Abstract
The aim of this work was to evaluate the conformity of intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT), and verify the accuracy of the planning and delivery system used in this work based on the AAPM TG-119 protocol. The Eclipse 13.6 treatment planning system (TPS) was used to plan the TG-119 test suite, which included four test cases: MultiTarget, Prostate, Head/Neck, and C-Shape for IMRT and VMAT techniques with 6 MV and 10 MV acceleration voltages. The results were assessed and discussed in terms of the TG-119 protocol and the results of previous studies. In addition, point dose and planar dose measurements were done using a semiflex ion chamber and an electronic portal imaging device (EPID), respectively. The planned doses of all test cases met the criteria of the TG-119 protocol, except those for the spinal cord of the C-Shape hard case. There were no significant differences between the treatment planning doses and the doses given in the TG-119 report, with p-values ranging from 0.974 to 1 (p > 0.05). Doses to the target volumes were similar in the IMRT and VMAT plans, but the organs at risk (OARs) doses were different depending on the test case. The planning results showed that IMRT is more conformal than VMAT in certain cases. For the point dose measurements, the confidence limit (CLpoint) of 0.030 and 0.021 were better than the corresponding values of 0.045 and 0.047 given in the TG-119 report for high-dose and low-dose areas, respectively. Regarding the planar dose measurements, the CLplanar value of 0.38 obtained in this work was lower than that given in the TG-119 report (12.4). It is concluded that the dosimetry measurements performed in this study showed better confidence limits than those provided in the TG 119 report. IMRT remains more conformal in certain circumstances than the more progressive VMAT. When selecting the method of delivering a dose to the patient, several factors must be considered, including the radiotherapy technique, energy, treatment site, and tumour geometry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intensity-modulated radiation therapy (IMRT) has become a very popular dynamic treatment technique in the clinical routine of radiotherapy in recent years (IAEA 2008; Yang et al. 2012; Kishimoto and Yamashita 2018; Kruszyna-Mochalska 2018; Rehman et al. 2018). It is extensively used in modern radiotherapy (Kaushik et al. 2016). IMRT has replaced conformal radiotherapy because of its capacity to deliver a more conformal dose to the target and to spare healthy tissues and organs. Many authors have reported the advantages of IMRT over three-dimensional radiotherapy (Bucci et al. 2005; Cheung 2006; IAEA 2008; Nithya et al. 2016). Unlike conventional three-dimensional conformal radiotherapy (3D-CRT), both the treatment planning and delivery are more complex and less intuitive to users in IMRT (Palta et al. 2008), because there are more parameters to coordinate and verify (Palta et al. 2008). For example, the motion of the multi-leaf collimators (MLCs) generated from intensity-modulated beams is complicated. As a result, IMRTs field often consist of many small irregular off-axis fields, making the iso-dose distribution for the IMRT plan more conformal. Therefore, strict requirements of QA for IMRT planning and delivery are needed (Palta et al. 2008; AAPM 2009). The Radiological Physics Center (RPC), which was an operations group of the Scientific Committee formed in 1968 in Houston, had reported on IMRT accreditation using a head and neck phantom, and it was found that 71 of 250 deliveries did not meet modest accuracy requirements because of the poor use of the IMRT system (Gordon et al. 2011). Based on RPC reports, a protocol to assess the accuracy of a given IMRT system (Gordon et al. 2011) was proposed by Task Group 119 of the American Association of Physicists in Medicine (AAPM) (Saminathan et al. 2011). The guidelines established a test suite consisting of two preliminary tests and four mock clinical models to verify the overall accuracy of IMRT planning and delivery of a 6 MV photon beam (Ezzell et al. 2009; Zhang et al. 2020). The guidelines request that an institution should be able to evaluate the quality of their IMRT commissioning as compared to a standard benchmark by using the proposed protocol including a certain confidence limit (CL) variation for a single IMRT system. If the local CLs exceed those proposed by AAPM TG-119, then this might be an indication that the IMRT modeling needs to be improved (Gordon et al. 2011).
Similar to IMRT, volumetric-modulated arc therapy (VMAT) is also a very popular treatment modality because of its clinical advantages (Teoh et al. 2011; Mynampati et al. 2012; San-Miguel et al. 2016; Sangaiah et al. 2017; Sharma et al. 2017; De Martin et al. 2018; Afrin and Ahmad 2021; Rashid et al. 2021). VMAT represents an enhanced version of IMRT delivery (Nithya et al. 2016). It is a technique where the gantry rotates around the patient with continuous changes in MLC shape, gantry speed, and dose rate while the radiation is being delivered. VMAT was recognized to be a useful technique in radiation therapy of cancer since its inception.
Nowadays, however, IMRT is still indispensable in radiotherapy. In some previous studies the advantages of VMAT compared to IMRT were investigated for various cancers (head and neck, breast, and lung cancer) (Holt et al. 2013; Chen et al. 2017; Redapi et al. 2022; Li et al. 2023). However, other studies reported that in some circumstances, IMRT was superior compared to VMAT (Kim et al. 2018; Akbas et al. 2019; Xie et al. 2020). Specifically, it was found that in treatment of synchronous bilateral breast cancer, the dose distribution in the VMAT treatment plans were not as good as that in the IMRT treatment plans: particularly in the VMAT plans, the edge of the target volume did not receive sufficient doses. Regarding the dose distribution to the organs at risk (OARs), IMRT was superior to VMAT in terms of all dosimetric parameters. Thus, IMRT was evaluated to be better compared to VMAT in terms of target volume and OAR dose distribution, while VMAT was evaluated to show better treatment efficiency (Kim et al. 2018).
In the investigation of advanced techniques used for postmastectomy radiotherapy, based on the dose distribution to the target volume and OARs, it was shown that non-coplanar volumetric modulated arc therapy (NC-VMAT), fixed-beam IMRT, and mixed-beam therapy could offer the optimal radiation technique for certain breast cancer patients who are prone to develop radiogenic side effects. It was shown that fixed-beam IMRT displayed the best sparing of the contralateral breast, but increased the dose to the lungs and heart, while NC-VMAT provided the best sparing of the lungs, and mixed beam therapy exhibited the best sparing of the heart and good sparing of the contralateral breast (Xie et al. 2020).
For nasopharyngeal carcinoma (NPC) radiotherapy, a hybrid technique including both IMRT and VMAT was used (Akbas et al. 2019), because in difficult cases the use of neither IMRT nor VMAT alone might give adequate results. This hybrid technique significantly improved the target dose homogeneity and conformity compared to VMAT, but there was no significant difference compared to IMRT. The hybrid technique improved organ sparing, especially for serial organs such as the brainstem and spinal cord. The maximum dose to the brainstem was found to be significantly lower in IMRT and in hybrid plans compared to VMAT plans. Also, hybrid plans reduced the maximum dose of the spinal cord in most cases. However, the application of the hybrid technique may not be appropriate for every NPC patient, but it offers the planner a third choice besides IMRT and VMAT. New planning techniques may be developed by using new technologies in the future to address the fact that each patient has a unique anatomy for radiotherapy (Akbas et al. 2019).
These studies all showed that although VMAT has some advantages versus IMRT, IMRT still plays an important role in advanced radiotherapy techniques. In fact, it can be used alone or combined with VMAT, which can then (as a hybrid technique) offer optimal results for some special cases.
With the presence of VMAT, the TG 119 protocol has been used to evaluate the commissioning accuracy of both IMRT and VMAT systems and to understand the capability and quality of the two techniques (Mynampati et al. 2012; Thomas and Chandroth 2014; Sharma et al. 2017; Nainggolan and Pawiro 2019; Zhang et al. 2020). In these studies, results of IMRT and VMAT treatment planning were evaluated to meet the dose goals of the target volume and OARs in the TG 119 protocol, and the local confident limits were compared to those in the TG 119 report (Ezzell et al. 2009). However, none of those studies assessed which is the better technique for each case through the comparison between the IMRT doses and the VMAT doses. Thus, this was studied in the present work. Particularly, the C-shape hard case in TG 119 protocol was used for testing the treatment planning system. The core doses of the C-Shape hard case should be optimized to become as low as possible in order to explore the limit of the treatment planning system in the clinic. In previous studies, the dose goals of the core in this case could not be achieved (Ezzell et al. 2009; Saminathan et al. 2011; Mynampati et al. 2012; Thomas and Chandroth 2014; Kadam and Sharma 2016; Kaushik et al. 2016; Sharma et al. 2017; Nainggolan and Pawiro 2019; Zhang et al. 2020). Consequently, in the present study the core dose was investigated and optimized, to explore the ability of the system used here.
Thus, the aim of the present work was to analyze the conformity of IMRT compared to VMAT using the AAPM TG 119 protocol, with accelerator voltages of 6 MV and 10 MV. A detailed evaluation of the received doses was performed in an effort to understand which technique is more appropriate for each type of the TG 119 test cases that represented patient anatomies in different sites of the body. Besides, dosimetry measurements according to the instruction of the AAPM TG 119 protocol were also done to validate the commissioned IMRT treatment planning and dose delivery system.
Materials and methods
TG 119 test cases and treatment planning
According to the AAPM TG 119 instructions, the computed tomography (CT) and radiotherapy (RT) structure set consisting of four test cases were downloaded (AAPM 2009) and then transferred to the treatment planning system (TPS) used in the present study. The four test cases were the MultiTarget, Prostate, Head/Neck and C-Shape cases. The dose criteria were set into two versions for the mock C-Shape, so in total five clinical test cases were used called MultiTarget, Prostate, Head/Neck, and C-Shape easy and C-Shape hard involving different dose goals (Fig. 1). The MultiTarget case consisted of three cylindrical targets stacked along the center axis, namely, SuperiorTarget, CenterTarget, and InferiorTarget. The Prostate case included one target called Prostate CTV (Clinical Target Volume), and two OARs called Rectum, and Bladder. The Head/Neck case included one target volume Head/Neck PTV (Planning Target Volume), and three OARs, including the cord, the RT Parotid, and the LT Parotid. The C-Shape case included one target volume, the C-Shape PTV, surrounding the spinal cord, named the core. This case has two versions consisting of, namely, the C-Shape easy and the C-Shape hard with different dose goals for each case. The preliminary test P1 was generated as a verification plan using 6 MV with two parallel opposed 10 × 10 cm2 fields to deliver 2 Gy to the local slab phantom at the isocenter. The preliminary test P2 was the same as P1, but the MLC was used to plan five 3 cm bands with asymmetric jaws for receiving doses ranging from 0.4 to 2 Gy (AAPM 2009).
IMRT and VMAT plans for 6 and 10 MV and for the five test cases were created. The results were then compared with those given in the AAPM TG 119 report (Ezzell et al. 2009) and other studies (Nainggolan and Pawiro 2019; Zhang et al. 2020). All plans were optimized and calculated on the Eclipse TPS version 13.6 for the Varian TrueBeam STx linear accelerator (Varian Medical System Inc., USA), which was equiped with a ‘high definition’ 120-leaf multileaf collimator (HD120 MLC). The algorithm used was the Analytical Anisotropic Algorithm (AAA) dose calculation algorithm including a 2.5 mm calculation grid size and heterogeneity corrections at a dose rate of 600 MU/min. The clinical test cases were planned for IMRT and VMAT with 6 MV and 10 MV photon beams called IMRT 6X, IMRT 10X, VMAT 6X, and VMAT 10X. Most of the studies using TG 119 recommendations (Ezzell et al. 2009; Saminathan et al. 2011; Mynampati et al. 2012; Thomas and Chandroth 2014; Kadam and Sharma 2016; Kaushik et al. 2016; Nithya et al. 2016; Sharma et al. 2017; Nainggolan and Pawiro 2019; Zhang et al. 2020) involved conventional modes with a flattening filter (FF). Although the flattening filter free (FFF) mode is the crucial feature of the TrueBeam, in the present work the FF mode instead of the FFF mode was used, to find out whether there were any differences between the results obtained using the TrueBeam system and results reported by others. Dose prescriptions for the test suit were 75.6 Gy (1.8 Gy/fraction) for Prostate and 50 Gy (2 Gy/ fraction) for MultiTarget, Head/Neck and C-Shape. The IMRT and VMAT plans were created with the beam arrangement as given in Table 1.
Dose measurements
In the present study, point dose measurements were performed with an ion chamber, while composite planar dose and per field measurements were performed with an electronic portal imaging device (EPID).
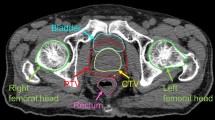
The QA plans for point dose measurement were created in the TPS for two test plans with the planned gantry and collimator angles at the positions as instructed in the TG 119 report. For the point dose measurements, a semiflex ion chamber (PTW, Freiburg, Germany) with a sensitive volume of 0.125 cm3 and a Dose 1 electrometer (IBA Dosimetry, Germany) were used. The SP34 solid plate phantom (IBA dosimetry) of 30 × 30 × 15 cm3 was set up for this measurement (Fig. 2). The test P1 geometry was measured at the isocenter position. Measurement with the test P2 geometry were done at five different locations, namely, at the isocenter and at four other locations at the center of each band (which is defined by measurement locations to the right and left sides from the isocenter, for measurements with asymmetric jaws as referred in TG 119 protocol). For the clinical test cases of TG 119, point dose measurements were done for all five test cases at different positions of the ion chamber (Fig. 3), to investigate the dose in two regions, including the high dose area in the target volume (except for the MultiTarget where all measurement points were target volumes) and the low dose area in the OARs. Before doing measurements of the P1 and P2 tests, the two instruments (i.e., the ion chamber and electrometer), were calibrated. The calibration factor was gained by dividing the dose (Gy) of calculating 100 MUs in the TPS by the dosimeter reading (nC) of delivering 100 MUs to the slab phantom, with 6 MV and 10 cm x 10 cm field size. Then the dosimeter reading (nC) received by irradiating the test plans including the slab phantom was multiplied with the calibration factor to get the delivered doses. The differences between the measured and planned point doses were calculated as the ratio of the prescribed dose as follows (AAPM 2009):
The prescribed dose is the expected dose to the target volume; the the planned dose is dose calculated with the TPS; the measured dose is the dose delivered by the linac. Typical measurement uncertainties were 3% (Gordon et al. 2011).
Measurements of the composite planar dose and per field measurements were carried out by using the 1200 electronic portal imaging device (EPID) mounted on the TrueBeam linac. Planar dose is the overall dose deduced from per field doses. It is automatically obtained from the composite function in the EPID. For all test cases, verification plans were created in Eclipse TPS at planned gantry and collimator angles with a 100 cm source-to-detector distance (SDD). In the Eclipse TPS, the measured planar dose distributions were analyzed using gamma index criteria of 3% dose difference (DD)/3 mm distance to agreement (DTA) and a 10% threshold dose.
Theory and calculation
With respect to planar dose measurements, the gamma passing rate was calculated using Eq. (2). As pass criteria for the gamma passing rate, the percentage of points with gamma value less than 1 was chosen (Low et al. 1998; Hussein et al. 2017; Diamantopoulos et al. 2019). Global normalization was used according to TG-218 AAPM (Miften et al. 2018).
Where \({\text{\varvec{\updelta}r}}\)is the distance difference criterion and \({\text{\varvec{\updelta}D}}\) is the dose difference criterion; \({\text{\varvec{\Delta}r}}\left( {{\mathbf{r}_{\text{R}}}{\text{, }}{\mathbf{r}_{\text{E}}}} \right)\) is the distance between the reference point rR and the evaluated point rE; \({\text{\varvec{\Delta}D}}\left( {{\mathbf{r}_{\text{R}}}{\text{, }}{\mathbf{r}_{\text{E}}}} \right)\)is the dose difference calculated using Eq. (3) (Low et al. 1998; Hussein et al. 2017):
Where \({{\text{D}}_{\text{E}}}\left( {{\mathbf{r}_{\text{E}}}} \right)\) and \({{\text{D}}_{\text{R}}}\left( {{\mathbf{r}_{\text{R}}}} \right)\) are the doses at a point in the evaluated dose distribution and reference dose distribution, respectively.
For point doses, the confidence limit (CL) defined by TG 119 was applied to evaluate the agreement between the planned dose and the measured dose. The confidence limit for the point dose measurement (CLpoint) was calculated as follows (AAPM 2009):
Where the mean is the average difference and σ is the standard deviation. It was expected that 95% of a large number of such measurements should fall within the confidence limit.
For the planar dose measurements, the confidence limit (CLplanar) was calculated as (AAPM 2009):
where the mean is the average percentage of points passing the gamma criterion and σ is the standard deviation.
It was expected that 95% of the results obtained in the test cases should result in gamma passing rates that exceed (100-CLplanar)% for a large number of gamma analyses. Then, as recommended in the TG 119 protocol, the local confidence limits were compared to those in the TG 119 report. If they were within the TG 119 limits, then the accuracy of the system was considered to have been verified.
Statistical analyses
Regarding the statistical analysis of the data, the analysis-of-variance (Anova) test (Lee 2016; Lee and Lee 2018) and the paired t-test were applied to study any differences among the variables including absorbed doses of the six groups of treatment plans, including 6 MV and 10 MV photon beams for IMRT (IMRT 6X and IMRT 10X) and VMAT (VMAT 6X and VMAT 10X), and those in the AAPM protocol and the AAPM report (Ezzell et al. 2009) (Goals and Report) with 26 cases per group as described in the Table 2. The command entitled Pairwise Comparisons of Means for Tukey’s Honest Significant Difference (Tukey’s HSD) was used with a p-value of < 0.05 to identify any differences in groups, while any differences between planning and measurement doses were tested by the paired t-test command of STATA Software (StataCorp 2021) with p-values < 0.05. In the ideal case, planned and measured doses are the same. In practice, however, they are not completely the same. If the p-value (P > |t| value) is higher than 0.05, then the planned and measured doses do not show any statistically significant difference. In contrast, if the p-value is less than 0.05, then both are considered statistically different.
Results
For the point dose measurement results, the differences between the measured and planned doses in this work were 0.0072 for the test P1 geometry, and in the range of -0.001 to 0.012 for the test P2 geometry, with a p-value of 0.077 (i.e., p > 0.05) (Table 3). The average dose difference for the high-absorbed dose region in Table 4 was 0.002 ± 0.014 with a confidence limit (CLpoint) of 0.030, while the value of the TG 119 report was − 0.002 ± 0.022 with a CLpoint of 0.045. Similarly, in Table 5, for the low absorbed-dose region, the average difference was − 0.0003 ± 0.011 with a CLpoint of 0.021. The result for this region in the TG 119 report was 0.003 ± 0.022 with a CLpoint of 0.047.
The dose statistics of the IMRT and VMAT treatment plans for five test cases, namely, MultiTarget, Prostate, Head/Neck, C-Shape easy, and C-Shape hard, are illustrated in Fig. 4. Absorbed doses ranged from 12.68 Gy to 51.65 Gy for the MultiTarget, from 50.19 Gy to 78.97 Gy for the Prostate, from 16.21 Gy to 51.71 Gy for the Head/Neck, from 21.74 Gy to 53.04 Gy for the C-Shape easy, and from 15.47 Gy to 54.70 Gy for the C-Shape hard.
Absorbed doses calculated in this work compared to those provided in the TG 119 report (Ezzell et al. 2009); The notation “DXX” means the minimum absorbed dose covering XX% of the volume; IMRT (intensity-modulated radiation therapy) and VMAT (volumetric modulated arc therapy); PTV (Planning Target Volume); LT (Left); RT (Right)
In Table 2, the maximum deviation was the absorbed dose difference between the TG 119 report and VMAT 6X with the mean dose of 4.46 ± 5.90 Gy and 95% confident interval in the range of -12.59 Gy to 21.51 Gy, while the minimum deviation was the absorbed dose difference between VMAT 10X and IMRT 10X with the mean absorbed dose of 0.01 ± 5.79 Gy and 95% confident interval in the range from − 16.70 Gy to 16.71 Gy. The values of P > |t| presenting the difference among the six groups of treatment plans ranged from 0.974 to 1 (p-value < 0.05).
In the MultiTarget case, the absorbed doses received by 10% of the considered volume (D10) including SuperTarget and InfTarget of the VMAT plans were 26.10 Gy ± 0.07 Gy and 13.50 Gy ± 0.18 Gy, respectively, while the absorbed doses of these parameters in the IMRT plans were 29.39 ± 0.39 Gy and 19.16 Gy ± 0.40 Gy, and were 34.12 ± 3.04 Gy and 24.18 ± 2.72 Gy, respectively in the TG 119 report.
For the Prostate case, the PTV D5 absorbed dose was 78.97 Gy ± 0.44 Gy in this work and 81.43 Gy ± 1.56 Gy in the TG 119 report. The PTV D5 absorbed doses were 79.34 Gy ± 0.01 Gy for the IMRT 6X and VMAT 6X plans and 78.61 Gy ± 0.18 Gy for the IMRT 10X and VMAT 10X plans. The Rectum D30 absorbed dose of 54.19 Gy for the IMRT 10X plan in this study was lower than that of 62.38 Gy for the VMAT 6X plan, and 6.303 Gy for the VMAT 10X plan. Similarly, the Bladder D30 absorbed dose in this study was 32.40 Gy for the IMRT 10X plan, while it was 35.49 Gy for the VMAT 6X plan, and 34.68 Gy for the VMAT 10X plan. In the IMRT 6X plan of this study, the Rectum D30 and Bladder D30 absorbed doses were 67.91 Gy and 51.47 Gy, respectively, while they were 65.36 Gy and 43.94 Gy, respectively, in the TG 119 report.
Regarding to the Head/Neck case, the cord absorbed doses were 32.34 Gy ± 0.70 Gy in the VMAT plans, 38.25 Gy ± 1.45 Gy in the IMRT plans and 37.41 Gy ± 2.50 Gy in the TG 119 report. The Parotid absorbed dose D50 was 18.11 Gy ± 0.04 Gy for the VMAT 10X plan and in a range from 14.61 Gy to 16.19 Gy for the three other plans, while it was 17.98 Gy ± 1.84 Gy in the TG 119 report.
For the C-Shape easy case, the Cord D10 and Cord D5 absorbed doses were 21.13 Gy ± 1.63 cGy and 22.36 Gy ± 1.44 Gy, respectively. The D10 absorbed dose of the cord in the VMAT plans of this work was 19.74 Gy ± 0.39 Gy, while it was 22.50 Gy ± 0.44 cGy in the IMRT plans and 22.00 Gy ± 3.14 Gy in the TG 119 report.
With the C-Shape hard case, Fig. 5 illustrates the absorbed dose distribution of the plans receiving 95% of the prescribed dose and the maximum absorbed doses of the plans for both IMRT and VMAT for the C-Shape easy and C-Shape hard cases. Figure 6 presents the cord absorbed doses D10 of the C-Shape hard which decreased to 13.52 Gy and 12.68 Gy for the IMRT 6X and VMAT 6X plans, respectively. For the TG 119 report (Ezzell et al. 2009) and some previous studies (Saminathan et al. 2011; Mynampati et al. 2012; Kaushik et al. 2016; Sharma et al. 2017; Nainggolan and Pawiro 2019; Zhang et al. 2020), the mean of Core D10 absorbed doses were 15.59 Gy ± 1.18 Gy (n = 6) for IMRT 6X and 16.03 Gy ± 3.11 Gy (n = 4) for VMAT 6X (Thomas and Chandroth 2014; Kadam and Sharma 2016; Kaushik et al. 2016; Sharma et al. 2017; Nainggolan and Pawiro 2019; Zhang et al. 2020). The Cord D5 absorbed doses of this work for the C-shape hard case were 15.52 Gy for the IMRT 6X plan and 14.14 Gy for the VMAT 6X plans, while the results of other works were 16.43 Gy ± 0.65 Gy (n = 3) for the IMRT 6X plan (Ezzell et al. 2009; Saminathan et al. 2011; Mynampati et al. 2012) and 16.77 Gy for the VMAT 6X plan (Mynampati et al. 2012).
The results of the composite planar absorbed dose measurements obtained in the present are work shown in Table 6. The percentage of the gamma passing rate with the evaluation criteria of 3% dose difference (DD) and 3 mm distance to agreement (DTA) were 100% for P1, and 99.7% for P2. The average percentage of passing gamma for the five test cases including MultiTarget, Prostate, Head/Neck, C-Shape easy and C-Shape hard was 99.90 ± 0.14% with a CLplanar value of 0.38 (i.e., 99.62% passing). In contrast, the average percentage of passing gamma of the TG 119 report is 96.3 ± 4.4%, with a CLplanar value of 12.4 (i.e., 87.6% passing).
The results for the per-field measurement are shown in Table 7. The maximum and the minimum percentage of gamma passing rates (3% DD/3 mm DTA) were 99.2% and 100%, respectively. The average percentage of passing gamma was 99.76 ± 0.56% with a CLplanar value of 1.34, while the percentage of passing TG 119 results was 97.9 ± 2.5% with a CLplanar value of 7.0.
Discussion
Regarding to point dose measurements, the absorbed dose differences for the P1 and P2 test geometries in Table 3 showed that the IMRT/VMAT system delivered similar absorbed doses, because the measured and planned absorbed doses in this work were not statistically significantly different. With the results of the point absorbed dose measurements given in Tables 4 and 5, the CLs obtained for the high-absorbed dose and low absorbed-dose areas were better than the CL of 0.045 and 0.047 in the TG 119 report.
Form the data of all treatment plans (Fig. 4) it can be seen that the absorbed doses for the IMRT and VMAT test cases obtained in this work achieved the TG 119 goals, except for the core of the C-shape hard case. Besides, the p values in Table 2 demonstrate that the results of all the IMRT and VMAT plans obtained in the present study are almost the same as the results reported in the TG 119 report.
In the present study, the results obtained for the MultiTarget case were better than those reported in the TG 119 report, because the absorbed doses of the CentralTarget D99 met the goals while the result of the TG 119 report did not achieve the required criteria. In Fig. 7 (A), for SuperTarget D10 the results of this work were similar to those reported in some previous studies (e.g., Mynampati (2012), and Nainggolan (2019), with the absorbed doses in VMAT plans being lower than those in IMRT plans. This means that high absorbed doses were somewhat better controlled in the VMAT plans than in the IMRT plans. However, in studies by Thomas 2014; Sharma 2017, and Jun Zhang (2021), the absorbed doses in IMRT plans were lower than those in VMAT plans. For the InferTarget D10, the present results were similar to those obtained in some previous studies (e.g., Mynampati (2012), Nainggolan (2019), and Jun Zhang (2021), with the absorbed doses in VMAT plans being lower than those in IMRT plans, while the opposite results were received in the studies of Thomas (2014), and Sharma (2017). In general, it can be seen that none of the results were identical, which can be due to the decision or expectation of the involved planners or dosimetrists (Ezzel 2009). However, the results showed that in most of the studies, the VMAT technique controlled high absorbed doses better than the IMRT technique, because in the high-absorbed dose area, the absorbed doses in the VMAT plans of this work and in various other studies (e.g., Mynampati (2012), Thomas 2014; Seema Sharma 2017, Nainggolan (2019), and Jun Zhang (2021) were lower than those in IMRT plans. Thus, in those cases the VMAT technique was more conformal than the IMRT technique.
Comparison of the planning absorbed doses obtained in the present work and those reported in other studies; IMRT (Intensity-Modulated Radiation Therapy); VMAT (Volumetric Modulated Arc Therapy); The notation “DXX” means the minimum absorbed dose covering XX% of the volume; PTV (Planning Target Volume); LT (Left); RT (Right); SuperTarget (Superior target); InferTarget (Inferior target)
For the Prostate case (Fig. 7 (B), the present study demonstrated that in the high-absorbed dose area the absorbed doses to the target were controlled better than those in the TG 119 report, because the present study provided lower PTV D5 values. In addition, it was found that in the high-absorbed dose area the absorbed doses were lower for higher energies, because the PTV D5 absorbed doses of the IMRT 10X and VMAT 10X plans were lower than those of the IMRT 6X and VMAT 6X plans (data not shown in Fig. 8). The Rectum D30 and the Bladder D30 absorbed doses for the IMRT 10X plan in this study were better than the corresponding values in the VMAT 6X and VMAT 10X plans, so the IMRT technique was more suitable for the Prostate case. This finding shows that the IMRT technique is still useful compared to VMAT, at least in some cases. In the IMRT 6X plan, the Rectum D30 and Bladder D30 absorbed doses in this study were higher compared to those provided in the TG 119 report. The reason was that in the present work the involved dosimetrist decided that the plan should achieve a higher absorbed dose in the target volume PTV D95, leading to higher absorbed doses in OARs. Thus, the results for the Prostate obtained in the present study suggest that the absorbed doses depended not only on the radiotherapy technique and energy, but also on the decision of the dosimetrists (Ezzell et al. 2009).
For the Head/Neck case (Fig. 7 (C)), the cord absorbed doses in the VMAT plans were significant lower compared to those in the IMRT plans and to the TG 119 results. This illustrates again that VMAT is better than IMRT for protecting the spinal cord. However, the parotid absorbed dose D50 for the VMAT 10X plan was quite higher than the absorbed doses in the other plans of this work and in the TG 119 report (data not shown in Fig. 7). This suggests that, although the VMAT technique provided impressive results with the PTV and spinal cord absorbed doses, the parotid absorbed doses of the VMAT 10X plan were unfavorable compared with those of the IMRT plans. Consequently, the absorbed doses obtained in the present study for the Head/Neck case indicated that the VMAT technique is not always better than the IMRT technique. In other words, the usefulness of the IMRT technique was proven here in some cases.
For the C-Shape easy case (Fig. 7 (D)), the Cord D10 absorbed doses of the VMAT plans obtained in this work were lower compared to the absorbed doses of the IMRT plans and the TG 119 report. In fact, VMAT gave better results, because for the same absorbed dose to the target absorbed doses were smaller for the high absorbed dose area and the cord. So, VMAT was more conformative than IMRT, for the C-shape geometry.
For the C-Shape hard case (Fig. 7 (E) the absorbed dose goals in the TG 119 protocol are very strict, so they have not yet been met until now. Nevertheless, despite not meeting these goals, in the present study the Cord D10 values of all 6X IMRT and 6X VMAT plans were lower than those in the TG 119 report (Ezzell et al. 2009) and those reported in previous studies (Thomas and Chandroth 2014; Kadam and Sharma 2016; Kaushik et al. 2016; Sharma et al. 2017; Nainggolan and Pawiro 2019; Zhang et al. 2020). Particularly, the Cord D5 values of the C-shape hard geometry obtained in the present study for the IMRT 6X and VMAT 6X plans were the lowest compared to those obtained in previous studies (Thomas and Chandroth 2014; Kadam and Sharma 2016; Kaushik et al. 2016; Sharma et al. 2017; Nainggolan and Pawiro 2019; Zhang et al. 2020). It can be seen that the absorbed dose distribution in Fig. 5 given by the IMRT technique is much less homogeneous compared to that given by the VMAT technique. In addition, the absorbed dose distributions of the C-Shape hard case were more heterogenous than those of the C-Shape easy case, because it was designed to meet the stricter goals. This demonstrates that the absorbed dose distribution is affected by the type of technique and the rigidity of the applied criteria. This means that the Cord D10 and D5 absorbed doses can be reduced more now. In the present study, the cord absorbed dose calculated using VMAT 6X was lower than that using IMRT 6X. This suggests that VMAT is more appropriate than IMRT in the case of a tumour with a “C”-letter shape.
Finally, the measured composite planar absorbed doses in Table 6 were lower than the absorbed doses given in the TG 119 report. This demonstrates that the dosimetry equipment, including the ion chamber and the EPID, and the treatment delivery system was commissioned with adequate accuracy. This means that the linac delivers an accurate absorbed dose and the EPID is calibrated correctly.
Conclusions
Based on the TG 119 test suites used in the present study, the following conclusions can be drawn: (i) VMAT controlled the absorbed doses in the high-absorbed dose area better than IMRT; (ii) IMRT is still more conformal compared to VMAT for certain treatment sites; (iii) VMAT is more conformal than IMRT for a target with the shape of letter C; and (iv) Treatment planning results were affected by the techniques used (VMAT or IMRT) and photon beam energies.
The overall results obtained in the present study met the AAPM TG 119 requirements, and were even better. It is concluded that IMRT is still more conformal compared to the more advanced VMAT in some cases. However, VMAT has of course its advantages. At present, IMRT and VMAT are parallelly used. Some aspects should be considered, such as radiotherapy technique, beam energy, treatment site, and tumour geometry, to choose the best conformal way to deliver a dose to the patient. Therefore, to get the optimal treatment modality, in the future a more detailed conformity evaluation of these techniques should be performed including other treatment sites, tumour geometries, tumour control probability (TCP), and normal tissue complication probability (NTCP), etc.
References
AAPM (2009) TG – 119 IMRT commissioning tests instructions for planning, measurement, and analysis. Differences 21:308–310
Afrin KT, Ahmad S (2021) Is IMRT or VMAT superior or inferior to 3D conformal therapy in the treatment of lung cancer? A brief literature review. J Radiother Pract 21:416–420. https://doi.org/10.1017/S146039692100008X
Akbas U, Koksal C, Kesen ND et al (2019) Nasopharyngeal carcinoma radiotherapy with hybrid technique. Med Dosim 44:251–257. https://doi.org/10.1016/j.meddos.2018.09.003
Bucci MK, Bevan A, Roach M (2005) Advances in Radiation Therapy: conventional to 3D, to IMRT, to 4D, and Beyond. CA Cancer J Clin 55:117–134. https://doi.org/10.3322/canjclin.55.2.117
Chen W, Yang X, Jiang N et al (2017) Intensity-modulated radiotherapy, volume-modulated arc therapy and helical tomotherapy for locally advanced nasopharyngeal carcinoma: a dosimetric comparison. Transl Cancer Res 6:929–939. https://doi.org/10.21037/tcr.2017.09.48
Cheung KY (2006) Intensity modulated radiotherapy: advantages, limitations and future developments. Biomed Imaging Interv J 2:1–19. https://doi.org/10.2349/biij.2.1.e19
De Martin T, Moutrie Z, Tighe D et al (2018) Volumetric modulated arc therapy (VMAT) for skin field cancerisation of the nose - A technique and case report. Int J Radiol Radiat Ther 5:142–148. https://doi.org/10.15406/ijrrt.2018.05.00152
Diamantopoulos S, Platoni K, Patatoukas G et al (2019) Treatment plan verification: a review on the comparison of dose distributions. Phys Med 67:107–115. https://doi.org/10.1016/j.ejmp.2019.10.029
Ezzell GA, Burmeister JW, Dogan N et al (2009) IMRT commissioning: multiple institution planning and dosimetry comparisons, a report from AAPM Task Group 119. Med Phys 36:5359–5373. https://doi.org/10.1118/1.3238104
Gordon JD, Krafft SP, Jang S et al (2011) Confidence limit variation for a single IMRT system following the TG119 protocol. Med Phys 38:1641–1648. https://doi.org/10.1118/1.3555298
Holt A, Van Gestel D, Arends MP et al (2013) Multi-institutional comparison of volumetric modulated arc therapy vs. intensity-modulated radiation therapy for head-and-neck cancer: a planning study. Radiat Oncol 8:1–11. https://doi.org/10.1186/1748-717X-8-26
Hussein M, Clark CH, Nisbet A (2017) Challenges in calculation of the gamma index in radiotherapy – towards good practice. Phys Med 36:1–11. https://doi.org/10.1016/j.ejmp.2017.03.001
IAEA (2008) International Atomic Energy Agency: Transition from 2-D Radiotherapy to 3-D Conformal and Intensity Modulated Radiotherapy,. In: Iaea-Tecdoc-1588. pp 1–61
Kadam A, Sharma S (2016) Estimation of local confidence limit for 6 MV photon beam IMRT system using AAPM TG 119 test protocol. Int J Cancer Ther Oncol 4:4110. https://doi.org/10.14319/ijcto.41.10
Kaushik S, Tyagi A, Kumar L et al (2016) Validation of intensity-modulated radiotherapy commissioning as per recommendations in test plans of the American Association of Physicists in Medicine task group 119 report. Radiat Prot Environ 39:138–145. https://doi.org/10.4103/0972-0464.194960
Kim SJ, Lee MJ, Youn SM (2018) Radiation therapy of synchronous bilateral breast carcinoma (SBBC) using multiple techniques. Med Dosim 43:55–68. https://doi.org/10.1016/j.meddos.2017.08.003
Kishimoto S, Yamashita M (2018) A successive LP approach with C-VaR type constraints for IMRT optimization. Oper Res Heal Care 17:55–64. https://doi.org/10.1016/j.orhc.2017.09.007
Kruszyna-Mochalska M (2018) EPID-based daily verification of reproducibility of patients’ irradiation with IMRT plans. Rep Pract Oncol Radiother 23:309–314. https://doi.org/10.1016/j.rpor.2018.05.003
Lee DK (2016) Alternatives to P value: confidence interval and effect size. Korean J Anesthesiol 69:555–562. https://doi.org/10.4097/kjae.2016.69.6.555
Lee S, Lee DK (2018) What is the proper way to apply the multiple comparison test? Korean J Anesthesiol 71:353–360. https://doi.org/10.4097/kja.d.18.00242
Li C, Luo H, Song W et al (2023) Dosimetric comparison of four radiotherapy techniques for stage III non–small cell lung cancer. Oncol Lett 26:1–13. https://doi.org/10.3892/ol.2023.13933
Low DA, Harms WB, Mutic S, Purdy JA (1998) A technique for the quantitative evaluation of dose distributions. Med Phys 25:656–661. https://doi.org/10.1118/1.598248
Miften M, Olch A, Mihailidis D et al (2018) Tolerance limits and methodologies for IMRT measurement-based verification QA: recommendations of AAPM Task Group 218. Med Phys 45:e53–e83. https://doi.org/10.1002/mp.12810
Mynampati DK, Yaparpalvi R, Hong L et al (2012) Application of AAPM TG 119 to volumetric arc: therapy (VMAT). J Appl Clin Med Phys 13:108–116. https://doi.org/10.1120/jacmp.v13i5.3382
Nainggolan A, Pawiro SA (2019) Dosimetric evaluation of volumetric modulated arc therapy (VMAT) and intensity modulated radiotherapy (IMRT) using AAPM TG 119 protocol. J Biomed Phys Eng 9:395–408. https://doi.org/10.31661/jbpe.v0i0.839
Nithya L, Arunai Nambi Raj N, Rathinamuthu S, Pandey MB (2016) Analyzing the performance of the planning system by use of AAPM TG 119 test cases. Radiol Phys Technol 9:22–29. https://doi.org/10.1007/s12194-015-0328-z
Palta JR, Liu C, Li JG (2008) Quality Assurance of Intensity-Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys 71:108–112. https://doi.org/10.1016/j.ijrobp.2007.05.092
Rashid A, Ahmed Z, Memom MA, Hashim ASM (2021) Volumetric Modulated Arc Therapy (VMAT): a modern radiotherapy technique - A single institutional experience. Pakistan J Med Sci 37. https://doi.org/10.12669/pjms.37.2.2647
Redapi L, Rossi L, Marrazzo L et al (2022) Comparison of volumetric modulated arc therapy and intensity-modulated radiotherapy for left-sided whole-breast irradiation using automated planning. Strahlentherapie Und Onkol 198:236–246. https://doi.org/10.1007/s00066-021-01817-x
Rehman Jur, Zahra, Ahmad N et al (2018) Intensity modulated radiation therapy: a review of current practice and future outlooks. J Radiat Res Appl Sci 11:361–367. https://doi.org/10.1016/j.jrras.2018.07.006
Saminathan S, Manickam R, Chandraraj V (2011) Plan evaluation and dosimetric comparison of IMRT using AAPM TG119 test suites and recommendations. Australas Phys Eng Sci Med 34:55–61. https://doi.org/10.1007/s13246-011-0058-4
San-Miguel I, Carmona R, Luque L et al (2016) Volumetric Modulated Arc Therapy (VMAT) make a difference in retro-orbital irradiation treatment of patients with bilateral Graves’ ophthalmopathy. Comparative analysis of dosimetric parameters from different radiation techniques. Rep Pract Oncol Radiother 21:435–440. https://doi.org/10.1016/j.rpor.2016.03.001
Sangaiah A, Ganesh KM, Ramalingam K et al (2017) Dosimetric validation of volumetric modulated arc therapy (VMAT) using AAPM TG-119 Benchmark plans in an upgraded CLINAC 2100CD for flattening filter free (FFF) photon beams. Asian Pac J Cancer Prev 18:2965–2970. https://doi.org/10.22034/APJCP.2017.18.11.2965
Sharma S, Chander S, Subramani V et al (2017) IMRT and VMAT commissioning for Versa Hd Linear Accelerator using aapm TG-119. Int J Adv Res 5:352–362. https://doi.org/10.21474/ijar01/3819
StataCorp (2021) Stata: Release 17. Statistical Software. StataCorp LLC, College Station, Texas
Teoh M, Clark CH, Wood K et al (2011) Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol 84:967–996. https://doi.org/10.1259/bjr/22373346
Thomas M, Chandroth M (2014) Local confidence limits for IMRT and VMAT techniques: a study based on TG119 test suite. Australas Phys Eng Sci Med 37:59–74. https://doi.org/10.1007/s13246-014-0241-5
Xie Y, Bourgeois D, Guo B, Zhang R (2020) Postmastectomy radiotherapy for left-sided breast cancer patients: comparison of advanced techniques. Med Dosim 45:34–40. https://doi.org/10.1016/j.meddos.2019.04.005
Yang B, Zhu L, Cheng H et al (2012) Dosimetric comparison of intensity modulated radiotherapy and three-dimensional conformal radiotherapy in patients with gynecologic malignancies: a systematic review and meta-analysis. Radiat Oncol 7. https://doi.org/10.1186/1748-717X-7-197
Zhang J, Jiang D, Liu H et al (2020) Analyzation of the local confidence limits for IMRT and VMAT based on AAPM TG119 report. Med Dosim 45:66–72. https://doi.org/10.1016/j.meddos.2019.06.001
Acknowledgements
The authors would like to express their thanks to the anonymous reviewers of this manuscript for their critical review and helpful discussions.
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tam, D.T.M., Ho, P.L., Uy, P.Q. et al. Evaluation of the conformity of intensity-modulated radiation therapy and volumetric modulated arc therapy using AAPM TG 119 protocol. Radiat Environ Biophys (2024). https://doi.org/10.1007/s00411-024-01091-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00411-024-01091-2