Abstract
The aim of the study is to investigate factors that may cause radiation-induced lung disease (RILD) in patients undergoing stereotactic body radiotherapy (SBRT) for lung tumors. Medical records of patients treated between May 2018 and June 2019 with SBRT were retrospectively evaluated. All patients should have a diagnosis of either primary non-small cell lung cancer (NSCLC) or less than three metastases to lung from another primary. The median treatment dose was 50 Gy in 4–5 fractions. Tumor response and RILD were evaluated in thoracic computer tomography (CT) using RECIST criteria. 82 patients with 97 lung lesions were treated. The median age was 68 years (IQR = 62–76). With a median follow-up of 7.2 months (3–18 months), three patients had grade 3 radiation pneumonitis (RP). RILD was observed in 52% of cases. Patients who had RILD had a higher risk of symptomatic RP (p = 0.007). In multivariate analyses older age, previous lung radiotherapy history, and median planning treatment volume (PTV) D95 value of ≥ 48 Gy were associated with RILD. Local recurrence (LR) was observed in 5.1% of cases. There was no difference in overall survival and LR with the presence of RILD. Older age, previous lung radiotherapy history, and median PTV D95 value of ≥ 48 Gy seems to be associated with post-SBRT RILD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiation therapy (RT) is a major treatment modality for the management of non-small cell lung cancer (NSCLC). Recent advances in RT technology allow delivering higher doses of radiation to the tumor. However, the lung is a radiosensitive organ, and the delivery of high doses of radiation has been largely limited by normal tissue injury that is defined as radiation-induced lung disease (RILD).
In conventional (chemo) radiotherapy radiological RILD has been classically described as having two phases; the acute phase, referred to as radiation pneumonitis (RP) (within 1–3 months after RT) and the chronic phase, referred to as fibrosis (observed several months after RT and may progress slowly for months to years).
Stereotactic body RT (SBRT) enables the delivery of a very high radiation dose to the target volume while minimizing the dose to the adjacent normal tissues. Similar to conventional RT-induced changes detected by computerized tomography (CT), CT findings after lung SBRT can be classified into two phases: the acute phase (within 6 months after SBRT) and the chronic phase (later than 6 months). In most cases, radiologic changes of normal lung tissue do not occur before 3 months after SBRT. Clinical symptoms of acute radiation-induced lung injury develop within approximately 3–6 months after treatment (Linda et al. 2011; Zhu et al. 2019).
As a summary, CT findings after SBRT have a different appearance, geographic extent, and progression timeline compared to those following conventional RT for lung cancer. In particular, SBRT-induced changes are in the shape of consolidation, thus the differentiation from tumor recurrence can be very difficult (Linda et al. 2011). The aim of this study was to analyze follow-up thoracic CT images to identify factors that may cause RILD in patients undergoing SBRT for primary lung cancer or lung metastases.
Materials and methods
Our institution is performing lung SBRT since 2007. From our prospective lung SBRT database, medical records and treatment plans of 82 patients with 97 lung lesions at a single treatment machine were retrospectively evaluated. All patients had a diagnosis of either non-small cell lung cancer (NSCLC) or any oligometastatic cancer with less than three metastases to the lung parenchyma.
The cases underwent a free-breathing, end inspiration and end-expiration four-dimensional computed tomography (4DCT) planning scan of 1 mm slice thickness. The image data sets were transferred to Raystation Treatment Planning System (TPS) (RaySearch Laboratories, Stockholm, Sweden) and were reconstructed by a maximum intensity projection algorithm to generate internal target volume (ITV). Subsequently, a 3 mm isotropic expansion margin was applied to ITV to create the planning target volume (PTV). The number of fractions was chosen according to the location of the tumor. SBRT was delivered with 4DCT-based volumetric modulated arc therapy (VMAT) using the Elekta Versa HD system (Elekta, Sweden).
Treatment planning was made by Raystation TPS version 8A®. VMAT plans with two arcs were optimized to ensure coverage of 95% of prescription dose by PTV using 6 MV flattening filter-free (FFF) photon beams. Dose limitations for critical structures were determined according to institutional SBRT protocol (Hurmuz et al. 2020). All patients received 50 Gy in four or five fractions with 4D cone-beam CT (4D CBCT) image guidance. 4D CBCT acquisitions were performed with the Elekta Versa HD linac imaging system (XVI System, Elekta AB, Stockholm, Sweden) using Symmetry (Elekta AB, Stockholm, Sweden) algorithm.
Patients were generally seen at 1 month following SBRT, every 3 months for 2 years, every 6 months for year 3–5 and once yearly thereafter, according to our follow-up protocol. History and physical exam, and I.V. Thorax CT scan were obtained at each visit. Tumor response and RILD were evaluated using Response Evaluation Criteria in Solid Tumors (RECIST 1.1) (Eisenhauer et al. 2009), at a single center by two doctors, each with minimum 18 years of experience in thoracic radiotherapy (P.H. and M.C.). Local recurrence (LR) is defined as recurrence at the irradiated tumor nodule confirmed by FDG/PET-CT. RILD was defined as the presence of diffuse/patchy consolidation or ground-glass opacity in follow-up thoracic CTs (Linda et al. 2011). The toxicity was evaluated by the Common Terminology Criteria for Adverse Events (CTCAE v 4.0). The analysis was approved by the Ethics committee of Hacettepe University Faculty of Medicine (GO 19–667).
For statistical analyses, SPSS 22 (SPSS Inc., Chicago IL, USA) was used. Chi-square/Fischer’s exact test and Mann–Whitney U test were used for univariate analyses of categorical and non-normally distributed numerical variables, respectively. All covariates with a p value ≤ 0.25 in the univariate analysis were included in multivariate logistic regression models. In the multivariate analyses, p value of ≤ 0.10 was accepted as statistically significant (Mickey and Greenland 1989).
Results
Between May 2018 and June 2019, 97 lesions were treated with ITV-based SBRT using the Elekta Versa HD© treatment machine. The median patient age was 68 years (IQR = 62–76). Tumors were mostly located at the periphery of the lung (89%). Metastatic lesions constituted 54% of all lesions. The major primary sources of metastatic lesions were colorectal cancer (44%), sarcoma (13%), and lung cancer (10%). The characteristics of the patients are shown in Table 1. Median PTV was 15.3 cm3 (IQR = 7.2–27.9). For total lung, dose constraints were within the accepted limits as follows; Dmean = 2.6 Gy (IQR = 2.0–4.2), median V5 = 12.6% (IQR = 7.4–18.5), V10 = 7.3% (IQR = 4.7–11.0), and V20 = 3.2% (IQR = 1.9–5.8).
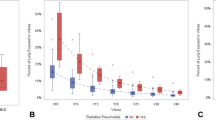
None of the patients had acute grade > 3 toxicity. With a median follow-up of 7.2 months (3–18 months), five patients had grade 2, three patients had grade 3 radiation pneumonitis (RP). Grade 3 RP was observed 18 to 24 weeks after SBRT and these patients needed to use short term steroids and intermittent oxygen. RILD in the treated volume was observed in 52% of cases. All patients had consolidation as RILD. A case with T2N0M0 primary NSCLC and his post-SBRT CT scans are shown in Fig. 1 and Fig. 2. Patients who had RILD had a higher risk of symptomatic RP (p = 0.007). We evaluated the effect of age, gender, primary diagnosis, disease status (primary/metastatic), history of chronic obstructive pulmonary disease (COPD), smoking, tumor location, tumor size, previous lung RT to the lung, chemotherapy, SBRT dose, median PTV D95 value, V10–V20 of total lung, and Dmean of the total lung on RILD.
73-year-old male patient diagnosed with T2N0M0 primary lung adenocarcinoma (a). Patient has previous smoking history (30 pack/year). SBRT of 50 Gy in five fractions was delivered to the lesion located in the left upper lobe (b). Red line indicates internal target volume and light blue line indicates planning target volume
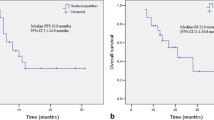
The results of univariate analyses are shown in Table 2. In the multivariate analyses, high age, previous lung RT history, and median PTV D95 value of ≥ 48 Gy were associated with RILD (Table 3). LR was observed in five cases. Two patients with LR were treated due to lung metastases (pancreas and thymic carcinoma primaries) and the three patients had primary early stage NSCLC. Presence of RILD did not affect overall survival (80.9% vs. 93.1%; p = 0.869). There was no difference in LR (5.7% vs. 10.7%; p = 0.648) and local recurrence-free survival (LRFS) rates (1-y LRFS 74.1% vs. 71.9%; p = 0.330) between patients with and without RILD.
Discussion
Due to the higher dose per fraction and radiobiological mechanisms, RILD represents different patterns in patients receiving SBRT to the lung. In conventional RT, clinical symptoms of RILD are related to age, history of smoking, presence of pre-existing lung disease, the volume of irradiated lung, pre-treatment pulmonary function, and use of systemic therapies (Hanania et al. 2019; Giuranno et al. 2019). In the current study, we found that high age, previous lung RT and median PTV D95 value of ≥ 48 Gy were associated with RILD in patients who were treated with lung SBRT.
Liu et al. analyzed 72 patients treated with SBRT after previous thoracic RT and found 20.8% of patients developed grade ≥ 3 RP. It was shown that poor performance status, pre-SBRT FEV1 ≤ 65%, V20 ≥ 30% of the composite plan, and an initial PTV in the bilateral mediastinum were significantly associated with increased risk of RP (Liu et al. 2012). Though previous thoracic RT is not a contraindication for SBRT to recurrent or secondary lung tumors, patient selection criteria should be strict to prevent RILD. In our study, the previous RT term includes cases who received treatment to the ipsilateral or contralateral lung. None of the patients had SBRT to the previously irradiated lesion. Although composite treatment plans were successful to meet the dose criteria for organs at risk including lung, RILD was higher in the group with previous RT history. This might be due to the reaction of the previously irradiated lung to radiation that induces cascade of molecular pathways.
In our study, 52% of patients had RILD following SBRT. It is known that 60–100% of patients treated with lung SBRT can be expected to have radiographic changes (Bradley 2007; Aoki et al. 2004; Matsuo et al. 2007). Matsuo et al. (2007) reported mass-like consolidation in 68% of cases at a median time of 5 months after lung SBRT. The time of appearance of the LR was median 7 months and there was no significant difference in the time of appearance between RILD and LR. Follow-up examination revealed that 89% of the mass-like consolidations were RILD and 11% were LR. The authors stated that the size of the mass-like consolidations did not increase in any RILD cases after 12 months.
In the current study, there was no correlation between regular dosimetric factors (V10 of the total lung, V20 of the total lung, and Dmean of the total lung) and development of RILD. This might be due to homogenous and low OAR doses due to strict dosimetric constraints in our planning protocol. LR was observed in five cases and there was no difference in LR between patients with and without RILD. We did not observe any effect of the number of treatment fractionations on the outcome. However, it seems that the PTV D95 value of ≥ 48 Gy was associated with RILD.
All patients in the study were treated according to a homogeneous treatment protocol with a margin of 3 mm to ITV for PTV in the same treatment planning system. Flattening filter free (FFF) beams with a high dose rate were used for SBRT. FFF allows for approximately a 2–6 fold increase in the instantaneous dose of X-ray pulse delivered compared with conventional flattening filter (FF) photon beam (Prendergast et al. 2013). FFF method has the advantage of reduction in the dose to surrounding normal tissue, in particular lungs, by drawing sharp slope on the periphery of the tumor that might lead to lower side effects (Cashmore 2008; Vassiliev et al. 2006). However, physical properties of these beams together with potentially unknown radiobiological effects are the question of interest. Navarria et al. (2013) reported results in patients treated with SBRT for medically inoperable early-stage NSCLC. All the 86 patients receiving FF beams were treated with 3D technique whereas all the 46 patients receiving FFF beams were treated with VMAT Rapid Arc technique. There was no difference between the two groups in toxicity. In the FFF group, 4% of patients experienced grade 3 pulmonary toxicity. However, there was a significant earlier radiological response in the FFF group with a 1-year local control rate of 100% vs. 92.5% compared with the FF beams group (p = 0.03).
Aoki et al. (2018) compared the FFF technique in volumetric modulated arc therapy with a FF method for lung SBRT in 65 cases. There was no difference in treatment plans, outcomes, and toxicity between groups. One-year local control rates were 97.1 and 90.0% in the FF group and FFF groups, respectively (p = 0.33). Grade 3 pneumonitis was observed in 5.8% of FF patients and 3.4% of FFF patients (p = 1.00). No other adverse events ≥ grade 3 were observed. The results of the study suggest that VMAT-SBRT using the FFF technique shortens the treatment time for lung SBRT while maintaining a high local control rate with low toxicity. We also delivered VMAT-SBRT using the FFF technique and our toxicity rates seem to be low and compatible with the literature.
In the current study, patients with RILD and suspicious CT findings underwent FDG-PET-CT to evaluate LR or metastases. We do not have tissue confirmation of LR. However it is known that in detecting residual or recurrent NSCLC, FDG-PET-CT has sensitivity and specificity of 100% and 96–98%, respectively; when performed 1 year after SBRT or the time of suspicious metastases (Takeda et al. 2013; Pastis et al. 2014). It is also known that most of the patients referred to lung SBRT lack tissue diagnosis due to medical reasons. Currently, there are no robust predictors for pathological complete response (pCR) after SBRT. However, advances in functional imaging, radiomics, and liquid-based genomics may be helpful in non-invasive assessment of tumor response to treatment. Use of artificial intelligence (AI)-based CT data analysis on the diagnosis of lung injury pattern after SBRT is on the way and the differentiation would be more accurate by combining theoretical knowledge, visual judgment and experience (Prayer et al. 2020).
Our study has some limitations: the small patient numbers and its retrospective nature make it difficult to draw definitive conclusions. Median follow-up time of 7.2 months might appear short, however it seems a proper time for evaluation of acute RILD that occurs median 3–6 months after SBRT. Although the treatment was delivered homogeneously with a standard protocol, the patients represent a heterogeneous group. We included patients with early-stage primary NSCLC and metastases to lung as the primary purpose of the study was to evaluate RILD and tumor control in the irradiated area. However, future studies evaluating RILD, LC, and overall survival relationships could be planned in a homogenous patient group.
In conclusion, RILD is not an unusual consequence of lung SBRT. Increased age, previous RT history, and higher PTV D95 doses are potential risk factors for RILD. With the increase in the use of SBRT in the oligometastatic disease concept, prospective studies using modern non-invasive response evaluation methods will be helpful for further understanding RILD.
Availability of data and material
Data are available upon reasonable request.
References
Aoki T, Nagata Y, Negoro Y, Takayama K, Mizowaki T, Kokubo M, Oya N, Mitsumori M, Hiraoka M (2004) Evaluation of lung injury after three-dimensional conformal stereotactic radiation therapy for solitary lung tumors: CT appearance. Radiology 230(1):101–108. https://doi.org/10.1148/radiol.2301021226
Aoki S, Yamashita H, Haga A, Nawa K, Imae T, Takahashi W, Abe O, Nakagawa K (2018) Flattening filter-free technique in volumetric modulated arc therapy for lung stereotactic body radiotherapy: a clinical comparison with the flattening filter technique. Oncol Lett 15(3):3928–3936. https://doi.org/10.3892/ol.2018.7809
Bradley J (2007) Radiographic response and clinical toxicity following SBRT for stage I lung cancer. J Thorac Oncol 2(7 Suppl 3):S118-124. https://doi.org/10.1097/JTO.0b013e318074e50c
Cashmore J (2008) The characterization of unflattened photon beams from a 6 MV linear accelerator. Phys Med Biol 53(7):1933–1946. https://doi.org/10.1088/0031-9155/53/7/009
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Giuranno L, Ient J, De Ruysscher D, Vooijs MA (2019) Radiation-induced lung injury (RILI). Front Oncol 9:877–877. https://doi.org/10.3389/fonc.2019.00877
Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M (2019) Radiation-induced lung injury: assessment and management. Chest 156(1):150–162. https://doi.org/10.1016/j.chest.2019.03.033
Hurmuz P, Cengiz M, Ozyigit G, Akkas EA, Yuce D, Yilmaz MT, Yildiz D, Zorlu F, Akyol F (2020) Stereotactic body radiotherapy in patients with early-stage non-small cell lung cancer: does beam-on time matter? Jpn J Clin Oncol 50(10):1182–1187. https://doi.org/10.1093/jjco/hyaa093
Linda A, Trovo M, Bradley JD (2011) Radiation injury of the lung after stereotactic body radiation therapy (SBRT) for lung cancer: a timeline and pattern of CT changes. Eur J Radiol 79(1):147–154. https://doi.org/10.1016/j.ejrad.2009.10.029
Liu H, Zhang X, Vinogradskiy YY, Swisher SG, Komaki R, Chang JY (2012) Predicting radiation pneumonitis after stereotactic ablative radiation therapy in patients previously treated with conventional thoracic radiation therapy. Int J Radiat Oncol Biol Phys 84(4):1017–1023. https://doi.org/10.1016/j.ijrobp.2012.02.020
Matsuo Y, Nagata Y, Mizowaki T, Takayama K, Sakamoto T, Sakamoto M, Norihisa Y, Hiraoka M (2007) Evaluation of mass-like consolidation after stereotactic body radiation therapy for lung tumors. Int J Clin Oncol 12(5):356–362. https://doi.org/10.1007/s10147-007-0691-9
Mickey RM, Greenland S (1989) The impact of confounder selection criteria on effect estimation. Am J Epidemiol 129(1):125–137. https://doi.org/10.1093/oxfordjournals.aje.a115101
Navarria P, Ascolese AM, Mancosu P, Alongi F, Clerici E, Tozzi A, Iftode C, Reggiori G, Tomatis S, Infante M, Alloisio M, Testori A, Fogliata A, Cozzi L, Morenghi E, Scorsetti M (2013) Volumetric modulated arc therapy with flattening filter free (FFF) beams for stereotactic body radiation therapy (SBRT) in patients with medically inoperable early stage non small cell lung cancer (NSCLC). Radiother Oncol 107(3):414–418. https://doi.org/10.1016/j.radonc.2013.04.016
Pastis NJ Jr, Greer TJ, Tanner NT, Wahlquist AE, Gordon LL, Sharma AK, Koch NC, Silvestri GA (2014) Assessing the usefulness of 18F-fluorodeoxyglucose PET-CT scan after stereotactic body radiotherapy for early-stage non-small cell lung cancer. Chest 146(2):406–411. https://doi.org/10.1378/chest.13-2281
Prayer F, Röhrich S, Pan J, Hofmanninger J, Langs G, Prosch H (2020) Artificial intelligence in lung imaging. Radiologe 60(1):42–47. https://doi.org/10.1007/s00117-019-00611-2
Prendergast BM, Fiveash JB, Popple RA, Clark GM, Thomas EM, Minnich DJ, Jacob R, Spencer SA, Bonner JA, Dobelbower MC (2013) Flattening filter-free linac improves treatment delivery efficiency in stereotactic body radiation therapy. J Appl Clin Med Phys 14(3):4126. https://doi.org/10.1120/jacmp.v14i3.4126
Takeda A, Kunieda E, Fujii H, Yokosuka N, Aoki Y, Oooka Y, Oku Y, Ohashi T, Sanuki N, Mizuno T, Ozawa Y (2013) Evaluation for local failure by 18F-FDG PET/CT in comparison with CT findings after stereotactic body radiotherapy (SBRT) for localized non-small-cell lung cancer. Lung Cancer 79(3):248–253. https://doi.org/10.1016/j.lungcan.2012.11.008
Vassiliev ON, Titt U, Pönisch F, Kry SF, Mohan R, Gillin MT (2006) Dosimetric properties of photon beams from a flattening filter free clinical accelerator. Phys Med Biol 51(7):1907–1917. https://doi.org/10.1088/0031-9155/51/7/019
Zhu X, Li X, Gu H, Yu W, Fu X (2019) Radiation-induced lung injury patterns and the misdiagnosis after SBRT of lung cancer. Eur J Radiol 121:108708. https://doi.org/10.1016/j.ejrad.2019.108708
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hurmuz, P., Cengiz, M., Esen, C.S.B. et al. Factors affecting post-treatment radiation-induced lung disease in patients receiving stereotactic body radiotherapy to lung. Radiat Environ Biophys 60, 87–92 (2021). https://doi.org/10.1007/s00411-020-00878-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-020-00878-3