middle phase, IL 6 could be produced by keratinocytes [12] and fibroblasts [28] in response to TNF α, and has also been reported to express in endothelial cells, vascular smooth muscle cells [25], and macrophages [5].
Factors mainly detectable from the middle phase to the late phase were IL 2, IL 4, and IL 8. IL 8, which was the most plentiful cytokine in the present study, has been demonstrated to originate from keratinocytes [18], fibroblasts, endothelial cells [29], and neutrophils [30]. In addition, IL 8 proliferates keratinocytes and works as a potent chemokine for neutrophils [26] and lymphocytes [34]. IL 2 promotes T lymphocyte proliferation [19] and interacts with IFN γ on IL 8 mRNA production in keratinocytes [18]. On the other hand, IL 4 is thought to proliferate fibroblasts [7]. Significant expressions of IL 6, IL 8, IFN γ, and TNF α were suggestive of their having significant effects on dermal wound healing.
Quantitative analyses of cytokines at the wound site can be quite useful for wound age estimation, and multiplex analyses would be a promising method. Previous reports of cytokine interactions were performed with data collected by different methods and from different samples [8, 27], whereas the present multiplex data were gained from the same samples with a single method. Therefore, it is considered that more accurate interactions of cytokines could be demonstrated. Moreover, unlike previous dermal quantitative studies on cytokines mainly conducted by means of animal experiments, human penetrating wounds were analyzed directly in the present study. On the other hand, difference of age, sex, and type of wound, which might affect the cytokine pattern, could not been take into account in this study, because of relatively limited number of samples. Further investigations with more detailed classifications are required. Although the present study was oriented to practical forensic pathology, the data obtained would also be informative for various fields of medicine.
References
Betz P (1994) Histological and enzyme histochemical parameters for the age estimation of human skin wounds. Int J Legal Med 107:60–68
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Breuhahn K, Mann A, Muller G et al (2000) Epidermal overexpression of granulocyte-macrophage colony-stimulating factor induces both keratinocyte proliferation and apoptosis. Cell Growth Differ 11:111–121
Bussolino F, Wang JM, Defilippi P et al (1989) Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 337:471–473
Castells-Rodellas A, Castell JV, Ramirez-Bosca A et al (1992) Interleukin-6 in normal skin and psoriasis. Acta Derm Venereol 72:165–168
Dedhar S, Gaboury L, Galloway P et al (1988) Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proc Natl Acad Sci USA 85:9253–9257
Fertin C, Nicolas JF, Gillery P et al (1991) Interleukin-4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol 37:823–829
Gillitzer R, Goebeler M (2001) Chemokines in cutaneous wound healing. J Leukoc Biol 69:513–521
Hayashi T, Ishida Y, Kimura A et al (2004) Forensic application of VEGF expression to skin wound age determination. Int J Legal Med 118:320–325
Hernandez-Cueto C, Girela E, Sweet DJ (2000) Advances in the diagnosis of wound vitality: a review. Am J Forensic Med Pathol 21:21–31
Ishida Y, Kondo T, Takayasu T et al (2004) The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol 172:1848–1855
Kirnbauer R, Kock A, Schwarz T et al (1989) IFN-beta 2, B cell differentiation factor 2, or hybridoma growth factor (IL-6) is expressed and released by human epidermal cells and epidermoid carcinoma cell lines. J Immunol 142:1922–1928
Kock A, Schwarz T, Kirnbauer R et al (1990) Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med 172:1609–1614
Kondo T, Ohshima T (1996) The dynamics of inflammatory cytokines in the healing process of mouse skin wound: a preliminary study for possible wound age determination. Int J Leg Med 108:231–236
Kondo T, Ohshima T, Mori R et al (2002) Immunohistochemical detection of chemokines in human skin wounds and its application to wound age determination. Int J Leg Med 116:87–91
Kondo T (2007) Timing of skin wounds. Legal Med 9:109–114
Kowanko IC, Ferrante A (1987) Stimulation of neutrophil respiratory burst and lysosomal enzyme release by human interferon-gamma. Immunology 62:149–151
Li J, Ireland GW, Farthing PM et al (1996) Epidermal and oral keratinocytes are induced to produce RANTES and IL-8 by cytokine stimulation. J Invest Dermatol 106:661–666
Malek TR (2003) The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol 74:961–965
Nathan CF, Murray HW, Wiebe ME et al (1983) Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med 158:670–689
Newman SL, Henson JE, Henson PM (1982) Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med 156:430–442
Ohshima T (2000) Forensic wound examination. Forensic Sci Int 113:153–164
Ohshima T, Sato Y (1998) Time-dependent expression of interleukin-10 (IL-10) mRNA during the early phase of skin wound healing as a possible indicator of wound vitality. Int J Leg Med 111:251–255
Ortiz-Rey JA, Suarez-Penaranda JM, Da Silva EA et al (2002) Immunohistochemical detection of fibronectin and tenascin in incised human skin injuries. Forensic Sci Int 126:118–122
Paquet P, Pierard GE (1996) Interleukin-6 and the skin. Int Arch Allergy Immunol 109:308–317
Rennekampff HO, Hansbrough JF, Kiessig V et al (2000) Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J Surg Res 93:41–54
Schroder JM (1995) Cytokine networks in the skin. J Invest Dermatol 105:20S–24S
Seitz M, Loetscher P, Dewald B et al (1995) Interleukin-10 differentially regulates cytokine inhibitor and chemokine release from blood mononuclear cells and fibroblasts. Eur J Immunol 25:1129–1132
Sticherling M, Hetzel F, Schroder JM et al (1993) Time- and stimulus-dependent secretion of NAP-1/IL-8 by human fibroblasts and endothelial cells. J Invest Dermatol 101:573–576
Strieter RM, Kasahara K, Allen RM et al (1992) Cytokine-induced neutrophil-derived interleukin-8. Am J Pathol 141:397–407
Takamiya M, Saigusa K, Nakayashiki N et al (2003) Studies on mRNA expression of basic fibroblast growth factor in wound healing for wound age determination. Int J Leg Med 117:46–50
Takamiya M, Kumagai R, Nakayashiki N et al (2006) A study on mRNA expressions of fibronectin in dermal and cerebral wound healing for wound age estimation. Legal Med 8:214–219
Wanidworanun C, Strober W (1993) Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol 151:6853–6861
Wilkinson PC, Newman I (1992) Identification of IL-8 as a locomotor attractant for activated human lymphocytes in mononuclear cell cultures with anti-CD3 or purified protein derivative of Mycobacterium tuberculosis. J Immunol 149:2689–2694
Acknowledgment
This study was supported by grants-in-aid for health care research from Iwate prefecture, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takamiya, M., Fujita, S., Saigusa, K. et al. Simultaneous detection of eight cytokines in human dermal wounds with a multiplex bead-based immunoassay for wound age estimation. Int J Legal Med 122, 143–148 (2008). https://doi.org/10.1007/s00414-007-0183-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-007-0183-5