Abstract
The purpose of this study was to systematically investigate how high doses of sparsely and densely ionizing radiations influence the proliferation time of lymphocytes in short-term cultures and, consequently, the observed frequencies of dicentric and centric ring chromosomes. Peripheral blood samples from five volunteers were irradiated with high doses of 200 kV X-rays and with neutrons with a mean energy of <E n>=2.1 MeV. First division metaphase cells were collected after different culture times of 48, 56, and 72 h and dicentrics, centric ring chromosomes, and acentric fragments were determined. The data hint at considerable mitotic delay. The main increase in the number of chromosome aberrations occurred between 48 and 72 h after an X-ray exposure and between 56 and 72 h after neutron exposure. When the data were used for a calibration of aberration frequency versus dose, subsequent dose estimations resulted, however, in comparable values. Thus, in spite of the influence of mitotic delay on observable chromosome aberrations, at least for the radiation types investigated here, a culture time of 48 h is acceptable for biological dosimetry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The analysis of dicentric and centric ring chromosomes in human lymphocyte metaphases is the most reliable and sensitive method to quantify exposure to ionizing radiation for purposes of biological dosimetry [1–4]. It is known that several factors can influence the yield of chromosomal aberrations observed in the first post-irradiation metaphase after low or high linear energy transfer (LET) radiation. One of these factors is the applied culture time; in general 48 h is used.
The yield of aberrant cells depends on the cell cycle kinetics of the exposed cell population. The cell cycle of aberrant cells may be delayed in a way that the cell division cannot be completed within a culture time of 48 h. Consequently, these aberrant cells would not be observed during the chromosome aberration analysis. Such a mitotic delay was described by Lloyd et al. [5] after a high-dose irradiation. In extended studies with high-LET particle exposure, a drastic increase in chromosomal damage was observed with prolonged culture times, and especially the highly damaged cells reached mitosis significantly later than the undamaged cells [6–9]. Low-LET irradiation was reported to exert only a small effect on the cell cycle [10, 11]. This difference between the high and low-LET irradiation is important for the estimation of the relative biological effectiveness (RBE) of densely ionizing radiation. When highly aberrant cells cannot reach or complete their mitosis within 48 h of culture time, the damage originating from high-LET radiation may be underscored [8]. In the case of biological dosimetry, this may lead to a systematic underestimation of the dose applied.
The purpose of this study was the systematic investigation of how high doses of sparsely and densely ionizing radiation influence the proliferation time and, as a consequence, the observed aberration frequency. Blood samples from five male volunteers were exposed to doses of 1.0, 2.0, 3.0, 4.0, and 5.0 Gy for 200 kV X-rays and to doses of 0.1, 0.3, 0.5, 0.7, and 1.0 Gy for neutrons with a mean energy of <E n>=2.1 MeV. Chromosomal aberrations were scored in metaphases reaching the first mitosis at culture times of 48 h, 56 h, and 72 h. To reduce statistical uncertainties on the aberration yields, large samples of metaphase cells were analyzed.
Material and methods
Ethics committee and selection of blood donors
The Committee of Ethics of the General Medical Council in Bremen, Germany, approved the project on 23 November 2002. Five healthy male volunteers (42–53 years of age) were selected considering the following exclusion criteria: (1) previous or ongoing radiotherapy and/or therapy with cytotoxic drugs; (2) previous or current smoking; (3) nuclear-medical examinations during the past 2 years; (4) diagnostic medical radiation exposure higher than average; (5) classified and/or badge-monitored occupational radiation exposure. The donors were informed in detail about the aim of the project and all of them gave their written consent.
Blood collection and in vitro irradiation
The peripheral blood samples were taken from the volunteers by venipuncture late in the evening before irradiation and constantly kept at 37°C. The irradiation of the blood samples with 200 kV X-rays (RT 200 Müller; 15 mA; filtration: first half-value layer: 1.5-mm Cu, second half-value layer: 2.4-mm Cu; 1-mm Cu; dose rate: 0.52 Gy/min) was carried out at the Centre of Environmental Research and Technology (UFT) of the University of Bremen. The blood samples were left in 7.5-ml standard Lithium–Heparin polyethylene tubes (Sahrstedt, Germany) with a 15.3-diameter. These were positioned exactly at a depth of 5.0 cm in a 23.0 cm×23.0 cm acrylic-glass water phantom. Doses of 1.0, 2.0, 3.0, 4.0, and 5.0 Gy were applied. The X-ray dose was determined in the centre of the blood samples. The dosimetry was carried out with a calibrated 1 cm3 ionization chamber type nr. M 23331-338 [Physikalisch-Technische Werkstätten Dr. Pychlau GmbH (PTW)] at the same position. According to the manufacturer the uncertainty of the calibration factor for the range 140–280 kV was u=±2%. The irradiation took place on three different dates. Before setting up lymphocyte cultures the samples were kept at 37°C for 4 h in order to ensure conditions comparable to those of the neutron exposure experiments and to allow some time for repair.
The neutron irradiations were performed at the ion accelerator facility of the Physikalisch-Technische-Bundesanstalt (PTB) in Braunschweig, Germany [12]. The intense neutron field was produced using the (9Be+d)-reaction with a deuteron energy of E d=3.4 MeV on a thick Be-target within a collimator [13, 14]. The neutron spectrum had a broad energy distribution [15], which extended from about 0.1 to 8 MeV, with about 75% of all neutrons in a maximum between 0.5 and 2.5 MeV. This resulted in a fluence-averaged mean neutron energy of <E n>=2.1 MeV. The distribution of LET in water ranged about 10–90 keV/μm with a fluence-averaged mean of about 40 keV/μm. The blood samples were also taken late in the evening before irradiation, kept at 37°C, and transferred to Braunschweig the next morning. The tubes containing the blood samples and a tissue-equivalent (TE) ionization chamber were located ‘free in air’ at 585-mm from the Be-target and 180-mm from the collimator, which defined a neutron field of 75 mm by 75 mm.
The absorbed dose to water was determined by means of the tissue-equivalent ionization chamber, consisting of A150-plastic and filled with TE-gas (EXRADIN, Type T2, Standard Imaging, USA). This ionization chamber reading was compared at the same position with a water calorimeter measurement in a neutron field with an average energy of <E n>=5.3 MeV. The absolute doses from both the instruments agreed within the relative standard uncertainty of 1.8% for the calorimeter [14]. The dose rate of the neutron field with <E n>=2.1 MeV is not sufficient for direct measurement with the water calorimeter. However, the above comparison at <E n>=5.3 MeV can be applied to the present measurements at <E n>=2.1 MeV, as the differences in neutron sensitivity of the ionization chamber, 0.939±5% [14] versus 0.96±10% [16], and in the ratios of kerma factors for A150-plastic and water, R=1.058 [14] versus R=1.067 [17], are much smaller than the overall measurement uncertainties of 7%.
A relative photon dose component of about 11% for this radiation field was determined in separate measurements with the ‘neutron-sensitive’ TE chamber and a ‘neutron-insensitive’ Geiger–Müller tube. A total dose rate of about 1.8 Gy h−1 could be achieved with a deuteron current of 80 μA. The relative standard uncertainty (k=1) of the doses quoted is 7%.
Nominal doses of 0.1, 0.3, 0.5, 0.7, and 1.0 Gy were applied. The actual doses are shown in Table 1. For greater simplicity we will refer in the following to the nominal doses. However, for estimating the coefficients of the calibration curve the actual doses of each experiment were used. After irradiation at room temperature, the samples were transferred back to Bremen in a portable incubator at 37°C. Immediately after arriving in Bremen (about 4 h after the irradiation) lymphocyte cultures were set up.
Lymphocyte culture
Lymphocyte cultures were set up in the Bremen laboratory according to a standardized cell-cycle controlling method described elsewhere [18]. Briefly, 0.5-ml peripheral blood was cultured in 5.6 ml RPMI 1640 medium (Seromed), supplemented with 17% foetal calf serum (Seromed), 0.5 mg streptomycin, 500 IU penicillin (Boehringer), 500 IU heparin-sodium (Ratiopharm), 0.036 mg phytohaemagglutinin (Seromed), and 0.027 mg bromodeoxyuridine (BrdU, Sigma). Following a 3 h treatment with 0.33 μg ml−1 colcemid, the lymphocyte metaphases were harvested after 48, 56 and 72 h in culture.
Cytogenetic analysis
After fluorescence plus Giemsa (FPG) staining [19], the chromosome aberration analysis was restricted to first division metaphases. Collection of metaphases was facilitated by an automated computerized system including a data management tool (MetaSystems, Altlussheim, Germany). According to the Bremen laboratory standards [20], only complete metaphases with 46 centromers were included in the analysis. All structural chromosome aberrations like dicentric chromosomes (dic), centric ring chromosomes (cRing), polycentric chromosomes such as tricentric (tric) and tetracentric (tetra) chromosomes, acentric fragments (excess ace), which comprised minutes and acentric rings, chromosome gaps and structural chromatid aberrations like triradial and quadriradial exchanges, chromatid breaks, and chromatid gaps were recorded. In the present analysis only dic, cRing, and ace were taken into account. Dic and cRing had to be accompanied by an acentric fragment. Polycentric chromosomes with n centromeres were counted as n-1 dicentrics. The identification and description is based on the criteria of the International System for Human Cytogenetic Nomenclature [21].
A total of 15,000 metaphases (5,000 each for the three different culture times) were scored for the 1.0 Gy dose of X-rays, 7,500 metaphases for 2.0 Gy, 4,500 metaphases for 3.0 Gy, 3,000 metaphases for 4.0 Gy and 3,000 metaphases for 5.0 Gy. For neutrons, 15,000 metaphases were scored for the 0.1 Gy dose, 7,500 metaphases for 0.3 Gy, 6,000 metaphases for 0.5 Gy, 4,500 metaphases for 0.7 Gy, and 3,000 metaphases for 1.0 Gy. For the determination of control values, 1,000 metaphases per volunteer were analyzed for each of the three different culture times.
To determine the proliferation index (PI) the proportion of cells in their first, second, and third or higher cell division were analyzed from 200 successive metaphases. The PI was calculated according to the following formula:
Statistical analysis
The dispersion index (ratio variance/mean) was used to test for Poisson distribution. Values of variance/mean<1 indicate underdispersion, while values>1 indicate overdispersion. Overdispersion is expected after exposure to densely ionizing radiation. The u test was applied to test for overdispersion, which is considered significant (P<0.05) when variance/mean exceeds 1.96 [22–24]. The standard error of the mean (SEM) was calculated for all aberration frequencies in the different experiments. The t -test was applied for analyzing whether the means of the dic+cRing frequencies between the three different culture times are statistically significant from each other. The parameters for the dose effect curve were obtained by least squares regression, using iteratively reweighted inverse Poisson variances as weights. Goodness of fit was tested with the Pearson χ2 value.
Results
Cell cycle delay after exposure to 200 kV X-rays
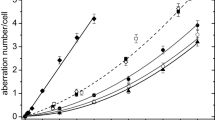
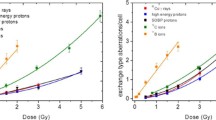
Chromosomal aberrations after exposure to 200 kV X-rays were analyzed in a total of 33,000 cells. Table 2 and Fig. 1 show the dose and culture time dependent increase in the frequency of dic + cRing. Significant differences can be observed between the culture times of 48 h and 72 h at all doses ≥2.0 Gy [2.0 Gy (P<0.01), 3.0 Gy (P<0.01), 4.0 Gy (P<0.01), and 5.0 Gy (P<0.05)], and after 2.0 and 4.0 Gy also between 56 and 72 h (P<0.01). The intercellular distribution of dic + cRing was found to be compatible with a Poisson distribution (Table 2); with one exception (3.0 Gy, 48 h). There is a slight, but statistically not significant, tendency towards underdispersion.
At all doses, cells with two or more dic + cRing (Cd2+) were observed more frequently after 56 and 72 h than after 48 h culture time (Table 2), suggesting that the analysis of highly damaged cells profits most from an extension of the culture time. Statistically significant differences between the culture times were observed after 2.0 Gy (more Cd2+ cells at 72 h than at 56 h; P<0.05) and after 4.0 Gy (more Cd2+ cells after 72 h than after 48 h; P=0.05). Considering all doses and a total of 33,000 analyzed cells, 1,628 Cd2+ cells (4.9%) were observed after 48 h culture time, 1,665 Cd2+ cells (5.0%) after 56 h, and 1,821 Cd2+ cells (5.5%) after 72 h. The proportion of Cd2+ cells is significantly (P<0.01) higher after 72 h than after the shorter culture times.
Tricentric chromosomes occurred at all doses and culture times (data not shown). Tetracentric chromosomes occurred also at all culture times, but only after 4.0 and 5.0 Gy. The highest yield was observed after 5.0 Gy. In the case of Cd3+ cells (cells with three or more dic + cRing) a significant difference (P<0.05) only showed between 48 and 72 h of culture time.
Excess acentric fragments not associated with a dic or cRing increase significantly after doses ≥3.0 Gy (Table 2). After irradiation with 3.0 Gy, excess fragments increased significantly between 48 and 72 h (P<0.01) and between 56 and 72 h (P<0.01). After 4.0 Gy, excess ace differed between 48 and 56 h (P<0.01) and between 48 and 72 h (P<0.01), after 5.0 between 48 and 56 h (P<0.01), 48 and 72 h (P<0.01), and 56 and 72 h (P<0.01).
The proliferation index confirmed the impact of high doses on mitosis. With increasing dose the PI approached a value of 1.0 for all culture times. Table 3 shows the yield of metaphases in first, second and third or higher cell divisions. At all doses, after 48 h of culture time 99.3–100% of the lymphocytes in metaphase were in their first cell division, after 56 h the main proportion (84.9–96.9%) of lymphocytes was also in their first cell division, and after 72 h the proportion of the second division metaphases exceeded the yield of the first division metaphases, except for the 5.0 Gy dose.
A linear-quadratic dose effect model (y=c + αD + βD²) was fitted to the observed frequencies of dicentrics and centric rings. Using the Bremen laboratory control value of 0.0005±0.0001 the model resulted in the following coefficients:
While the observed aberration frequencies after 48 h were compatible with a linear-quadratic dose effect curve, the agreement between the observed data and the fitted curve was poor after 56 and 72 h.
Cell cycle delay after exposure to <E n>=2.1 MeV neutrons
Chromosomal aberrations were analyzed in a total of 36,000 cells after exposure to <E n>=2.1 MeV neutrons. Table 2 and Fig. 2 show the dose-dependent increase in the frequency of dic + cRing with prolonged culture time. Significant differences could be observed after 0.1 and 0.5 Gy between 48 and 72 h culture time (P<0.05) and after 0.7 and 1.0 Gy between 48 and 72 h (P<0.01) and 56 and 72 h (0.7 Gy: P<0.05; 1.0 Gy: P<0.01). The intercellular distribution of dic and cRing in the investigated group deviates from Poisson distribution and shows a significant overdispersion (variance/mean >1.96) (Table 2).
The number of heavily damaged cells increased with dose and time of culture. At 0.7 and 1.0 Gy the number of Cd and Cd2+ cells increased significantly (P<0.01) between 48 and 72 h culture time. In addition the number of Cd3+ cells is significantly different between 48 and 72 h at 1.0 Gy. The number of cells with only one dic + cRing remained relatively constant at prolonged culture times.
Tricentric chromosomes appear at all doses and culture times, after applying X-rays. Tetracentric chromosomes appear only after 0.5, 0.7, and 1.0 Gy and only at 56 and 72 h culture time. Considering all the applied doses and 36,000 analyzed cells, a total of 443 Cd2+ cells (1.2%) is observed at 48 h culture time, 471 Cd2+ cells (1.3%) at 56 h and 603 Cd2+ cells (1.7%) at 72 h. There are significant differences between 48 and 72 h (P<0.01) and 56 and 72 h (P<0.01) of culture time. In the case of Cd3+ cells, significant differences remain between 48 and 72 h (P<0.01) and 56 and 72 h (P<0.05). An extension of culture time from 48 to 56 h does not considerably increase the number of cells with multiple aberrations. The most heavily damaged cells induced by neutrons require 72 h culture time to become apparent.
The number of excess ace increases significantly: after 0.5 Gy between 48 and 56 h (P<0.05) and 48 and 72 h (P<0.01), after 0.7 Gy between 48 and 72 h (P<0.01), and after 1.0 Gy between 48 and 56 h (P<0.01) and 48 and 72 h (P<0.01).
The PI decreased with increasing dose towards 1.0. Table 3 shows the yield of metaphases in their first, second, and third or higher cell divisions. At 48 h culture time 98.5–100% of lymphocytes in metaphase are in their first cell division, at 56 h the main proportion (76.1–86.9%) of lymphocytes is in their first cell division, at 72 h still a large part of metaphases is in the first cell division (47.6–65.7%). The percentage of the second division metaphases ranges between 26.7 and 42.3% and the percentage of the third and higher division metaphases varies between 6.0 and 10.1%.
The observed frequencies of dicentrics and centric rings after 48 and 56 h were fitted to a linear dose effect model: y=c + αD, since applying a linear-quadratic model resulted in negative coefficients for the quadratic term. After 72 h, however, a better fit was obtained using the linear-quadratic dose effect model: y=c + αD + βD². Applying the Bremen laboratory control value of 0.0005±0.0001 the models resulted in the following coefficients:
Discussion and conclusions
A number of investigations raised doubts about the common notion that lymphocytes show consistent aberration frequencies which are independent of the duration of culture time. Bender and Brewen [25] postulated two subpopulations of lymphocytes which can be distinguished by radiation sensitivity and cell cycle progression. Beek and Obe [26] described higher aberration frequencies in the early first metaphases compared to the late ones. In contrast, Hoffmann et al. [27] observed increasing frequencies of chromosomal aberrations in first metaphases from 48 h, over 70 h, and up to 94 h of culture time. Similar data were obtained by Boei et al. [28], who detected this increase after FPG staining as well as after applying fluorescence-in-situ-hybridization (FISH). However, others observed constant aberration frequencies in FISH experiments [29]. Krishnaja and Sharma [30] reported increasing frequencies of dicentrics after 60Cobalt irradiation and culture times between 50 and 96 h.
In various studies it has been postulated that sparsely ionizing radiation, like gamma and X-rays, as well as particle radiation with an LET of up to some 30 keV/μm, exert only a small effect on cell cycle kinetics [9]. Irradiated cells should yield either a stable aberration frequency [8, 31–33] or a limited increase in aberration frequency with sampling time [5, 10, 11]. However, strong cell cycle disturbances and a considerable increase in aberrant metaphases with prolonged culture times were observed after densely ionizing radiation with LET values exceeding 100 keV/μm [8, 11, 34]. It is likely that these differences between the sparsely and densely ionizing radiations are based on the different patterns of local energy deposition in the cell nucleus. A uniform energy distribution with a homogenous aberration distribution in an exposed cell population is typical of sparsely ionizing radiation, while a densely ionizing radiation induces an inhomogeneous energy distribution with the occurrence of non-hit, slightly hit and heavily hit cell populations. The heavily hit cells should reach mitosis later than the other groups.
In the present study, the dic + cRing frequency in human lymphocytes was found to increase with prolonged culture times, and significant differences between 48 and 72 h could be observed after exposure to 200 kV X-rays with doses ≥2 Gy. There were also significant differences between culture times of 48 and 72 h after neutron irradiation at 0.1, 0.5, 0.7, and 1.0 Gy. After X-ray exposure, the main increase in chromosomal aberration frequency occurred between 48 and 72 h, after neutron exposure between 56 and 72 h. In comparison to the X-rays, neutron radiation causes an enhanced cell cycle delay, which even after 56 h can clearly be seen and can also be inferred from the yields of metaphases in their different division phases. The number of second metaphases exceeded the number of first metaphases in culture times of 72 h after X-ray exposure up to 4.0 Gy, whereas after neutron exposure the number of lymphocytes in first metaphases at 72 h was still higher than the number of second metaphases.
If mitotic delay were mainly due to heavily damaged cells, as assumed by Lloyd et al. [5], an enhanced yield of cells with multiple aberrations might be expected in metaphases arising late (after 56 or 72 h) as compared to metaphases arising after 48 h culture time. The FISH data of Hoffmann et al. [27] after high doses of X-rays showed such a trend, which was, however, not significant. Considering all the doses and culture times in this study, neutron exposure was found to cause significant differences in the number of Cd2+ cells as well as Cd3+ cells between culture times of 48 and 72 h and between 56 and 72 h. After an X-ray exposure also significant differences exist in the number of Cd2+ cells between 48 and 72 h, as well as between 56 and 72 h, whereas the number of Cd3+ cells differs significantly only between 48 and 72 h. This shows that neutrons cause a more pronounced cell cycle delay than the X-rays. Especially after neutron exposure highly damaged cells require up to 72 h of culture time to become visible in first metaphases. This observation is corroborated by chemically induced premature chromosome condensation (PCC) experiments [8, 35] which showed that heavily damaged cells require longer periods of time to pass the G2-phase of the cell cycle than lightly damaged cells. The initial molecular damage induced by high-LET radiation differs qualitatively from the damage induced by low-LET radiation, which implies that the repair is more difficult and slower [10, 36, 37].
The extent of the observed delay probably depends on the dose and on the different mean LET of the applied radiation types. For X-rays the mean LET is 1.7 keV/μm [38]; for the neutron spectrum the mean value is 40 keV/μm. Even in the dose range ≤1.0 Gy neutrons induce mitotic delay compared to the X-ray dose range: the smallest X-ray dose exceeds the smallest neutron dose by a factor of 10. Prolonged culture times after particle irradiation with a LET of up to 30 keV/μm only showed a minimal effect on the aberration frequency [9], neutrons with a broad LET distribution (10–90 keV/μm) with an average of about 40 keV/μm (as used in the present study) already affect significantly the cell cycle of damaged lymphocytes.
Excess acentric fragments, which may hint at uncompleted repair, increased significantly after the three highest doses of X-rays and neutrons as the culture time lengthened. An increase in the frequency of excess acentric fragments has been associated with exposure to high-LET radiation [39, 40]. Here, a direct comparison is only possible between 1.0 Gy of X-rays and 1.0 Gy of neutrons. After 1.0 Gy and 48 h culture time neutrons induce 3.5 times more excess ace than 1.0 Gy of X-rays; after 56 h culture time this ratio becomes 4.5 times and after 72 h culture time it is 4.7. These data support the notion of LET-dependent increases in the yield of excess acentric fragments.
In vitro calibration curves are a prerequisite to the estimation of absorbed radiation doses by biological dosimetry. The data of the present study are compatible with a linear-quadratic dose effect model for 200 kV X-rays and 48 h culture time. However, after 56 and 72 h the data agreed poorly with a linear-quadratic effect curve. This may be due to the saturation effects after high doses: heavily damaged cells die by interphase death or do not reach metaphase. The dic + cRing data after X-ray exposure tend towards a non-significant underdispersion; this observation complies with the data of Lloyd et al. [41]. To investigate the influence of mitotic delay on dose estimations in biological dosimetry, dose estimations were performed with three different, culture time specific calibration curves, using the observed aberration frequencies as input data. Although different dose values were obtained for the different calibration curves, they had overlapping confidence intervals and were thus not considered significantly different.
Fitting of the <E n>=2.1 MeV neutron data after 48 and 56 h culture time to a linear dose effect model or a linear-quadratic model, respectively, resulted in rather poor agreements, which may be explained by the observed mitotic delay, especially after doses ≥ 0.7 Gy. After 72 h, the data were compatible with a linear-quadratic dose effect model. This might be an indication that the observed mitotic delay was no longer in effect after 72 h of culture time. Again, dose estimates based on these calibration curves did not yield significant differences, since the confidence intervals of the estimated dose values overlap.
Thus, in spite of the significant influence of mitotic delay on the yield of chromosome aberrations observable after 48 h culture time, it can be concluded that dose response curves established after this time provide reliable dose estimations. This even holds in the high dose range, and significantly biased estimations of the equivalent whole body dose for biological dosimetry would not be expected. It has to be emphasized, however, that this conclusion may only be valid for the radiation types investigated here; especially neutrons with higher LET values may result in drastically different aberration patterns. If feasible, dose estimates should be carried out using dose response curves that had been established under conditions which are strictly comparable to those of the sample investigated for biological dosimetry.
References
Bender MA, Awa AA, Brooks AL, Evans HJ, Groer PG, Littlefield LG, Pereira C, Preston RJ, Wachholz BW (1988) Current status of cytogenetic procedures to detect and quantify previous exposures to radiation. Mutat Res 196:103–159
Bauchinger M (1995) Quantification of low-level radiation exposure by conventional chromosome aberration analysis. Mutat Res 339:177–189
Hoffmann W, Schmitz-Feuerhake I (1999) How radiation-specific is the dicentric assay? J Exp Anal Environ Epidemiol 2:113–133
Schröder H, Heimers A (2002) Chromosome aberrations induced in human lymphocytes by in vitro and in vivo X-rays. Mutat Res 517:167–172
LLoyd DC, Dolphin GW, Purrott RJ, Tipper PA (1977) The effect of X-ray induced mitotic delay on chromosome aberration yields in human lymphocytes. Mutat Res 42:401–412
Scholz M, Kraft-Weyrather W, Ritter S, Kraft G (1994) Cell cycle delays by heavy ion irradiation of synchronous mammalian cells. Int J Radiat Biol 66:59–75
Ritter S, Nasonova E, Kraft-Weyrather W, Kraft G (1994) Influence of radiation quality on the expression of chromosomal damage. Int J Radiat Biol 66:625–628
Ritter S, Nasonova E, Furusawa Y, Ando K (2002) Relationship between aberration yield and mitotic delay in human lymphocytes exposed to 200 MeV/u Fe-ions or X-rays. J Radiat Res 43:S175–S179
Nasonova E, Ritter S (2004) Cytogenetic effects of densely ionising radiation in human lymphocytes: impact of cell cycle delays. Cytogenet. Genome Res 104:216–220
Ritter S, Nasonova E, Scholz M, Kraft-Weyrather W, Kraft G (1996) Comparison of chromosomal damage induced by X-rays and Ar ions with an LET of 1840 keV/μm in G1 V79 cells. Int J Radiat Biol 69:155–166
Anderson RM, Marsden SJ, Wright EG, Kadhim MA, Goodhead DT, Griffin CS (2000) Complex chromosome aberrations in peripheral blood lymphocytes as a potential biomarker of exposure to high-LET α-particles. Int J Radiat Biol 76:31–42
Brede HJ, Cosack M, Dietze G, Gumpert H, Guldbakke S, Jahr R, Kutscha M, Schlegel-Bickmann D, Schölermann H (1980) The Braunschweig accelerator facility for fast neutrons research I Building design and accelerators. Nucl Instrum Methods 169:349–358
Dietze G, Brede HJ. Schlegel-Bickmann D (1984) Dosimetry for neutron therapy at the Physikalisch-Technische Bundesanstalt (PTB). In: advances in dosimetry for fast neutrons and heavy charged particles for therapy applications, IAEA-Report-AG-371/14, pp 203–215
Brede HJ (2004) Darstellung der Wasserenergiedosis im kollimierten, gemischten Neutronen-Photonenfeld der PTB. PTB-Bericht: PTB-N-45, pp 170–179
Meadows JW (1993) The 9Be(d,n) thick-target neutron spectra for deuteron energies between 2.6 and 7.0 MeV. Nucl Instrum Methods A324:239–246
Waterman FM, Kuchnir FT, Skaggs LS, Kouzes RT, Moore WH (1979) Energy dependence of the neutron sensitivity of C-Co2, Mg-Ar and TE-TE ionisation chambers. Phys Med Biol 24:721–733
Caswell RS, Coyne JJ, Randolph ML (1980) Kerma factors for neutron energies below 30 MeV. Radiat Res 83:217–254
Heimers A, Schröder H, Lengfelder E, Schmitz-Feuerhake I (1995) Chromosome aberration analysis in aircrew members. Radiat Prot Dosimetry 60:171–175
Perry P, Wolff S (1974) New Giemsa method for the differential staining of sister chromatids. Nature 251:156–158
Hoffmann W, Dannheim T, Grell-Büchtmann I, Heimers A, Nahrmann A, Schröder H, Schmitz-Feuerhake I, Tomalik P (1991) Quantitative Chromosomenaberrationsanalyse zur retrospektiven Dosisermittlung nach Exposition mit ionisierender Strahlung: Biologische Dosimetrie. BIOforum 14:381–385
Mitelmann F (ed) (1995) [ISCN. An international system for human cytogenetic nomenclature]., Basel, Karger
Radakrishna RC, Chakravarti J (1956) Some small sample tests of significance for a Poisson distribution. Biometrics 12:264–282
Savage JRK (1970) Sites of radiation induced chromosome exchanges. Curr Top Radiat Res 6:89–100
Edwards AA, Lloyd DC, Purrott RJ (1979) Radiation induced chromosome aberrations and the Poisson distribution. Radiat Environ Biophys 16:89–100
Bender MA, Brewen JG (1969) Factors influencing chromosome aberration yields in the human peripheral leucocyte system. Mutat Res 8:383–399
Beek B, Obe G (1976) The human leucocyte test system: X Higher sensitivity to X-irradiation in the G0 stage of the cell cycle of early as compared to late replicating cells. Hum Genet 35:57–70
Hoffmann GR, Sayer AM, Littlefield LG (2002) Higher frequency of chromosome aberrations in late-arising first-division metaphases than in early-arising metaphases after exposure of human lymphocytes to X-rays in G0. Int J Radiat Biol 78:765–772
Boei JJWA, Vermeulen S, Natarajan AT (1996) Detection of chromosomal aberrations by fluorescence in situ hybridization in the first three postirradiation divisions of human lymphocytes. Mutat Res 349:127–135
Guerrero-Carbajal YC, Moquet JE, Edwards AA, Lloyd DC (1998) The persistence of FISH translocations for retrospective biological dosimetry after simulated whole or partial body irradiation. Radiat Prot Dosim 76:159–168
Krishnaja AP, Sharma NK (2004) Transmission of γ-ray-induced unstable chromosome aberrations through successive mitotic divisions in human lymphocytes in vitro. Mutagenesis 19:299–305
Leonard A, Decat G (1979) Relation between cell cycle and yield of aberrations observed in irradiated human lymphocytes. Can. J Genet Cytol 21:473–478
Scott D, Lyons CY (1979) Homogeneous sensitivity of human peripheral blood lymphocytes to radiation-induced chromosome damage. Nature 278:756–758
Buul v PPW. Natarajan AT (1980) Chromosomal radiosensitivity of human leucocytes in relation to sampling time. Mutat Res 70:61–69
George K, Wu H, Willingham V, Furusawa Y, Kawata T, Cucinotta FA (2001) High- and low-LET induced chromosome damage in human lymphocytes: a time-course of aberrations in metaphase and interphase. Int J Radiat Biol 77:175–183
Durante M, Yamada S, Ando K, Furusawa Y, Kawata T, Majima H, Nakano T, Tsujii H (1999) Measurements of the equivalent whole-body dose during radiation therapy by cytogenetic methods. Phys Med Biol 44:1289–1298
Peak MJ, Wang L, Hill CK, Peak JG (1991) Comparison of repair of DNA double strand breaks caused by neutron or gamma radiation in cultured human cells. Int J Radiat Biol 60:891–898
Loucas BD, Geard CR (1994) Kinetics of chromosome rejoining in normal human fibroblasts after exposure to low- and high-LET radiations. Radiat Res 138:352–360
International Commission on Radiation Units and Measurements Inc. ICRU (1970) Linear Energy Transfer. ICRU Report 16. Bethesda, Maryland, USA
Griffin CS, Marsden SJ, Stevens DL, Simpson P, Savage JRK (1995) Frequencies of complex chromosome exchange aberrations induced by 238Pu α-particles and detected by fluorescence in situ hybridization using single chromosome-specific probes. Int J Radiat Biol 67:431–439
Hahnfeldt P, Hlatky LR, Brenner DJ, Sachs RK (1995) Chromosome aberrations produced by radiation: the relationship between excess acentric fragments and dicentrics. Radiat Res 141:136–152
Lloyd DC, Edwards AA, Prosser JS (1986) Chromosome aberrations induced in human lymphocytes by in vitro acute X and gamma radiation. Radiat Prot Dosimetry 15:83–88
Acknowledgements
This study was financed by the German Federal Office of Radiation Protection, StSch 4321. The conclusions presented here are those of the authors and do not necessarily represent the official position of the German Federal Minister for the Environment, Nature Conservation and Nuclear Safety The authors thank the five volunteers: F. Berger, P. Drewes, R. Hecking, A. Nöske, and G. Uelzen for considerable quantities of peripheral blood; H. Schröder for blood sampling; L. Röhrs for excellent technical assistance; H. Braselmann for statistical advice; E. Eggestein, H. Eggestein, O. Döhr, T. Heldt, and M. Hoffmann for performing the neutron measurements and operation of the accelerators at the PTB and H. v. Boetticher and I. Bareth for calibrating the X-ray source at the UFT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heimers, A., Brede, H.J., Giesen, U. et al. Influence of mitotic delay on the results of biological dosimetry for high doses of ionizing radiation. Radiat Environ Biophys 44, 211–218 (2005). https://doi.org/10.1007/s00411-005-0014-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-005-0014-3