Abstract
Garnet granulite xenoliths from the Nurbinskaya diatreme in the central part of the Archean Anabar province in Siberia are fragments of the local lower crust that experienced multiple metamorphic events in the Paleoproterozoic and reheating events in the Mesoproterozoic and later. This study addresses the timing of metamorphic transformations, and constrains the cooling rate and the time of stabilization of the lower crust. The observed metamorphic mineral assemblage of garnet, clinopyroxene, plagioclase, amphibole, rutile and ilmenite was formed at ~ 800 °C, 1.1–1.2 GPa under water-undersaturated conditions at ~ 1.88 Ga. However, the mineral assemblage is not well equilibrated and retains evidence of earlier and subsequent metamorphic stages. Late titanite formed in response to hydrous fluid influx according to phase equilibria modeling. U-Pb dating shows two events of titanite formation at 1850 ± 5 Ma and at 1788 ± 2 Ma. After deformation, which led to the porphyroclastic rock textures, the granulites underwent near-isobaric cooling. The cooling rate was higher than ~ 6 °C/Myr, to retain the garnet compositional zoning. Rutile ages are discordant, with 207Pb/206Pb dates ranging from 1.43 to 1.53 Ga. However, rutile may have responded to earlier thermal pulses, and was also reset later, so it does not record the stabilization of the crust. Crustal stabilization after Paleoproterozoic orogenic events may have occurred shortly after titanite formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
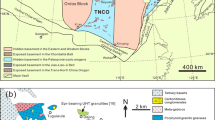
Lower crustal xenoliths are known from many Devonian kimberlites in the Anabar province of Siberia (Fig. 1). They are predominantly mafic garnet granulites. According to Cherepanova and Artemieva (2015), the Moho temperature in the central part of the Anabar province is 380–580 °C. These temperatures are low enough to retain evidence of ancient magmatic and metamorphic events, but are not too low for the stable metamorphic mineral assemblage of garnet, pyroxenes and plagioclase under dry conditions. However, the observed mineral compositions suggest significantly higher formation temperatures. Moreover, the xenolith lithologies are not dry, since amphibole and biotite are common in Siberian garnet granulite xenoliths (e.g., Koreshkova et al. 2011; Moyen et al. 2017; Perchuk et al. 2021; Shatsky et al. 2018, 2022). This situation is ubiquitous for Precambrian areas around the world (Rudnick and Gao 2014).
Geological sketch map of Siberia showing the main features of the surface geology and the locations of kimberlite fields (after Rosen et al. 2006; Gladkochub et al. 2006). Shaded areas are outcropping Precambrian rocks; white area is post-Paleoproterozoic sedimentary cover; dashed areas are Phanerozoic mobile belts. Kimberlite fields are marked with circles. The Nakyn kimberlite field is marked with a star
Unlike outcropping granulite-facies terranes, granulite xenoliths represent the lower crust at the time of eruption of the host magma (Rudnick and Gao 2014). Beneath Archean areas, the lower crust is composed predominantly of metaigneous rocks with Archean protoliths, which experienced multiple episodes of metamorphism during the Archean and the Paleoproterozoic (e.g., Koreshkova and Downes 2021; Zhao et al. 2024). By combining geochronological data and pressure and temperature estimates, part of the metamorphic history from the time of the earliest recorded event to the time of eruption of the host magma can be reconstructed. This allows comparison to upper crustal metamorphic and tectonic history, evaluation of the cooling rate after metamorphism, and an estimation of the time of stabilization of the local lower crust. In other words, thermal history reconstruction yields information about how the lower crust reached its present state.
Here data are presented for a xenolith suite from the Nurbinskaya diatreme (the Nakyn kimberlite field), situated in the central part of the Markha terrane of the Anabar province of the Siberian craton (Fig. 1). Three mineral geochronometers are present in these xenoliths: zircon, titanite and rutile. In general, magmatic and metamorphic generations of titanite and zircon can date the corresponding events in the history of the lower crust. U-Pb dating of rutile can give the age of the onset of a steady-state cratonic geotherm within the crust due to its low closure temperature (Schmitz and Bowring 2003; Blackburn et al. 2011). Under these conditions, rutile retains its Zr-in-rutile formation temperature (Ewing et al. 2013).
Titanite is rarely observed in lower crustal xenoliths. It was reported in xenoliths from the southern Wyoming craton (Farmer et al. 2005) and the Slave craton (Davis et al. 2003), where it gives ages of 2.6 and 1.7 Ga, and in a few other localities. The older date coincides with the U-Pb age of metamorphic zircon and corresponds to the formation of the granulite-facies assemblages. The younger one is interpreted as the result of transient heating related to mafic dyke swarm events (Davis et al. 2003). Titanite has not been dated yet in xenoliths from the Siberian craton.
Rutile from lower crustal xenoliths shows significantly younger ages than those from upper crustal rocks from the same locality (Apen et al. 2022; Blackburn et al. 2011; Davis et al. 2003). The interpretation of U-Pb rutile dating results involves either slow cooling from the time of metamorphism (Blackburn et al. 2011; Schmitz and Bowring 2003) or reheating events, which can be correlated with the upper crustal geological record (Davis et al. 2003; Förster et al. 2017).
Metamorphic zircon does not necessarily date the observed mineral assemblage. Zircon can be either inherited from earlier stages of metamorphism or formed later in the metamorphic history (Rubatto 2017). It is necessary therefore to determine which events a particular zircon generation belongs to. The data on lower crustal xenoliths from the Siberian craton show the formation of metamorphic zircon from 1.97 to 1.66 Ga. Zircon generations (mostly overgrowths) with the age of 1.87–1.84 Ga were equilibrated with the observed garnet and thus date the granulite-facies assemblage (Koreshkova and Downes 2021; and references therein).
Estimates of the cooling rate for lower crustal xenoliths worldwide vary from < 0.25 to 4°/Myr (Blackburn et al. 2011; Davis et al. 2003; Koreshkova et al. 2017; Scherer et al. 2000; Schmitz and Bowring 2003). At a rate of 0.25°/Myr, a lower crustal garnet granulite would exist at ~ 800 °C for a 100 Myr, sufficient to completely erase major- and trace element-zoning in garnet. However, garnet in the xenoliths usually shows zoning in High Field Strength Elements (HFSE).
In granulite xenoliths from the Nurbinskaya diatreme, ilmenite, rutile and titanite are in a reaction relationship, and rock-forming minerals are zoned. Therefore, these xenoliths provide an opportunity to determine how the conditions changed and to date the sequence of metamorphic transformations. This study considers in detail the metamorphic history of several mafic garnet granulite xenoliths and reports new data on U-Pb dating of rutile and titanite from them. The timing of the metamorphic transformations and constraints on the cooling rate are considered, and the time of stabilization of the local lower crust is discussed. These results are important for understanding the formation of the lower crust and its preservation to the present day beneath Archean areas around the world.
Geological background
The Archean (2.9–2.5 Ga) Markha terrane has a thick Mesoproterozoic-Mesozoic sedimentary cover containing Triassic flood basalts (e.g., Rosen et al. 2006). Unlike other kimberlite fields, the Nakyn field is located away from the main suture zones (Fig. 1). The age of the Nurbinskaya diatreme in this field is ~ 371 Ma (Tretiakova et al. 2017). The nearest boreholes to the Nakyn field revealed granites, granite-gneisses, Bt-Pl-gneisses and actinolite schist in the uppermost basement (Rosen et al. 2002). However, the geological history of the basement beneath the region is not constrained in detail. The Markha terrane was welded together with other Archean terrains of the Siberian craton between 2.1 Ga and 1.87 Ga (Rosen et al. 2006; Pisarevsky et al. 2008). The Moho depth in this area is 42–44 km; the thickness of the lower crust is 15–16 km and the Moho temperature is about 500–540 °C (Cherepanova and Artemieva 2015).
The main wealth of data on xenoliths from the Siberian craton comes from the Daldyn (the Udachnaya diatreme) and Alakit fields, located approximately 300 km north and northeast of the Nakyn field. Garnet granulite xenoliths from these fields contain varying amounts of orthopyroxene, amphibole (pargasite) and scapolite. Garnet and pyroxenes are zoned. The PT-conditions for grain rims compositions are thoroughly constrained using phase equilibria modeling and correspond to 600–650 °C and 0.8-1.0 GPa (Perchuk et al. 2021). Those for the cores were estimated using conventional thermobarometry and Ti-oxide oxythermometry that give 800–990 °C and 1.0-1.2 GPa (Koreshkova et al. 2011; Perchuk et al. 2021). According to Perchuk et al. (2021), pyroxenes preserved their compositions from a magmatic stage, whereas Koreshkova et al. (2011) consider all mineral assemblages to be metamorphic.
In xenoliths from the Daldyn and Alakit fielads, relics of magmatic zircon in grain cores yield ages between ~ 3.15 and ~ 2.66 Ga (Koreshkova and Downes 2021; and references therein). Metamorphic zircons are widespread. Most of them show Paleoproterozoic ages of 1.97–1.78 Ga and some have two metamorphic generations. From REE-composition of co-existing garnet and zircon, it follows that granulite-facies assemblages formed at 1.87–1.84 Ga (Koreshkova and Downes 2021; and references therein).
Rutile and apatite from lower and upper crustal xenoliths from the Udachnaya diatreme were dated by Apen et al. (2022), who showed that these minerals from upper crustal rocks retain Paleoproterozoic ages. In lower crustal rocks, apatite and rutile have a wide range of dates from 360 to 1600 Ma. Apen et al. (2022) concluded that lower crustal rocks cooled below 350–450 °C by ~ 1.6 Ga and were reheated shortly before the host kimberlite eruption in the Devonian.
Among crustal xenoliths from the Nakyn field, Grt-bearing Bt-Amp-Pl-gneisses are most common (Rosen et al. 2002). (Mineral abbreviations are from Whitney and Evans (2010).They have Archean TDM Nd model ages, but the age of metamorphism is not well constrained, although Rosen et al. (2006) reported Sm-Nd mineral isochron ages of 1.78–1.83 Ga. Shatsky et al. (2018) studied crustal xenoliths from the Botuobinskaya diatreme in the same kimberlite field. They obtained pressure and temperature estimates of 0.7–0.9 GPa and 600–750 °C for garnet granulite xenoliths, which correspond to middle-lower crustal depths. U-Pb age data for igneous zircon from these xenoliths are 2.9–2.7 Ga. Shatsky et al. (2022) also studied two xenoliths of garnet-biotite gneiss and a mafic garnet granulite xenolith from the Nurbinskaya pipe. They obtained U-Pb ages of 3.1–2.6 Ga for igneous zircons and 2.3–1.8 Ga ages for metamorphic zircons. Temperature estimates using Ti-in-zircon calibration of Watson et al. (2006) vary from 940 to 650 °C; the latter may correspond to metamorphic zircon. However, the metamorphic history of the xenoliths was not within the scope of these studies.
Koreshkova and Downes (2021) reported U-Pb ages of metamorphic zircon from a xenolith of Bt-Amp-Pl-gneiss Nur26 (2.76 Ga) and mafic garnet granulite xenoliths Nur27 and Nur21. In sample Nur27, five of the seven spots in six grains show concordant ages that do not differ within analytical uncertainties, yielding an average age of 1876 ± 15 Ma. Two spots within blurred and bleached areas gave younger ages, around 1.83 Ga. Four grains from sample Nur21 gave an age of 1848 ± 30 Ma, which, within the uncertainty, does not differ from the date from sample Nur27.
In general, U-Pb zircon dates reflect metamorphic events during the amalgamation of the Siberian craton. However, lower crustal rocks demonstrate systematically younger zircon dates compared to upper crustal rocks. The age of formation of granulite-facies mineral assemblages in xenoliths from the Udachnaya diatreme coincides with the intrusion of post-tectonic granites (Rosen et al. 2006).
Description of samples
Garnet granulite xenoliths from the Nurbinskaya pipe are small (5–10 cm in diameter), ellipsoidal and show alteration zones (< 1 cm) along contacts due to interaction with the host kimberlite. The xenoliths consist of garnet, clinopyroxene, plagioclase and varying amounts of amphibole. Data are reported for seven least altered samples and one highly altered xenolith. Their modal mineralogy is presented in Table 1.
Grt-granulites are foliated porphyroblastic rocks that exhibit varying degrees of recrystallization related to deformation, such that porphyroblastic textures grade to porphyroclastic ones. Recrystallization mostly affected clinopyroxene and plagioclase, which were transformed into fine-grained aggregates (Fig. 2a-c). Rare clinopyroxene porphyroclasts with deformation bands are surrounded by smaller recrystallized grains (neoblasts, 100–200 μm in size) (Fig. 2c). Garnet porphyroblasts are also recrystallized in some samples but to a much lesser extent (Fig. 2d). We assume that small garnet grains in the groundmass are mainly neoblasts that have separated from outer parts of large garnet grains. Amphibole is present in the groundmass as small grains; it often surrounds garnet but rarely occurs in inclusions in garnet. Rare small irregular grains of biotite are associated with amphibole in the groundmass. Granulites have accessory quartz, pyrrhotite, rutile, ilmenite, apatite, zircon and titanite (Table 1). Most samples contain individual grains of rutile and ilmenite. Rutile grains often have lamellae and rims of ilmenite (Fig. 2f-g). Titanite is confined to the groundmass, where it often surrounds rutile and ilmenite grains (Fig. 2e-g). Garnet has no inclusions of titanite. Scapolite is found only in xenolith Nur30, forming aggregates of polygonal grains. Small (< 30 μm) polygonal zircon grains are present in the groundmass and as inclusions in ilmenite. Several larger grains were separated from two samples.
Microphotographs and BSE images of xenoliths illustrating main textural features. a Porphyroclastic texture of sample Nur30 with preserved garnet porphyroblasts and rare clinopyroxene porphyroclasts in fine-grained groundmass, plane-polarized light. b Porphyroclastic texture of sample Nur28; dark color of diopside-pargasite fine-grained lenses is due to alteration, plane-polarized light. c Clinopyroxene porphyroclast with deformation bands and surrounding neoblasts, sample Nur30, crossed polars. d Garnet grain with neoblasts along its boundary, sample Nur28, plane-polarized light. e Rutile, typically surrounded by titanite if not shielded by garnet, sample Nur30, BSE image. f Rutile relic in titanite at the boundary with garnet; rutile has ilmenite lamellae and a rim with tiny zircon inclusions; ilmenite and zircon grains trace the former boundary of rutile, sample Nur30, BSE image. g Rutile with ilmenite lamellae and tiny zircon inclusions; rutile is surrounded by titanite, sample Nur1, BSE image
Many samples show high degrees of alteration, mostly chlorite after clinopyroxene and potassic feldspar after plagioclase. Calcite, barite, pyrite and chalcopyrite are common. The alteration is related to a kimberlite-derived fluid, since the amount of these minerals increases towards the contact with the host kimberlite and these minerals constitute the outer part of a xenolith.
Methodology
The outer parts of the xenoliths were removed by sawing, after which the samples were washed and then crushed to ∼2.5 cm size with a screw press and reduced to fragments ∼0.4 mm size with a stainless steel jaw crusher. The crushed material was divided into two parts, one part for mineral separation and the other was powdered in an agate mill for bulk rock analyses. Mineral separation was performed using standard magnetic and heavy liquid techniques. The purity of the mineral separates was achieved by hand-picking. Despite the small sample sizes and high degree of alteration, sufficient amounts of rutile, titanite and zircon were obtained for geochronology.
Analytical methods
Bulk-rock analyses for major and trace elements were obtained at A.P. Karpinsky Russian Geological Research Institute in St Petersburg (Table 2). Major element data were obtained by X-ray fluorescence (XRF) on fused glass discs with an ARL 9800 X-ray fluorescence spectrometer. Fusion with LiBO2 was used to homogenize the powders. The uncertainty of the XRF analysis is 0.5% for SiO2, 1.5% for Al2O3 and CaO, 5% for Na2O, and 2-2.5% for the rest of the oxides.
Trace element concentrations were determined by solution ICP-MS with a quadrupole ICP-MS ELAN-6100-DRC (Perkin Elmer). Sample solutions were prepared from fused glasses using ultrapure HNO3. Certified multi-element solutions were employed to construct calibration graphs. The data were processed using TOTALQUANT software. An internal Rh standard was used to correct signal drift. Interference corrections were routinely applied to correct the analyte isotopes 151Eu (from BaO, BaOH) and 159Tb (from NdO, NdOH). Oxide production ratio was < 3%, and uncertainties are estimated to be better than 2–10%.
Modeling of phase equilibria using measured bulk-rock compositions does not adequately reproduce the observed mineral assemblages. To avoid the compositional distortion caused by secondary alteration, the major and trace element whole-rock compositions of samples Nur1, Nur21 and Nur30 were calculated using their modal abundances, average mineral compositions and mineral density values (Deer et al. 2013). The results are given in Table 2. The compositions of other samples were not reconstructed due to the high degree of alteration. When calculating the bulk-rock composition, the water content in amphibole and apatite was included. The water content was obtained from the recalculated compositions of these minerals, based on the assumption of their stoichiometry. CO2 and SO3 contained in scapolite were also included for Nur30. Modeling of phase equilibria using the calculated compositions gave satisfactory results (section “Thermobarometry and phase equilibria modeling”).
Major element analyses of primary garnet, clinopyroxene, amphibole, mica, plagioclase, K-feldspar, rutile, ilmenite, titanite, zircon, scapolite and sulfides, as well as secondary minerals, were obtained by using a JEOL 8100 Superprobe at Birkbeck/UCL, using an accelerating voltage of 15 kV, current of 2.5 nA and a beam diameter of 1 μm and an Energy Dispersive System. The analyses were calibrated against standards of natural silicates, oxides and Specpure metals with the data corrected using a ZAF program provided by Oxford Instruments (Inca and Aztec software).
Trace element analyses of garnet, clinopyroxene, amphibole, plagioclase, rutile, ilmenite, apatite and titanite were obtained at the Geological Institute, BAS, Bulgaria, using a New Wave UP193FX Laser Ablation System and PerkinElmer SCIEX ELAN DRC-e ICP-MS. Time-resolved analysis was employed during data acquisition and the raw data were processed using Sills software (Guillong et al. 2008). Internal standards based on EPMA measurements were as follows: Al2O3 (garnet), SiO2 (garnet, clinopyroxene, pargasite, titanite, plagioclase), CaO (clinopyroxene, apatite), TiO2 (rutile, ilmenite). The synthetic glass reference material NIST610 was used for external calibration (Jochum et al. 2011). A laser diameter of 50 μm was used in most cases. For grains < 70 μm size, the diameter was reduced to 35 μm. Ablation was carried out in He mixed with Ar carrier gas before entering the plasma torch. The samples were analyzed at a repetition rate of 5 Hz and a laser energy density of 6.1–7.2 Jcm− 2. The analyses comprised ~ 30 s of gas blank acquisition and ~ 60 s of sample ablation. The intensities of all isotopes were acquired with a dwell time of 20 ms for 139La-175Lu, 232Th and 238U, and 10 ms for the others. The reproducibility of analyses was checked using the synthetic glass reference material NIST612 (Jochum et al. 2011), analyzed as an unknown. Deviations are within 0.2–4.2% for most isotopes and 8% for 24Mg, 31P and 118Sn. Average measured values are reported in supplementary Table ESM 2.
U-Pb (ID TIMS) dating of titanite and rutile was carried out at the Institute of Precambrian Geology and Geochronology, Russian Academy of Sciences (IPGG RAS). Handpicked titanite and rutile fractions were subjected to multi-stage pre-treatment with 3 N HCl in an ultrasonic bath and on a hotplate. After each step, the grains were washed with ultrapure water. Decomposition of samples and subsequent chemical separation of U and Pb were carried out following the modified methods described in Stifeeva et al. (2020). The isotopic composition of Pb and U was determined on a Triton TI multicollector mass spectrometer in static or dynamic modes using an ion counter. The spike 235U-202Pb was used for isotopic studies. The uncertainty in determining U-Pb ratios and U and Pb contents was 0.5%. Procedural blanks did not exceed 15 pg for Pb and 1 pg for U. Correction for common Pb was applied using the model of Stacey and Kramers (1975). The results are reported in Table 3 and in Fig. 7a-b. All uncertainties are at the level of 2σ (standard deviation).
Results
Major and trace element whole-rock compositions
Sample Nur30 is the least altered xenolith, and its measured and calculated compositions are similar except for Ba and Sr (Table 2; Fig. 3). In Nur1, plagioclase is replaced to some extent by secondary potassic feldspar. Because the calculated composition excluded K-feldspar, it resulted in a higher CaO and Na2O, and a lower K2O content than the measured values. The calculated composition of Nur21 shows a higher CaO and Na2O, and a lower MgO content than the measured one, which is expected due to the replacement of clinopyroxene by chlorite. The calculated REE abundances are higher than the measured ones. Alteration is assumed to have caused the removal of LREE from clinopyroxene.
The measured compositions of samples Nur27, Nur28 and Nur31 are similar to that of Nur21. Their REE contents reflect the same character of alteration. Measured compositions of samples Nur13 and Nur21 are similar in major elements (except for P2O5) but differ in trace elements. Nur13 is enriched in P2O5 and LREE. No REE-carbonates and phosphates, or any unusual minerals, were found among alteration products in this sample. Its measured composition likely reflects both the elevated content of apatite and the high degree of alteration, so that the measured REE composition is controlled by apatite due to the lack of contribution of other minerals.
In general, the rocks are basic in composition, and olivine-normative. Nur1 is rich in Al2O3 and Na2O. It has a LREE-enriched and HREE-depleted pattern and a positive Eu anomaly, suggesting that its protolith was a plagioclase-rich gabbroid. The remaining rocks are generally similar to basalts. Nur30 has a high Mg-number (0.63), a relatively high Cr content (650 µg/g) and an almost flat REE pattern, about 10 times that of chondrite. Nur21 resembles a Fe-rich tholeiite with an N-MORB-like LREE-depleted pattern and a small negative Eu anomaly (Fig. 3).
Composition of minerals
Data for mineral compositions are reported in supplementary Tables ESM1 and ESM2.
Garnet
Garnet is largely almandine with 25–38% pyrope and 15–26% grossular components. The calculated Fe2O3 content is in the range of 0.7–2.1 wt% and corresponds to 3–8% of the andradite molecule. Garnet grains show prominent zoning. Traverses across single grains demonstrate that their compositions change within the outermost 150–300 μm, whereas the central parts have nearly constant abundances of Ca, Mg and Fe (Fig. 4). In all samples, the FeO content increases, while the MgO content and Mg-number decrease from cores to rims. No change in the MnO content was detected in garnet from any sample. The CaO content decreases from cores to rims in all samples except for Nur21 and Nur30 (Fig. 4). In Nur30, garnet shows an increase in CaO (Fig. 4a), and in Nur21, the CaO content increases from the cores to the peripheral parts and then decreases within the outermost rims (Fig. 4b). Samples Nur1 and Nur30 also show differences in the composition of the rims adjacent to plagioclase and those adjacent to mafic minerals (the latter described above). In Nur1, the rims adjacent to plagioclase have the same compositions as the cores, whereas in Nur30, these rims are the richest in CaO (Table ESM1). Small grains (newly formed and neoblasts, 100–200 μm in size) continue the compositional trends observed in the larger grains, but in samples Nur21 and Nur27 their composition varies greatly and on average demonstrates higher MgO content than in the rims.
Variations in XCa, XMg and XFe along traverses across garnet grains from xenoliths from the Nurbinskaya diatreme. a Sample Nur30. Graph for coarse grain 1 and smaller grain 2. b Sample Nur21. c Sample Nur27. Circles indicate average compositions of small grains (newly formed and neoblasts, 100–200 μm in size), which are plotted at the distance of 100 μm from large grains
Garnet has REE patterns with slight positive and negative slopes of HREE (Fig. ESM3). Large grains (> 1 mm) show a 2–3 times decrease in Yb from cores to rims. In smaller grains from sample Nur1, the zoning is insignificant. In Nur30, the small grains (newly formed and neoblasts, 100–200 μm in size) have the lowest HREE and Y contents, while the small grains and the outermost rims in Nur21, Nur27 and Nur28 show an increase in Yb (Fig. 5a). Garnet shows no clear zoning in Zr and Ti, although garnet from Nur21, Nur27 and Nur28 has a higher Ti content in the cores compared to the rims (Fig. 5b). The reduced Ti and Zr contents in garnet cores are probably due to exsolution of rutile needles.
Variations in trace element concentrations along traverses across garnet grains from xenoliths from the Nurbinskaya diatreme. a Yb contents in garnet from samples Nur21, Nur27, Nur28 and Nur30. b CaO (wt%), Ti, Zr and Yb (µg/g) contents in a garnet grain from sample Nur27. Open symbols correspond to the composition of small grains (newly formed and neoblasts, 100–200 μm in size), which are plotted at the distance of 50 μm from the rims of large grains
Clinopyroxene
Clinopyroxene is Na-Al-diopside having 3.3–6.3 wt% Al2O3 and 1.3–1.8 wt% Na2O. The ferric iron content was calculated assuming that trivalent ions in octahedra balance Al replacing Si in tetrahedra, and is about 20% of the total amount of iron in the clinopyroxene. In a few samples, clinopyroxene is rich in Al, so ferric iron content cannot be estimated this way. Assuming that the excess sum in the mineral formula is due to the presence of ferric iron, then its estimated content varies from 20 to 60% of the total amount of iron (Table ESM1). Sapegina et al. (2022) obtained similar values using Mössbauer spectroscopy for granulite xenoliths from the Udachnaya diatreme.
Clinopyroxene grains are zoned. Large grains (porphyroclasts) show a bell-shaped distribution of Al2O3 and Na2O. In all grains, an increase in the MgO content towards the rims is observed, the maximum in the rims adjacent to garnet (Fig. ESM4). Clinopyroxene inclusions in garnet are usually rich in both MgO and Al2O3, which is explained by their contemporaneous formation with the cores of large grains and later diffusional exchange of Mg and Fe with their host garnet.
Trace elements were analyzed in samples Nur21, Nur28 and Nur30 (Table ESM2). Clinopyroxene has LREE-enriched and HREE-depleted REE patterns with maxima at Pr and Nd (Fig. ESM4). In Nur21, compositions were only obtained for neoblasts and porphyroclast outer parts, due to the poor preservation of porphyroclast cores. In Nur28, the porphyroclasts show a decrease in Y and HREE from their cores to rims. Clinopyroxene from Nur30 shows only a slight increase in Y contents from the porphyroclast cores to the rims and neoblasts (Table ESM2).
Amphibole
Amphibole mainly forms small grains in the groundmass. Amphibole is rich in alumina and alkalis and is classified as pargasite (Hawthorne et al. 2012). Samples Nur1, Nur13 and Nur30 contain K-rich pargasite, whereas in Nur31, Nur27 and in the groundmass of Nur28, pargasite is poor in K2O. A large inclusion in garnet from sample Nur28 is K-rich pargasite. Only small relics have been found in sample Nur2; they vary in composition from K-bearing pargasite to magnesio-hornblende. Similar variations are observed in Nur30, where the amphibole is unaltered. Amphibole shows an increase in MgO and decrease in FeO from grain cores to rims (Table ESM1).
Trace elements were analyzed in pargasite from samples Nur1, Nur27 and Nur28. In Nur1, amphibole is enriched in LREE and LILE and poor in Cr and Ni abundances. Pargasite from Nur27 and Nur28 have lower LREE and LILE contents and much higher Cr and Ni. In Nur28, pargasite in the groundmass is richer in Nb, Y and HREE but poorer in K, Rb and Ba than in the inclusion in the garnet (Table ESM2).
Feldspars and scapolite
Plagioclase is oligoclase (An20 − 28) in all samples except Nur30, where it is andesine (An41 − 43). Plagioclase forms small grains, which are mostly unzoned. A small increase in CaO content towards the boundaries of the plagioclase aggregates was observed in samples Nur1, Nur2 and Nur30. Plagioclase was analyzed for trace elements in samples Nur1, Nur21, Nur28 and Nur30. It concentrates LILE; REE abundances are low (Tables ESM1 and ESM2).
Potassic feldspar occurs as diffuse rims along plagioclase boundaries and as irregular grains within pseudomorphs together with chlorite, calcite, barite and sulfides. Therefore, potassic feldspar is considered to be a secondary mineral.
Scapolite is found in xenolith Nur30. It forms polygonal grains, which are distributed in stripes and patches. Scapolite is Ca-rich and contains SO3, CO2 and a minor amount of Cl (Table ESM1).
Titanite, ilmenite and rutile
All xenoliths except Nur30 contain rutile and ilmenite as individual grains and intergrowths in the groundmass and as inclusions in garnet. Sample Nur30 contains only rutile. Rutile grains have ilmenite lamellae ranging in size from less than a micrometer to tens of micrometers. Ilmenite in turn has rutile lamellae. In sample Nur21, ilmenite has titanomagnetite lamellae. Rutile grains are usually surrounded by ilmenite rims, indicating that ilmenite replaces rutile. These rims and large lamellae sometimes contain small zircon inclusions (Fig. 2f). Rutile is rich in Zr, Hf, Nb, Ta and U. The U content varies from 2 to 80 µg/g. In mafic garnet granulites, rutile is rich in Cr (660–1980 µg/g) (Table ESM2).
Ilmenite contains 1.3–3.8 wt% MgO (Table ESM1). Ilmenite inclusions in garnet have a higher MgO content than ilmenite grains in the groundmass. In Nur27, ilmenite rims on rutile grains are richest in MgO (5.1 wt%). In Nur1, ilmenite inclusions in titanite have a lower MgO content than in the groundmass (1.0 vs. 1.4 wt%). Ilmenite has similar amounts of V, Cr and Ni to rutile, but it is poor in Zr and Hf. Nb and Ta contents vary significantly from grain to grain in each sample (Table ESM2).
Titanite is found in xenoliths Nur1 and Nur30, where it occurs as small grains in the groundmass and reaction rims around rutile and ilmenite grains. No titanite inclusions in garnet and clinopyroxene were observed. Titanite makes a significant contribution to the budget of REE, Th and U (Table ESM2, Fig. ESM4 c).
Apatite
Is fluorapatite in samples Nur1, Nur13 and Nur27, whereas in other samples, it is hydroxylapatite. Apatite is enriched in LREE and hosts 5–15% of the total amount of LREE and Th. In Nur1, apatite has abundant micrometer-sized inclusions of monazite.
Sulfides
Occur as rare small (< 50 μm) rounded inclusions in rock-forming minerals and in ilmenite. Ni-bearing pyrrhotite predominates but Ni-bearing chalcopyrite is sometimes observed together with pyrrhotite. In Nur30, a few larger grains are resorbed and partially replaced with secondary pyrite and chalcopyrite.
Zircon
Single small (< 30 μm) polygonal zircon grains were observed in thin sections, in the groundmass, in samples Nur1, Nur21, Nur27 and Nur30. However, no zircon was separated from samples Nur1 and Nur30. Only a few zircon grains were separated from samples Nur21 and Nur27. These grains are small (50–150 μm), pale pink, polygonal to irregular in shape. Inclusions in zircon are altered to chlorite.
In sample Nur21, zircon has blurred fir-tree sector texture. Th and U contents are relatively low, on average 46 and 62 µg/g, respectively. Th/U ratio varies from 0.50 to 1.06 (Koreshkova and Downes 2021). The REE composition is characterized by low HREE contents with YbN/DyN=0.9–3.2 (Fig. 9), typical for zircon in garnet-rich rocks.
In sample Nur27, zircon shows fir-tree sector texture, which is blurred in some grains. One grain has thin discontinuous CL-bright rim and a bleached domain. Th and U contents in the cores are uniform, with average values of 66 and 214 µg/g, respectively; Th/U = 0.17–0.41. The bleached domain has lower concentrations of Th and U: 37 and 127 µg/g, respectively; Th/U = 0.30 (Koreshkova and Downes 2021). The REE pattern shows low HREE contents with YbN/DyN=1.8 (Fig. 9).
Thermobarometry and phase equilibria modeling
P-T parameters were calculated using the average compositions of minerals with and without correction for ferric iron content (Table 1), by the formulations of the Grt-Cpx geothermometer of Ravna (2000) and the Grt-Pl-Cpx-Qtz geobarometer of Newton and Perkins (1982). We obtained 640–850 °C, 1.0-1.5 GPa for grain cores and 510–650 °C, 0.8-1.0 GPa for grain rims with correction for ferric iron content, and 710–840 °C, 1.0-1.5 GPa for grain cores and 580–770 °C, 0.8–1.2 GPa for grain rims without the correction.
The Zr-in-rutile geothermometers (Ferry and Watson 2007; Watson et al. 2006; Tomkins et al. 2007) gave similar estimates, varying between samples from 710 to 850 ± 55 °C. The temperature of titanite crystallization from the Zr-in-titanite geothermometer (Hayden et al. 2008) was 660–790 °C at 1.1 GPa and aSiO2 and aTiO2 = 0.5-1.0.
For phase equilibria modeling, the three least altered xenoliths Nur1, Nur21 and Nur30 were chosen. Their compositions cover the variations observed in the suite. The calculated bulk compositions (Table 2) were used to avoid the composition distortion caused by alteration. To construct phase diagrams, we used the PERPLE_X software (Connolly 2005) (version 7.1.4, updated on 26.10.2023), including database files ‘hp62ver.dat’ with a thermodynamic dataset (Holland and Powell 2011) and ‘solution_model.dat’ for solid-solution models. The latter are as follows: Augite(G) for clinopyroxene, cAmph(G) for amphibole (Green et al. 2016); Gt(W) for garnet, Opx(W) for orthopyroxene, Mica(W) for white micas, Chl(W) for chlorite, Bi(W) for biotite, Ilm(WPH) for ilmenite, (White et al. 2014), Pl(I1,HP) and Fsp(C1) for plagioclase and K-feldspar (Holland and Powell 2003); O(HP) for olivine, Sp(HP) for spinel, Do(HP) for dolomite, M(HP) for calcite (Holland and Powell 1998); Scap for scapolite (unpublished, https://www.perplex.ethz.ch/PerpleX_solution_model_glossary.html), CORK EoS for fluid (Holland and Powell 1991), and melt(G) for melt (Green et al. 2016). Whole-rock analyses were recalculated to 100% on a volatile-free basis; MnO and P2O5 contents were excluded; total iron was ferrous iron (FeOt).
The resulting phase diagrams in P-T coordinates reproduce the observed primary mineral assemblages in the xenoliths. The fields that correspond exactly to the observed assemblages are shown in red in Fig. 6b-c. The only exception is the stability of titanite in sample Nur30, which is absent at a given YCO2 (Fig. 6a) and appears with increasing water proportion. Several values of YCO2 (YCO2 = CO2/(CO2 + H2O) in molar proportions) in the range of 0.30–0.85 were used to simulate water-undersaturated conditions. No correspondence was obtained in wet conditions. In the calculated bulk composition of sample Nur30, YCO2 = 0.13, however, the best agreement with both the observed mineral assemblage and the composition of garnet was obtained at YCO2 = 0.70. For the same reason, we chose YCO2 = 0.85 for Nu1 and YCO2 = 0.70 for Nur21. The isopleths XCa and XMg for the model garnet compositions are shown in Fig. 6a-c for comparison with the observed garnet compositions.
The compositions of plagioclases and clinopyroxenes are close to the model ones under conditions of stability of the observed mineral assemblages. However, the compositions of garnets differ significantly. Discrepancies between the thermobarometric estimates and the modeling results reflect the lack of equilibrium between rock-forming minerals, as discussed below.
Phase equilibria modeling results for calculated compositions of xenoliths from the Nurbinskaya diatreme. PT-diagrams for a sample Nur30 at YCO2 = 0.85; b sample Nur21, YCO2 = 0.70 and c sample Nur1, YCO2 = 0.70. Average compositions of cores, rims and small grains of garnets are plotted using the XCa and XMg isopleths of model compositions of garnet. Numbers correspond to garnet formation stages (see section “Zoning in garnet” and Fig. 8)
U-Pb dating of rutile and titanite
The U-Pb isotopic analysis of six microweights (0.1–0.8 mg) of rutile from samples Nur1, Nur21, Nur27 and Nur30 and five microweights of titanite from Nur1 and Nur30 was performed (Table 3). All analyses of rutile yielded discordant ages, with 207Pb/206Pb ages ranging from 1426 to 1527 Ma. Three determinations from sample Nur21 show average 207Pb/206Pb age of 1481 ± 27 Ma (MSWD = 6.2) (Fig. 7).
Three analyses (5–10 grains per analysis) of titanite of the highest optical quality from Nur30 define a Discordia intersecting the Concordia at 1867 ± 33 Ma (lower intersection is at 1191 ± 630 Ma, MSWD = 0.17). The analysis #3 gives a concordant age of 1850 ± 5 Ma. In Nur1, two individual fractions were analyzed, each consisting of 2–10 grain fragments. Titanite #4 yields a concordant age of 1785 ± 4 Ma (MSWD = 1.8). Both analyses have a weighted average of 207Pb/206Pb ratios corresponding to the value of 1788 ± 2 Ma (MSWD = 0.02) (Fig. 7).
U-Pb Concordia diagrams for a titanite and b rutile from xenoliths from the Nurbinskaya diatreme. Data-point uncertainty ellipses are 2 standard deviation values (σ). Decay constants uncertainties are included. Numbers inside and near the ellipses correspond to the numbers of analyses in Table 3
Discussion
Zoning in garnet
Zoning patterns of Ca, Mg and Fe are characterized by constant compositions of central parts of grains and variations within rims (Fig. 4). Mn content does not change along traverses across grains, whereas HREE and Y show a bell-shaped distribution along the traverses (Fig. 5). Prolonged exposure of garnets to high temperature could affect the compositional profiles of divalent elements, which have higher diffusivities than tri- and tetravalent elements (Ganguly 2010). It would take 6–7 Myr at ~ 800 °C or 25–30 Myr at ~ 750 °C to erase growth zoning of Ca, Mg and Fe but retain zoning of trivalent and tetravalent HFSE. This estimate is obtained for a grain with a radius of 0.025 cm, using the expression t ≈ 1.11a2/D (Bloch et al. 2015), where t is time in seconds, a is the grain radius in centimeters, and D is the diffusion coefficient. Diffusivities of Lu and Hf (DHf ≈ DZr) are from Bloch et al. (2015), D of Fe-Mg interdiffusion is from Ganguly et al. (1998) and Ganguly (2010), DCa is from Perchuk et al. (2009) and Ganguly (2010). Ca diffusion and Fe-Mg interdiffusion in garnet would have “closed” at 600–670 °C, using the equation of Dodson (1973), the peak T = 750–800 °C and a cooling rate of 0.5–5.0 °C/Myr). In the case of slower diffusion of Ca (Ganguly 2010) than that determined by Perchuk et al. (2009), the growth zoning of Ca may be partially preserved. Mn has even faster diffusivity than Mg and Fe. The lack of Mn-zoning may suggest that conditions with T > 600–670 °C followed by fast cooling.
The observed zoning patterns of trivalent HREE and Y with a gradual decrease from cores to rims can be explained by Rayleigh fractionation during growth. The same is probably true for Ti and Zr. The content of Ti and Zr in the cores is relatively low and is reduced due to the exsolved rutile needles. However, no increase in Ti and Zr content in the rims is observed, which is consistent with the assumption (Fig. 5b). In samples Nur21, Nur27 and Nur28, an increase in HREE contents (1.5-2 times, less prominent in Y) in the edges (< 150 μm from grain boundaries) and in small grains in the groundmass is observed (Fig. 5), which indicates resumed garnet growth under new conditions.
The phase diagrams (Fig. 6a-c) show how the major element composition of garnet changes with changing conditions. They indicate an increase in XCa with decreasing temperature, as well as with increasing pressure and water content. XMg decreases with decreasing temperature. In the absence of clinopyroxene, a rapid increase in XMg and decrease in XCa with decreasing temperature is observed. A possible change in fO2 has minor effect on garnet composition: decreasing XCa with increasing oxygen fugacity (Perchuk et al. 2021). With increasing water proportion, the garnet compositions become richer in CaO and less magnesian.
In sample Nur30, the compositions of garnet cores can be modeled under high-temperature conditions (1000 °C, 1.0 GPa) within the Grt-Cpx-Amp-Pl-Scp-Ilm field or at ~ 720 °C, 1.1 GPa in the Grt-Dol-Amp-Pl-Scp-Qz-Rt field, at YCO2 = 0.85 (Fig. 6a). The rims plot at 800–840 °C, and small grains plot at ~ 770 °C, both at 1.1 GPa. Consequently, the enrichment of CaO in rims and small grains can be achieved by either decreasing or increasing the temperature. However, the latter possibility contradicts other observations.
Assuming that garnet cores did not participate in reactions at subsequent stages, we calculated the bulk-rock composition minus the estimated amount of garnet cores equal to 14 vol%. The compositions of rims and small grains are reproduced at ~ 720 °C, 1.2 GPa in the Grt-Dol-Amp-Pl-Scp-Qz-Rt field. This estimate is very approximate, but demonstrates that diffusional exchange with clinopyroxene and amphibole was able to produce the observed zoning during near-isobaric cooling.
These values of 720–770 °C are close to the maximum Zr-in-titanite temperature at this pressure. According to the modeling, garnet in equilibrium with titanite at this temperature, 1.1–1.2 GPa and YCO2 < 0.5 would have much higher Ca and Fe contents. However, it is likely that after titanite formation, the rims and small grains acquired the observed composition by diffusional exchange during cooling.
For Nur21, the simulation also gives two solutions for garnet core compositions: at 680–720 °C, > 1.15 GPa in the Grt-Dol-Pl-Ky-Qz-Rt or Grt-Dol-Pl-Qz-Rt fields or at ~ 1000 °C, 0.9 GPa in the Grt-Cpx-Amp-Pl-Ilm field, at YCO2 = 0.70 (Fig. 6b). The latter is more consistent with plagioclase composition, garnet amount, the presence of Ti-magnetite lamellae in ilmenite, and Zr-in-rutile temperature.
The stage of resumed garnet growth, which follows from the increase in HREE in grain edges and small grains, is consistent with the modeled change in conditions towards ~ 800 °C, 1.2 GPa. Under these conditions, the CaO content in garnet would be higher than observed (Fig. 6b). However, upon subsequent cooling the edges and small grains could acquire lower Ca contents due to exposure to temperatures ~ 660–670 °C (the values obtained from thermobarometry and phase equilibria modeling (Fig. 6b). It would take ~ 15 Myr to acquire a new composition for grains sized 100 μm, according to the expression t ≈ 1.11a2/D (Bloch et al. 2015)d of Fe-Mg interdiffusion from Ganguly et al. (1998) and Ganguly (2010), and DCa from Perchuk et al. (2009) and Ganguly (2010). The cooling must have occurred after the formation of small grains, that is, after deformation, which in turn occurred after the resumption of garnet growth. Assuming that cooling began at ~ 800 °C, and ~ 670 °C is the temperature at which the diffusion of Ca, Fe and Mg ceased in large grains of 0.5 mm in size, then the cooling rate will be 4–6 °C/Myr (using the equation of Dodson (1973); but it should be higher to maintain the observed zoning in large grains. This consideration explains the observed increase of CaO content from the cores to peripheral parts of porphyroblasts and the decrease within the outermost rims (Fig. 4b). Schematically, the stages of development of zoning in garnet grains are shown in Fig. 8.
Scheme of the stages of development of zoning in garnet grains from samples Nur21, Nur27 and Nur28. a Stage (1) Garnet growth. Gradual decrease in Yb content from cores to rims due to Rayleigh fractionation during growth. Exposure to high temperatures (e.g., 2–3 Myr at ~ 900 °C) erased growth zoning of Ca, Mg and Fe but retained zoning of Yb and other trivalent and tetravalent HFSE. b Stage (2) Resumed garnet growth during a new event resulted in grain edges with higher Yb content than in the pre-existing rims. At temperature ~ 800 °C, the edges had high Ca contents. c Stage (3) Cooling and deformation. After the formation of the edges, deformation occurred, leading to the formation of small grains (neoblasts). The small grains and rims acquired lower Ca contents due to exposure to temperatures about 660–670 °C. It would take 15–20 Myr to acquire a new composition for grains sized 100 μm. Cooling occurred after the formation of small grains
Garnet core compositions in sample Nur1 match the model compositions at ~ 870 °C, 1.1 GPa and YCO2 = 0.70 (Fig. 6c). The rims plot at ~ 800 °C and 0.9 GPa. These values are approximate because, for a given bulk-rock composition, the rims are not in equilibrium with the other phases. Cooling to a temperature of about 700 °C, as in other samples, would produce garnet with a significantly less magnesian composition than observed. However, in this sample, the garnet grains are mainly surrounded by plagioclase, which could have contributed to the preservation of its composition from the high-temperature stage. In addition, garnet did not recrystallize to form small grains.
Overall, it is assumed that the garnet cores inherited their major element compositions from an earlier stage, while the rim compositions reflect later diffusional modifications. The zoning in HREE and Y, which formed at earlier stages, was not affected during the cooling stage (Fig. 8).
U-Pb age of rutile, titanite and zircon
Zircon from samples Nur21 and Nur27 has low HREE abundances (Koreshkova and Downes 2021), which indicates their growth in the presence of garnet. The comparison of calculated compositions of zircon in equilibrium with garnet and silicic melt using distribution coefficients from Rubatto and Hermann (2007) with the measured compositions show that zircon equilibrated with garnet having low HREE abundancies, i.e., with outer parts of the cores or grain edges. The best agreement with the calculated compositions is observed for the composition of the edges at ~ 900–950 °C (Fig. 9a, b). In this case, zircon formation was associated with the late stages of garnet growth. In sample Nur27, the obtained age is 1876 ± 15 Ma, while that from sample Nur21 is 1848 ± 30 Ma and, within the limits of uncertainty, overlaps with that in Nur27 (Koreshkova and Downes 2021). Therefore, the age of 1.88 Ga is considered as the time of formation of a granulite-facies assemblage of garnet, clinopyroxene, amphibole, plagioclase, rutile and ilmenite (+ scapolite in Nur30).
REE patterns of zircons from xenoliths from the Nurbinskaya diatreme: a sample Nur27, b sample Nur21. Dashed lines show calculated compositions of zircon in equilibrium with garnet edges. DZrn/Grt from Rubatto and Hermann (2007). The best agreement with the calculated compositions is observed at ~ 900–950 °C. Values are normalized to chondrite CI (Sun and McDonough 1989)
Zircon from mafic garnet xenoliths from the Nakyn field, Siberia, studied by Shatsky et al. (2018, 2022) have relic magmatic cores with Archean ages and homogeneous metamorphic rims. In xenoliths from the Nurbinskaya pipe, the dates are in the range of 2807 − 1838 Ma. Hence, some of them may give the age of metamorphism. The single dated rim has a nearly concordant age of 1926 ± 68 Ma Ga. This rim shows a flat HREE pattern, so it was formed in the presence of garnet and may correspond to an early metamorphic event. In middle-lower crustal garnet granulite xenoliths from the neighboring Botuobinskaya diatreme, the rims were not dated. Nevertheless, in xenolith Bt-39-03, three homogeneous zircon grains with low Th and U (12 and 5–7 µg/g, respectively) were analyzed. Presumably, these grains are metamorphic, without magmatic cores. Their ages vary from 2.0 to 2.7 Ga and may belong to zircon recrystallized at 2.0 Ga or later, or to partially reset Archean metamorphic generations.
Zircon from the studied samples has a morphology and composition that are consistent with subsolidus growth in the presence of garnet and does not have relic magmatic cores. This zircon crystalized ~ 1.88 Byr ago during the formation of the observed granulite-facies assemblages. The dates of metamorphic zircon obtained by Shatsky et al. (2018, 2022) point to older metamorphic events, however, they need to be specified, and certainly, this zircon deserves further study.
Titanite has Pb diffusivity that is higher than (Cherniak 1993) or comparable to that of zircon (Kohn 2017). Thus, the “closure” temperature of Pb diffusion in titanite from the samples is comparable to the temperature of its formation. Consequently, titanite dates its formation event of at 1850 ± 5 Ma in sample Nur30 and at 1788 ± 2 Ma in Nur1.
The phase equilibria modeling and the Zr-in-titanite geothermometry (Hayden et al. 2008) place few constraints on the conditions of titanite formation. Titanite could form at temperature as high as 780–790 °C but not in equilibrium with garnet of the observed composition. Titanite stability requires higher water proportions than those for the modeled compositions of garnet cores. Under these conditions, garnets would be more calcic and less magnesian. Titanite formed after rutile and ilmenite. Therefore, the appearance of titanite is placed between the second and third stages of garnet formation.
Rutile is one of the earliest minerals to form, yet it shows the youngest 207Pb/206Pb dates of ~ 1.5 Ga. Rutile remained stable during the inferred metamorphic stages (Fig. 8a-c). This approximate value is interpreted as a “cooling age”, i.e., the time when ambient temperature was low enough to slow down isotope exchange. These data can be interpreted as the result of cooling either after titanite crystallization at ~ 1.79 Ga or after an unidentified younger thermal event. Taking into account the time span of ~ 300 Myr, a thermal event within this period is probable. Earlier we reported U-Pb age of 1656 ± 27 Ma of zircon from garnet granulite xenolith from the Komsomolskaya diatreme in Siberia (the Alakit field) (Koreshkova and Downes 2021). This age reflects a thermal event that may be related to the rifting of the Siberian craton in the late Paleoproterozoic-Mesoproterozoic. Rifting along the eastern and western margins of the Anabar Shield caused a rapid post-rift subsidence of the craton at around 1.6 Ga (Cherepanova and Artemieva 2015; and references therein).
The temperature of ~ 670 °C, inferred for garnet rims and small grains, is higher than the “closure” temperature of Pb diffusion in rutile. Starting at this temperature and with an assumed cooling rate of > 6 °C/Myr, the diffusion in rutile grains with a diameter of 100 μm would cease at ~ 580 °C, i.e., 15 Myr later than garnet rims and small grains acquired their major element compositions (using the equation of Dodson (1973) and Pb diffusivity from Cherniak (2000). With a lower cooling rate of 0.5 °C/Myr, a smaller grain size of 50 μm and a higher initial temperature of ~ 800 °C, the “closure” temperature of ~ 500 °C can be obtained. However, at such a low cooling rate, garnet would develop onspicuous rims with higher XFe than observed.
From U-Pb dating of rutile and apatites in xenoliths from the Udachnaya diatreme (the Daldyn field) Apen et al. (2022) concluded that lower crustal rocks cooled below 350–450 °C by ~ 1.6 Ga and were reheated shortly before to the host kimberlite eruption in Devonian times. This conclusion does not contradict our assumptions, given that lower crustal rocks at different depths will respond differently to thermal disturbances.
Metamorphic history
The interpretation of the modeling results must take into account the following observations: (1) the Zr-in-rutile temperature of ~ 800 °C, (2) the high-magnesian composition of garnet cores, (3) the coexistence of rutile and ilmenite, and (4) titanite formation after rutile in samples Nur1 and Nur30. With that, garnet rims and small grains cannot be reproduced on the phase diagrams (Fig. 8a-c). To model their compositions, it is necessary to exclude garnet and, possibly, clinopyroxene grain cores from the bulk-rock compositions, but the proportions are unknown. Nevertheless, the diagrams show how the major element composition of garnet would change with changing conditions.
Sample Nur1. The phase equilibria modeling reproduces the observed mineral assemblage in a narrow field at 820–920 °C, 1.1–1.3 GPa and YCO2 = 0.7 (Fig. 6c). The composition of garnet cores plots near this field. The modeled compositions of clinopyroxene and plagioclase, as well as the garnet mode, are consistent with those observed. However, the observed reaction relationship of titanite with rutile and ilmenite point to a lack of equilibrium. At higher YCO2, and in dry or wet conditions, no exact correspondence to the assemblage is found. At lower YCO2, the titanite stability field expands, and the area of coexistence of rutile and ilmenite diminishes, and the modeled garnet composition becomes more calcic and less magnesian.
The Zr-in-rutile temperature, plagioclase composition and garnet mode produce a point of ~ 800 °C and 1.05 GPa on the PT-path. However, the modeling predicts the disappearance of titanite along this path (Fig. 6c). Therefore, titanite either appeared after rutile and ilmenite during isobaric cooling, or was formed during an influx of hydrous fluid. In the latter case, titanite was not equilibrated with garnet.
The rock experienced deformation and acquired a foliated texture, but garnet grains were in a homogeneous plagioclase matrix, which prevented their recrystallization and limited the diffusional exchange of Mg and Fe between mafic minerals. This explains why garnet from this sample retained its composition from the high-temperature stage and did not record the stage of cooling after deformation.
Sample Nur21. The appropriate field for the observed mineral assemblage is located at 700–750 °C, 0.9-1.0 GPa at YCO2 = 0.7 (Fig. 6b). At higher water proportions, this field moves towards slightly higher pressures, and reduces in size, while the simulated garnet compositions become more calcic and less magnesian. At lower water proportions, there is no field corresponding to the observed assemblage.
The compositions of plagioclase and clinopyroxene plot near this field. However, the best approximation to the observed compositions of garnet cores is obtained at ~ 1000 °C, 0.8 GPa and YCO2 = 0.85. From the coexistence of rutile and ilmenite and the Zr-in-rutile temperature of ~ 810 °C (Table 1), it follows that, at some stage, the rock was at P > 1.1 GPa (Fig. 6b). The modal abundance of garnet is consistent with these conditions, but the garnet composition should be more calcic.
Along the path from high-temperature conditions of the garnet cores to ~ 810 °C and 1.1 GPa, the mineral assemblage of garnet, clinopyroxene, plagioclase, ilmenite and rutile was formed. At this stage, garnet growth was resumed. We assume that zircon grew together with the outer parts of garnet grains, namely the edges. Therefore, it dates this stage (stage 2 on Fig. 6b) to 1876 ± 15 Ma.
Deformation caused recrystallization of the plagioclase and clinopyroxene and, to a much lesser extent, garnet. The small garnet grains are neoblasts formed from the outer parts of the larger grains. The trace element composition of the garnet neoblasts is similar to the edges, while they have higher XMg and lower XCa than the outer parts. It is assumed that the major element compositions of the outer parts and small grains were changed by diffusion during later cooling.
Samples Nur13, Nur21, Nur27, Nur28 and Nur31 are similar in that they contain both ilmenite and rutile, and their garnets exhibit a decrease in CaO content towards the grain boundaries. Garnets from samples Nur27, Nur28 and Nur21 also have similar HREE-zoning. Therefore, it is assumed these rocks had the same PT-paths.
In sample Nur28, the difference in compositions of amphibole in inclusion in garnet and in the groundmass may be associated with the resumption of amphibole formation. The inclusion has low HREE and Y, consistent with its formation in the presence of garnet. In the groundmass, amphibole has significantly lower contents of Rb, Ba and K compared to the inclusion, which reflects its formation in an environment depleted in these elements. Higher Nb, Ta, HREE and Y contents indicate contributions from garnet, rutile and ilmenite. This amphibole may have formed after the second stage of granulite facies metamorphism.
The PT-path of sample Nur30 is generally similar to that of the other samples (Fig. 6a). The composition of garnet cores reflect high-temperature conditions in an early stage, whereas the assemblage of garnet, clinopyroxene, plagioclase, scapolite and rutile was formed along the path to the conditions of ~ 800 °C and 1.1 GPa. The pressure increase is consistent with the increase of CaO in the garnet rims. However, titanite is not present in the assemblage with these minerals. It appears with increasing water proportion in the fluid, and at these conditions, there are no suitable garnet compositions. Using the bulk-rock composition minus the estimated amount of garnet cores gives a plausible solution, with the stability of titanite in the assemblage of garnet, clinopyroxene, plagioclase, scapolite and quartz (rutile is absent) at 700–780 °C, 1.2 GPa, YCO2 = 0.5 and also reproduces the change of the compositions of garnet neoblasts during near-isobaric cooling to ~ 680 °C. In this case, titanite dates an event of fluid influx.
In summary, granulite xenoliths from the Nurbinskaya diatreme record three main metamorphic events under similar conditions in all samples: (i) an early high-temperature stage, (ii) the formation of the observed metamorphic mineral assemblage at ~ 800 °C, 1.1–1.2 GPa, and (iii) hydrous fluid influx events. A possible temperature evolution of the lower crust is presented in Fig. 10.
Garnet core compositions are the evidence of an early stage of growth at ~ 1000 °C, 0.8-1.0 GPa. The observed zoning patterns of HREE and Y with a gradual decrease from cores to rims can be explained by Rayleigh fractionation during growth at this stage or during the subsequent temperature decrease. This stage could not last longer than 1–5 Myr, otherwise the zoning would not be preserved even in grains with a diameter > 1 mm.
The conditions of ~ 800 °C, 1.1–1.2 GPa of the next stage were derived from the observed mineral assemblages and the Zr-in rutile thermometry. In samples Nur21, Nur27 and Nur28, an increase in HREE and Y contents in the edges of garnet grains suggests that garnet growth resumed. Zircons from samples Nur21 and Nur27 grew in equilibrium with garnet having low HREE abundances, i.e., with outer parts of the cores or grain edges. In this case, zircon dates the late stage of garnet growth, that is, the observed mineral assemblages, to ~ 1.88 Ga. This stage lasted no longer than 6–7 Myr to retain trace element zoning in the garnets.
The replacement of rutile and ilmenite by titanite in samples Nur1 and Nur30 requires an increase in water proportion in a fluid. Titanite is also not in equilibrium with garnet of the observed composition. We suggest that there was a distinct event of titanite formation at 700–780 °C by an influx of hydrous fluid. However, U-Pb titanite dates in these samples differ significantly: 1850 ± 5 Ma in Nur30 and 1788 ± 2 Ma in Nur1, suggesting that these are two different events. The other samples could also have experienced addition of a watery fluid. In Nur28, this may have caused the formation of the second generation of amphibole.
The garnet rims and small grains acquired their major element compositions during later near-isobaric cooling to ~ 660–680 °C. Deformation, which led to the observed rock textures and the formation of the small grains, occurred before this cooling stage. In the Udachnaya diatreme, garnet granulites appear to have experienced similar near-isobaric cooling (Perchuk et al. 2021). The conditions of formation of the garnet and clinopyroxene rims are 600–650 °C and 0.8-1.0 GPa, according to estimates by Perchuk et al. (2021).
If ~ 660–680 °C is taken as the temperature at which the diffusion of Ca, Fe and Mg ceased in large grains of 0.5 mm in size, the cooling rate was ~ 4–6 °C/Myr; but it should be higher to maintain zoning in large grains. The U-Pb system in rutile stayed open during cooling. Considering this cooling rate, the “closure” temperature of 590 °C would be reached ~ 15–20 Myr later than the garnet rims and small grains acquired their composition. The results of the U-Pb dating of rutile point to younger events, which could be reheating events during the Mesoproterozoic rifting of the Siberian craton and later in Devonian. Our data and those of Apen et al. (2022) demonstrate the susceptibility of the lower crust to reheating; each rifting event could reset the U-Pb system in rutile. According to Apen et al. (2022), lower crustal garnet granulites from the Udachnaya diatreme were also reheated shortly before the host kimberlite eruption.
Possible thermal history of the lower crust of the Anabar province of Siberia. The dashed line shows a possible change in temperature over time in the lower crust. The data for magmatic zircon are from (Koreshkova and Downes 2021; and references therein). The data for metamorphic zircon are from (Koreshkova and Downes 2021; and references therein). Not all zircon data are shown, only for xenoliths from the Nurbinskaya diatreme in the Nakyn field and from the Komsomolskaya diatreme (Koms) in the Alakit field; instead, age ranges are shown for metamorphic zircon from upper-middle and lower crustal xenoliths from kimberlites of the Daldyn and Alakit fields, with zircon symbols corresponding to the most frequent age values (Koreshkova and Downes 2021). U-Pb rutile data are from this study, and the data for rutile and apatite are from Apen et al. (2022). The data for the upper-middle crust are from Rosen et al. (2006). Tc is the “closure” temperature of isotopic exchange. The numbers in italics correspond to the stages of garnet formation, as in Fig. 8
Conclusion
Garnet granulite xenoliths from the Nurbinskaya diatreme are fragments of the local lower crust that experienced multiple metamorphic events in the Paleoroterozoic and reheating events in the Mesoproterozoic and later. Zircon and titanite U-Pb ages demonstrate involvement of the lower crust in the major tectonic events that constructed the Siberian craton, despite the great distance from the nearest regional suture zones.
The studied samples recorded three main events when the metamorphic mineral assemblages changed: (1) the early high-temperature stage of crystallization of the garnet grain cores, (2) the formation of the observed granulite-facies mineral assemblages at ~ 800 °C, 1.1–1.2 GPa, and (3) hydrous fluid influxes which formed titanite. Zircon was formed in the presence of the newly-grown garnet during the second granulite-facies stage and thus dates this event to ~ 1.88 Ga. Garnet cores inherited their compositions from an earlier stage, while the rim compositions reflect later diffusional modifications. Titanite is the youngest phase, and is not in equilibrium with other minerals. It formed at 1850 ± 5 Ma in sample Nur30 and at 1788 ± 2 Ma in Nur1 and postdates the granulite-facies stage.
After deformation, which led to the porphyroclastic rock textures, a near-isobaric cooling occurred. The cooling rate was ~ 4–6 °C/Myr, but may have been higher to retain garnet compositions from previous stages. The rocks probably also experienced later short-term thermal events that affected only the U-Pb system in rutile. The results of U-Pb dating show that Pb diffusion in rutile may have “closed” by ~ 1.5 Byr ago, however, rutile may have responded to the Mesoproterozoic thermal pulses. A slow cooling between ~ 1.8 and 1.5 Ga is inconsistent with the observed compositional zoning in garnet. Therefore, rutile does not show the time of stabilization of the crust after the Paleoproterozoic orogenic events, which may have occurred shortly after titanite formation.
References
Apen FE, Rudnick RL, Ionov DA, Cottle JM, Moyen J-F, Golovin AV, Korsakov AV (2022) Heat transfer and production in cratonic continental crust: U-Pb thermochronology of xenoliths from the Siberian craton. Geochemistry, Geophysics, Geosystems, 23, e2022GC010497. https://doi.org/10.1029/2022GC010497
Blackburn T, Bowring SA, Schoene B, Mahan K, Dudas F (2011) U-Pb thermochronology: creating a temporal record of lithosphere thermal evolution. Contrib Min Petrol 162:479–500. https://doi.org/10.1007/s00410-011-0607-6
Bloch E, Ganguly J, Hervig R, Cheng W (2015) 176Lu-176Hf geochronology of garnet I: experimental determination of the diffusion kinetics of Lu3+ and Hf4+ in garnet, closure temperatures and geochronological implications. Contrib Min Petrol 169:12. https://doi.org/10.1007/s00410-015-1109-8
Cherepanova Y, Artemieva IM (2015) Density heterogeneity of the cratonic lithosphere: a case study of the siberian Craton. Gondwana Res 28(4):1344–1360. https://doi.org/10.1016/j.gr.2014.10.002
Cherniak DJ (1993) Lead diffusion in titanite and preliminary results on the effects of radiation damage on Pb transport. Chem Geol 110:177–194. https://doi.org/10.1016/0009-2541(93)90253-F
Cherniak DJ (2000) Pb diffusion in rutile. Contrib Mineral Petrol 139:198–207. https://doi.org/10.1007/PL00007671
Connolly JAD (2005) Computation of phase equilibria by linear programming: a tool for geodynamic modeling and its application to subduction zone decarbonation. Earth Planet Sci Lett 236:524–541. https://doi.org/10.1016/j.epsl.2005.04.033
Davis JW, Canil D, MacKenzie JM, Carbno GB (2003) Petrology and U-Pb geochronology of lower crustal xenoliths and the development of a craton, Slave Province, Canada. Lithos 71:541–573. https://doi.org/10.1016/S0024-4937(03)00130-0
Deer WA, Howie RA, Zussman J (2013) An Introduction to the Rock-Forming Minerals (third edition). The Mineralogical Society, London, U.K., 498 pages + Crystal Viewer CD. ISBN 987-0903056-33-5
Dodson MH (1973) Closure temperature in cooling geochronological and petrological systems. Contrib Min Petrol 40:259–274. https://doi.org/10.1007/BF00373790
Ewing T, Rubatto D, Hermann J (2013) The robustness of the Zr-in-rutile and Ti-in-zircon thermometers during high-temperature metamorphism (Ivrea-Verbano Zone, northern Italy). Contrib Min Petrol 165:757–779. https://doi.org/10.1007/s00410-012-0834-5
Farmer GL, Bowring SA, Williams ML, Christensen NI, Matzel J, Stevens L (2005) Contrasting lower crustal evolution across an Archean–Proterozoic suture: physical, chemical and geochronologic studies of lower crustal xenoliths in southern Wyoming and northern Colorado. In: Karlstrom KE, Keller GR (eds) The Rocky Mountain Region: an evolving lithosphere. American Geophysical Union, Washigton, pp 139–162. https://doi.org/10.1029/154GM11
Ferry JM, Watson EB (2007) New thermodynamic models and revised calibrations for the Ti-in-zircon and Zr-in-rutile thermometers. Contrib Mineral Petrol 154:429–437. https://doi.org/10.1007/s00410-007-0201-0
Förster B, Aulbach S, Symes C, Gerdes A, Hofer HE, Chacko T (2017) A reconnaissance study of Ti-minerals in Cratonic Granulite Xenoliths and their potential as recorders of Lower Crust formation and evolution. J Petrol 58:2007–2034. https://doi.org/10.1093/petrology/egx080
Ganguly J (2010) Cation Diffusion Kinetics in Aluminosilicate garnets and Geological Applications. Rev Mineral Geochem 72:559–601
Gladkochub D, Pisarevsky SA, Donskaya T, Natapov LM, Mazukabzov A, Stanevich A, Slkyarov E (2006) Siberian Craton and its evolution in terms of Rodinia hypothesis. Episodes 29(3):169–174. https://doi.org/10.18814/epiiugs/2006/v29i3/002
Green ECR, White RW, Diener JFA, Powell R, Holland TJB, Palin RM (2016) Activity–composition relations for the calculation of partial melting equilibria in metabasic rocks. J Metamorph Geol 34:845–869. https://doi.org/10.1111/jmg.12211
Guillong M, Meier DL, Allan MM, Heinrich CA, Yardley BWD (2008) SILLS: a matlab-based program for the reduction of laser ablation ICP-MS data of homogeneous materials and inclusions. Mineral Assoc Can Short Course 40:328–333
Hawthorne FC, Oberti R, Harlow GE, Maresch WV, Martin RF, Schumacher JC, Welch MD (2012) Nomenclature of the amphibole supergroup. Am Min 97(11–12):2031–2048. https://doi.org/10.2138/am.2012.4276
Hayden LA, Watson EB, Wark DA (2008) A thermobarometer for sphene (titanite). Contrib Min Petrol 155:529–540. https://doi.org/10.1007/s00410-007-0256-y
Holland TJB, Powell R (1991) A compensated-redlich-kwong (CORK) equation for volumes and fugacities of CO2 and H2O in the range 1 bar to 50 kbar and 100–1600°C. Contrib Min Petrol 109:265–273. https://doi.org/10.1007/BF00306484
Holland TJB, Powell R (1998) An internally-consistent thermodynamic dataset for phases of petrological interest. J Metam Geol 16:309–344. https://doi.org/10.1111/j.1525-1314.1998.00140.x
Holland TJB, Powell R (2011) An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J Metam Geol 29:333–383. https://doi.org/10.1111/j.1525-1314.2010.00923.x
Jochum KP, Weis U, Stoll B, Kuzmin D, Yang Q, Raczek I, Jacob DE, Stracke A, Birbaum K, Frick DA, Günther D, Enzweiler J (2011) Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand Geoanal Res 35:397–429. https://doi.org/10.1111/j.1751-908X.2011.00120.x
Kohn MJ (2017) Titanite Petrochronology. Rev Mineral Geochem 83:419–441. https://doi.org/10.2138/rmg.2017.83.13
Koreshkova M, Downes H (2021) The age of the lower crust of the central part of the Columbia supercontinent: a review of zircon data. Gondwana Res 96:37–55. https://doi.org/10.1016/j.gr.2021.02.024
Koreshkova M, Downes H, Millar I, Levsky L, Larionov A, Sergeev S (2017) Geochronology of metamorphic events in the Lower Crust beneath NW Russia: a Xenolith Hf Isotope Study. J Petrol 58:1567–1590. https://doi.org/10.1093/petrology/egx065
Koreshkova MYu, Downes H, Levsky LK, Vladykin NV (2011) Petrology and geochemistry of granulite xenoliths from Udachnaya and Komsomolskaya kimberlite pipes, Siberia. J Petrol 52:1857–1885. https://doi.org/10.1093/petrology/egr033
Moyen J-F, Paquette JL, Ionov DA, Gannoun A, Korsakov AV, Golovin AV, Moine BN (2017) Paleoproterozoic rejuvenation and replacement of archaean lithosphere: evidence from zircon U-Pb dating and hf isotopes in crustal xenoliths at Udachnaya, siberian craton. Earth Planet Sci Lett 457:149–159. https://doi.org/10.1016/j.epsl.2016.09.046
Newton RC, Perkins D III (1982) Thermodynamic calibration of geobarometers based on the assemblage garnet-plagioclase-orthopyroxene (clinopyroxene)-quartz. Am Min 67:203–222
Perchuk AL, Burchard M, Schertl H-P, Maresch WV, Gerya TV, Bernhardt H-J, Vidal O (2009) Diffusion of divalent cations in garnet. Contrib Min Petrol 157:573–592. https://doi.org/10.1007/s00410-008-0353-6
Perchuk AL, Sapegina AV, Safonov OG, Yapaskurt VO, Shatsky VS, Malkovets VG (2021) Reduced amphibolite facies conditions in the precambrian continental crust of the siberian craton recorded by mafic granulite xenoliths from the Udachnaya kimberlite pipe, Yakutia. Precam Res 357:106122. https://doi.org/10.1016/j.precamres.2021.106122
Pisarevsky SA, Natapov LM, Donskaya TV, Gladkochub DP, Vernikovsky DA (2008) Proterozoic Siberia: a promontory of Rodinia. Precam Res 160:66–76. https://doi.org/10.1016/j.precamres.2007.04.016
Ravna EK (2000) The garnet-clinopyroxene Fe2+-Mg geothermometer: an updated calibration. J Metamorp Geol 18:211–219. https://doi.org/10.1046/j.1525-1314.2000.00247.x
Rosen OM, Serenko VP, Manakov AY, Zinchuk NN (2002) Yakutian Kimberlite province: position in the structure of the siberian craton and composition of the upper and lower crust. Russ Geol Geophys 43:6–26
Rosen OM, Levsky LK, Zhuravlev DZ, Rotman AYa, Spetsius ZV, Makeev AF, Zinchuk NN, Manakov AV, Serenko VP (2006) Paleoproterozoic accretion in the northeast siberian craton: isotopic dating of the Anabar collision system. Stratigr Geol Correl 14:581–601. https://doi.org/10.1134/S0869593806060013
Rubatto D (2017) Zircon: the Metamorphic Mineral. Rev Mineral Geochem 83:261–295. https://doi.org/10.2138/rmg.2017.83.9
Rubatto D, Hermann J (2007) Experimental zircon/melt and zircon/garnet trace element partitioning and implications for the geochronology of crustal rocks. Chem Geol 241:38–61. https://doi.org/10.1016/j.chemgeo.2007.01.027
Rudnick RL, Gao S (2014) 4.1 - Composition of the Continental Crust. In Holland HD, Turekian KK (Eds.) Treatise on Geochemistry (Second Edition), Elsevier, 1–51. https://doi.org/10.1016/B978-0-08-095975-7.00301-6
Sapegina AV, Voronin MV, Perchuk AL, Safonov OG (2022) Aegirine-Bearing Clinopyroxenes in Granulite Xenoliths from the Udachnaya Kimberlite Pipe, siberian Craton: comparison of the Mössbauer and Micropobe Data. Petrology 30(1):S119–S130. https://doi.org/10.1134/S0869591123010083
Scherer EE, Cameron KL, Blichert-Toft J (2000) Lu–Hf Garnet Geochronology: Closure temperature relative to the Sm–Nd system and the effects of trace mineral inclusions. Geochim Cosmochim Acta 64:3413–3432. https://doi.org/10.1016/S0016-7037(00)00440-3
Schmitz MD, Bowring SA (2003) Constraints on the thermal evolution of continental lithosphere from U-Pb accessory mineral thermochronometry of lower crustal xenoliths, southern Africa. Contrib Min Petrol 144:592–618. https://doi.org/10.1007/s00410-002-0419-9
Shatsky VS, Malkovets VG, Belousova EA, Tretiakova IG, Griffin WL, Wang Q, Ragozin AL, Gibsher AA, O’Reilly SY (2018) Multi-stage modification of Paleoarchean crust beneath the Anabar tectonic province (siberian craton). Precam Res 305:125–144. https://doi.org/10.1016/j.precamres.2017.11.017
Shatsky VS, Ragozin AL, Wang Q, Wu M (2022) Evidence of Eoarchean crust beneath the Yakutian kimberlite province in the siberian craton. Precam Res 369:106512. https://doi.org/10.1016/j.precamres.2021.106512
Stacey JS, Kramers JD (1975) Approximation of terrestrial lead isotope evolution by a two-stage model. Earth Planet Sci Lett 26:207–221. https://doi.org/10.1016/0012-821X(75)90088-6
Stifeeva MV, Salnikova EB, Arzamastsev AA, Kotov AB, Grozdev VY (2020) Calcic garnets as a source of information on the age of Ultramafic Alkaline intrusions in the Kola Magmatic Province. Petrology 28(1):62–33. https://doi.org/10.1134/S0869591120010063
Tomkins HS, Powell R, Ellis DJ (2007) The pressure dependence of the zirconium-in-rutile thermometer. J Metam Geol 25(6):703–713. https://doi.org/10.1111/j.1525-1314.2007.00724.x
Tretiakova IG, Belousova EA, Malkovets VG, Griffin WL, Piazolo S, Pearson NJ, O’Reilly SY, Nishido H (2017) Recurrent magmatic activity on a lithosphere-scale structure: crystallization and deformation in kimberlitic zircons. Gondwana Res 42:126–132. https://doi.org/10.1016/j.gr.2016.10.006
Watson EB, Wark DA, Thomas JB (2006) Crystallization thermometers for zircon and rutile. Contrib Min Petrol 151:413–433. https://doi.org/10.1007/s00410-006-0068-5
White RW, Powell R, Holland TJB, Johnson TE, Green ECR (2014) New mineral activity–composition relations for thermodynamic calculations in metapelitic systems. J Metam Geol 32:261–286. https://doi.org/10.1111/jmg.12071
Whitney DL, Evans BW (2010) Abbreviations for names of rock-forming minerals. Am Min 95(1):185–187. https://doi.org/10.2138/am.2010.3371
Zhao L, Jiang N, Guo J, Liu D, Hu J, Zhang X, Huang G (2024) Preservation of Archean mafic lower continental crust worldwide. Earth Planet Sci Lett 626:118556. https://doi.org/10.1016/j.epsl.2023.118556
Acknowledgements
We thank Dr Andy Beard, Dr Martin Rittner, Dr Stoyan Georgiev and Dr Elitsa Stefanova for technical help with microprobe and laser probe analyses. We thank Dr Francisco Apen and the anonymous reviewer for their thorough and constructive reviews. We thank the Resource center for microscopy and microanalysis and the Resource centre “Geomodel” in St Petersburg University for the help with sample preparation and preliminary microprobe investigation. This work was supported by RFBR grant 20-55-18017 Bolg-a and BAS grant KP-06-Russia/32.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Othmar Müntener.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koreshkova, M., Downes, H., Stifeeva, M. et al. Metamorphic history of the Precambrian lower cratonic crust from U-Pb dating of granulite xenoliths (Anabar province, Siberia). Contrib Mineral Petrol 179, 74 (2024). https://doi.org/10.1007/s00410-024-02156-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-024-02156-7