Abstract
Purpose
Pseudomonas aeruginosa is the predominant bacterial pathogen colonizing the cystic fibrosis (CF) lung. Mixed populations of nonmucoid and mucoid variants of P. aeruginosa have been isolated from the CF airway. While the association between mucoid variants and pulmonary function decline is well-established, their impact on inflammation and tissue damage in advanced CF lung disease remains unclear.
Methods
This pilot study utilized 1 non-CF and 3 CF lung explants to examine lobar distribution, inflammation, and histopathology related to nonmucoid and mucoid P. aeruginosa infection. To study tissue damage, we developed a novel lung histopathology scoring system, the first applied to human CF lung biopsies, which is comprised of five indicators: bronchiolar epithelial infiltrate, luminal inflammation, peribronchial/bronchiolar infiltrate, peribronchiolar fibrosis, and alveolar involvement.
Results
Mucoid P. aeruginosa variants were distributed throughout the CF lung but associated with greater concentrations of proinflammatory cytokines, IL-1β, TNF-α, IL-6, IL-8, and IFN-γ, and one anti-inflammatory cytokine, IL-10, compared to nonmucoid variants. CF lung explants exhibited higher histopathology scores compared to a non-CF lung control. In mixed-variant infection, nonmucoid constituents associated with increased bronchiolar epithelial infiltration, one indicator of histopathology.
Conclusion
This pilot study suggests ongoing interplay between host and bacterial elements in late-stage CF pulmonary disease. Mucoid P. aeruginosa infection correlates with inflammation regardless of lung lobe, whereas nonmucoid P. aeruginosa is associated with increased inflammatory cell infiltration. The development of a novel lung histopathology scoring system lays the groundwork for future large-cohort investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic pulmonary infections cause significant morbidity and mortality in people with cystic fibrosis (pwCF) [1]. Pseudomonas aeruginosa remains the predominant bacterial pathogen within the adult CF lung [2]. PwCF are initially colonized with nonmucoid variants of P. aeruginosa. However, over time, these bacteria acquire mutations leading to the emergence of mucoid variants, which overproduce the bacterial exopolysaccharide alginate [3]. When grown on agarose-containing media, colonies of mucoid P. aeruginosa are easily recognizable due to their unique “mucous-like” appearance [4]. Conversion to the mucoid phenotype correlates with enhanced antimicrobial resistance of P. aeruginosa and accelerated deterioration in pulmonary function [5, 6].
Intriguingly, mixed populations of nonmucoid and mucoid variants are often isolated from the CF airway [7,8,9,10]. We have previously shown that communities with both nonmucoid and mucoid P. aeruginosa constituents exhibit advantages in evading the innate immune response compared to isolated populations of each morphotype [11]. These results indicate a selective benefit for the co-existence of both variants within the CF lung environment, which is neutrophil-rich and hyperinflammatory.
Imaging studies suggest that tissue damage is often heterogeneously distributed within the CF lung, wherein the upper lobes are more severely injured [12,13,14]. We hypothesized that bacterial infection plays a role in lobe-specific inflammation and pathology in pwCF. Utilizing lobar bronchoalveolar lavage (BAL) fluid from pwCF with stable pulmonary disease, we recently demonstrated that mucoid P. aeruginosa infection is associated with greater regional inflammation compared to nonmucoid infection [10]. However, the impact of P. aeruginosa variants upon inflammation and tissue damage in the late stages of CF remains poorly understood.
This small cohort pilot study utilized human CF lung explants to determine how nonmucoid and mucoid P. aeruginosa infection influences lobar inflammation and histopathology. To systematically assess tissue damage, we developed a semi-quantitative, novel lung histopathology grading scheme incorporating five different indicators of tissue changes associated with CF. Although tested here in our limited cohort of clinical specimens, our long-term objective is the application of this method in larger-cohort investigations.
Methods
Procurement of Human Lung Explants
We procured four lung explants after lung transplantation: three were native CF lungs, and one was from a CF recipient undergoing re-transplant for chronic lung allograft dysfunction/bronchiolitis obliterans syndrome (“non-CF” lung) (Supplementary Table 1). Explanted lungs were made available to the research teams within 2–4 h of surgical explantation. All explants were stored in a large, sterile container at 4 °C prior to removal of lobar biopsies by our research team. 1cm3 superficial (parenchymal) and deep (airway) biopsies were obtained from all six lung lobes in each explant (n = 12 non-CF lung biopsies; n = 35 CF lung biopsies; a deep biopsy of the lingula from one patient was reserved for the hospital laboratory and not included in this study).
Each biopsy was split into two fractions and weighed. One biopsy fraction was homogenized (via the hand-held, PRO Scientific Bio-Gen PRO200 Tissue Homogenizer) for microbiology and cytokine quantitation; the other was formalin-fixed and processed for hematoxylin and eosin (H&E) staining, as described in-detail further below. All biopsy specimens were stored separately in labeled conical tubes on ice prior to and during specimen processing for microbiology, cytokines, and histopathology. Specimen collection was approved by the local Institutional Review Board (IRB07-00396) with informed consent obtained from all subjects.
Isolation and Quantitation of P. aeruginosa Variants
One-half of each explant biopsy was homogenized in Phosphate Buffered Saline [(PBS), Gibco™, pH 7.4], then serially diluted in PBS, before plating for colony forming units (CFUs) on Pseudomonas Isolation Agar (PIA) medium (BD™). After 48 h of incubation at 37 °C, colonies were counted for total P. aeruginosa growth. Additionally, colonies were visually identified as either mucoid or nonmucoid based on classically described colony morphology [3], counted separately, and expressed as “nonmucoid P.a. CFUs” or “mucoid P.a. CFUs.” PIA facilitates identification and counting of these P. aeruginosa variants as it contains Triclosan, which maintains the mucoid phenotype (otherwise prone to reversion back to a nonmucoid phenotype under laboratory conditions) [3, 15,16,17]. In specimens with mixed nonmucoid and mucoid colonies, “%mucoid P.a.” was calculated by taking mucoid P. aeruginosa CFUs as a percentage of total P. aeruginosa CFUs. For this study, a minimum of 10 colonies of growth on PIA was used as the limit of detection.
Multiplex Proinflammatory Cytokine Arrays
After plating for microbial populations, the homogenized tissue in PBS was centrifuged and supernatants were collected, filter sterilized, and stored at − 80 °C. Thereafter, these specimens were thawed on ice and applied to a multiplex cytokine array [V-PLEX Proinflammatory Panel 1 Human Cytokine Arrays (Meso Scale Diagnostics, LLC)] to determine concentrations of five pro-inflammatory cytokines, IL-1β, TNF-α, IL-6, IL-8, and IFN-γ, and one anti-inflammatory cytokine, IL-10. Cytokine arrays were performed according to manufacturer specifications by the Ohio State University Clinical Research Center (CRC) Laboratory.
Formalin-Fixation, H&E Staining, and Histopathology Scoring
One-half of each explant biopsy was formalin-fixed with 10% neutral buffered formalin (NBF) and stored in fixative for at least 72 h prior to further processing for histopathology. Specimens were paraffin embedded, sectioned, and stained with H&E. Each H&E-stained biopsy slide was scanned, digitized, and blindly scored by a board-certified pathologist for 5 indicators of tissue damage: bronchiolar epithelial infiltrate, luminal inflammation, peribronchial/peribronchiolar infiltrate, peribronchiolar fibrosis, and alveolar involvement. This scoring system was partially adapted from a mouse model of CF [18], though two of the histopathologic features used here, bronchiolar epithelial infiltrate and peribronchiolar fibrosis, are novel (Table 1).
The pathological scores for each of the five histopathology features were assessed as follows: bronchial/bronchiolar epithelial infiltrate and peribronchial/peribronchiolar infiltrate scores were obtained by counting the numbers of inflammatory cells and assessing their distribution (e.g., focal versus multifocal and partial versus complete cell collar) within the corresponding portion of the bronchial tree. Measurement of the thickness of peribronchiolar fibrosis and percentage of areas affected by intraluminal infiltrate and alveolar involvement was performed using Aperio ImageScope software (Leica, Germany). The calculation for the percentage of intraluminal infiltrate and alveolar involvement was performed by dividing the affected areas by the total area of the bronchial/bronchiolar lumen or alveoli, respectively. A histopathology composite score was calculated by taking the sum of the scores for all five metrics for any given specimen.
Of note, there were 5 superficial (parenchymal) biopsies within our cohort that did not include representative bronchi or bronchioles but did have alveoli; so for all histopathology indicators other than alveolar involvement, these specimens were scored as “not applicable (NA)” and not included in the corresponding statistical analyses. Conversely, all deep (airway) biopsies did include representative alveoli that could be scored for alveolar involvement.
Statistical Analyses
All statistical tests and linear regression analyses were performed via GraphPad Prism version 7.0 (GraphPad Software). Statistical significance was determined by p < 0.05 for all experiments. All testing using human explant biopsies were performed in duplicate. Non-parametric Kruskal–Wallis one-way analysis of variance (ANOVA) was used to determine statistical significance of the following: 1) differences in P. aeruginosa variant infection by patient and by CF lung lobe; 2) differences in cytokine concentrations and histopathology among three main groups of P. aeruginosa variants-No P. aeruginosa, Nonmucoid P. aeruginosa, and Mucoid or Mixed P. aeruginosa infection. When comparing histopathology scores across the five different indicators between the non-CF control and CF explants, statistical significance was determined via two-way ANOVA with Sidak’s multiple comparisons post hoc test. For the histopathology composite, the non-CF control and CF explants were compared via the unpaired t test with Welch’s correction.
Simple linear regression analysis was performed to assess the relationship between P. aeruginosa variants and cytokine concentrations or histopathology. Independent of microbial infection, the association between cytokines and histopathology was also examined. These data were plotted on a scatter plot, and the regression line was fitted to evaluate the magnitude and direction of the correlation. The goodness-of-fit was determined by the coefficient of determination (R2), and the significance of the regression model was assessed using the p-value.
Results
The distribution of P. aeruginosa variant populations across all lung explants was as follows: No P. aeruginosa was isolated from the non-CF lung (Fig. 1A) (n = 12 biopsies). Based on bacterial growth on Pseudomonas-selective medium, each CF biopsy was categorized as infected with no P. aeruginosa (n = 8), nonmucoid P. aeruginosa only (n = 15), mucoid P. aeruginosa only (n = 3), or mixed P. aeruginosa [i.e., infected with both mucoid and nonmucoid variants, (n = 9)]. There were no statistically meaningful differences in the distribution of P. aeruginosa variant populations across the three CF lung explants, likely due to the small sample size; although there were some notable findings, including the isolation of only nonmucoid variants from CF patient #3 (Fig. 1A). Additionally, all P. aeruginosa variant populations were distributed throughout the CF lungs, without preference for specific lung lobes (Fig. 1B). There were no differences in total P. aeruginosa burden (CFUs) or percentage of mucoid isolates across CF lung lobes (Fig. 1C and D).
P. aeruginosa nonmucoid and mucoid variants were distributed throughout the CF lung explants. Lobar biopsies from 3 CF lung explants and 1 non-CF lung explant control were homogenized and plated on selective media for P. aeruginosa growth. A Distribution of P. aeruginosa variants in each CF and the non-CF lung explant. B Distribution of P. aeruginosa variants by lung lobe in CF explants (n = 35 lobar biopsies). C Total P. aeruginosa burden (CFUs/mL/g tissue) by lung lobe in CF explants. D %Mucoid P. aeruginosa variants per lung lobe in CF explants. The mean and standard deviation are shown (C and D). Statistical significance was determined via non-parametric Kruskal–Wallis one-way analysis of variance (ANOVA). No statistically significant differences were observed (ns). P.a.-P. aeruginosa; No P.a.-No growth of P. aeruginosa; RUL-right upper lobe, RML-right middle lobe, RLL-right lower lobe, LUL-left upper lobe, LIN-lingula, LLL-left lower lobe. CFUs-colony forming units
To examine any association between P. aeruginosa infection and cytokine burden (or subsequently, histopathology), the non-CF lung was excluded since there was no growth of P. aeruginosa in all biopsies from that lung. Additionally, since there were only 3 lobar CF biopsies infected with mucoid variants only, this group was combined with specimens infected with mixed variants (i.e., both mucoid and nonmucoid P. aeruginosa, n = 9); for all subsequent analyses in this study, this new group was called “Mucoid or Mixed” (n = 12). Independent of regional location within CF lungs, mucoid or mixed P. aeruginosa infection was associated with greater concentrations of three proinflammatory cytokines, IL-1β, TNF-α, and IFN-γ, and one anti-inflammatory cytokine, IL-10 compared to nonmucoid P. aeruginosa infection (Fig. 2A–F). Mucoid or mixed P. aeruginosa infection also demonstrated non-statistically significant trends toward higher concentrations of IL-6 and IL-8. However, across all cytokines measured, there were no differences between mucoid or mixed P. aeruginosa and no P. aeruginosa infection (Fig. 2A–F).
Mucoid only or mixed-variant P. aeruginosa infection was associated with increased concentrations of proinflammatory cytokines compared to nonmucoid variant infection. Cytokines were measured in homogenized CF explant biopsy specimens (n = 35) via multiplex cytokine array. Data from each specimen was stratified by type of microbial population present [No P.a., nonmucoid P.a. only, and mucoid only or mixed P.a. variants (i.e., both mucoid and nonmucoid variants within the same specimen]. Cytokine concentrations (pg/mL) were adjusted for the weight of each biopsy [grams (g)]: A IL-1β. B TNF-α. C IL-6. D IL-8. E IL-10. F IFN-γ. Box and whisker plot showing all data points from minimum to maximum. The box represents the interquartile range (IQR), with the line inside the box indicating the median. Whiskers extend to the minimum and maximum values. Individual data points are displayed as dots. Statistical significance was determined via via non-parametric Kruskal–Wallis one-way analysis of variance (ANOVA). **p < 0.01, ***p < 0.001. P.a.- P. aeruginosa
Given the presence of mixed-variant P. aeruginosa populations, we wanted to differentially assess whether the mucoid or nonmucoid constituents within these communities were driving the inflammatory response. We performed simple linear regression analyses examining correlations between mucoid or nonmucoid P. aeruginosa CFUs and relative percentage of these variants with our cytokines of-interest. The proportion of mucoid variants and nonmucoid variants differed across the cohort and within the mixed-variant populations (Supplementary Table 2). Total P. aeruginosa CFUs positively correlated with concentrations of IL-1β, TNF-α, and IL-8 (Table 2). Mucoid variant CFUs and relative percentage positively correlated with all 5 proinflammatory cytokines and IL-10, whereas nonmucoid CFUs demonstrated no statistically significant correlations with any of the cytokines (Table 2). Furthermore, the relative percentage of nonmucoid variants were inversely correlated with IL-1β, IL-6, IFN-γ, and IL-10 (Table 2).
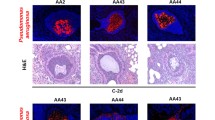
To investigate regional P. aeruginosa infection and tissue damage, we developed and used a novel histopathology scoring system (Table 1). As delineated above, H&E slides derived from each biopsy specimen were blindly scored by a board-certified pathologist, and then correlated with microbiologic data. Across each of five metrics, which were scored 0–4 in severity, we observed within-organ heterogeneity in tissue damage when comparing biopsies from each CF lung (Supplementary Fig. 1); there was greater luminal inflammation in the explant from CF patient #1 compared to CF patient #3, but no other statistically meaningful differences were observed for the other four histopathology metrics or composite among the 3 CF explants. Representative examples of maximum severity (score 4) for each of the five measures of histopathology are depicted here (Fig. 3).
Representative photomicrographs depicting the maximum severity of histopathology observed within CF lung explant biopsies. H&E-stained CF explant biopsies (n = 35) were blindly scored by a board-certified pathologist for 5 indicators of histopathology. A Representative photomicrograph of bronchial/ bronchiolar epithelium infiltrate (arrow; score 4) and peribronchial/ peribronchiolar infiltrate (arrowhead; score 4), scale bar = 50 µm. B Representative photomicrograph of intraluminal infiltrate (arrow; score 4), scale bar = 50 µm. C Representative photomicrograph of peribronchiolar fibrosis (arrow; score 4), scale bar = 100 µm. D Representative photomicrograph of alveolar involvement with septal fibrosis (arrowhead; score 3), scale bar = 100 µm
To validate this methodology, we compared the severity of tissue damage between the non-CF lung and the three native CF lungs. The CF explants demonstrated statistically greater histopathology compared to the non-CF control in three of five assessed features, including peribronchial/bronchiolar infiltrate, peribronchiolar fibrosis, and alveolar involvement (Fig. 4A, B). The overall summative histopathology composite score was also significantly higher in the CF explants compared to the non-CF lung (Fig. 4C).
CF lung explants exhibited significantly higher histopathology scores compared to the non-CF control. A Left panel- representative photomicrograph demonstrating alveolar histology of the non-CF lung. Right panel- representative photomicrograph with alveolar histology of the CF lung. Arrowhead indicates alveolar infiltrate and alveolar septal fibrosis. Scale bars = 300 µm (B). Blinded histopathology scoring of five metrics, bronchial/bronchiolar epithelium infiltrate, intraluminal infiltrate, peribronchial/peribronchiolar infiltrate, peribronchiolar fibrosis, and alveolar involvement in non-CF (n = 12) and CF (n = 35) explant specimens. Statistical significance was determined via two-way analysis of variance (ANOVA) with Sidak’s multiple comparisons post hoc test (C). Composite histopathology score (i.e., summation of scores across all five metrics) in non-CF and CF explant specimens. The mean and standard deviation are shown. Statistical significance was determined via unpaired t test with Welch’s correction. **p < 0.01, ***p < 0.001, ****p < 0.0001
When stratified by P. aeruginosa variants, mucoid or mixed-variant infection in CF lungs was associated with increased bronchiolar epithelial infiltrate compared to specimens without P. aeruginosa infection (Fig. 5A and Supplementary Fig. 2); but there was no difference in bronchiolar epithelial infiltrate between mucoid/mixed and nonmucoid infection (Fig. 5A). There were also no differences observed in the four other histopathologic markers (or the histopathology composite) among the P. aeruginosa infection groups (Fig. 5B–F). Given the presence of mixed-variant infection, we again wanted to assess which of the two constituents (mucoid or nonmucoid P. aeruginosa) was likely responsible for increased bronchiolar epithelial infiltrate. Via linear regression analysis, we found that total P. aeruginosa CFUs correlated with both increased bronchiolar epithelial infiltrate and luminal inflammation (Supplementary Table 3). Nonmucoid CFUs directly correlated with increased bronchiolar epithelial infiltrate, whereas mucoid CFUs did not. There were no correlations observed between relative percentage of mucoid or nonmucoid P. aeruginosa and histopathology (Supplementary Table 3). There was also no association between microbial populations and the histopathology composite.
Mucoid or mixed-variant P. aeruginosa infection is associated with greater bronchiolar epithelial infiltrate compared to no P. aeruginosa infection. Blinded histopathology scoring of five metrics, A bronchial/bronchiolar epithelium infiltrate, B luminal inflammation, C peribronchial/peribronchiolar infiltrate, D peribronchiolar fibrosis, E alveolar involvement, and F composite histopathology score (summative score of all 5 indicators) in CF explant specimens (n = 35). The mean and standard deviation are shown. Statistical significance was determined via non-parametric Kruskal–Wallis one-way analysis of variance (ANOVA). *p < 0.05. P.a.-P. aeruginosa
Of note, independent of microbial infection, we also examined correlations between all cytokines measured and histopathology. Only IL-1β was positively correlated with luminal inflammation (Supplementary Table 4). There were no other correlations observed between the other four indicators of tissue damage or the composite histopathology metric and proinflammatory cytokine concentrations in this study.
Discussion
Although the advent and utilization of novel modulator therapies has increased the life expectancy of pwCF, these drugs have not impacted the long-term microbial burden of P. aeruginosa within the CF lung [19]. As such, chronic bacterial infection and an associated hyperinflammatory response will likely continue to influence lung pathophysiology throughout the lifetime of pwCF. Both nonmucoid and mucoid P. aeruginosa variants, often in consortia, are commonly isolated from the CF airway [7,8,9,10]. The difficulty in eradicating mucoid P. aeruginosa variants with antimicrobials as well as their correlation with adverse clinical outcomes is well-established [5, 6]. However, the interface between these phenotypic variants, the innate immune response, and tissue damage in late-stage disease is still under-studied within human lung tissue.
This limited-cohort pilot study suggests that mucoid P. aeruginosa infection is associated with increased lobar inflammation compared to nonmucoid infection in advanced CF lung disease. In mixed-variant populations of P. aeruginosa, mucoid isolates predominantly contribute to inflammatory cytokine burden, whereas nonmucoid isolates are associated with immune cell infiltration, one marker of tissue damage. Furthermore, our observed findings provide a detailed description of a novel, semi-quantitative histopathology scoring system that can be applied to future larger-cohort and mechanistic studies examining host-bacterial interactions within the CF airway. A tool examining local host responses in the CF lung to microbiota and their interactions is crucial as significant research efforts by others and our group continue.
Our findings suggest that mucoid P. aeruginosa infection is associated with ongoing inflammation, even in the later stages of CF lung disease. Both mucoid P. aeruginosa CFUs and relative percentage of mucoid variants were associated with increased concentrations of all five proinflammatory cytokines measured (IL-1β, TNF-α, IL-6, IL-8, and IFN-γ) as well as the anti-inflammatory cytokine, IL-10 (Table 2). In contrast, there was a negative correlation between the relative proportion of nonmucoid variants and levels of IL-1β, IL-6, IL-10, and IFN-γ. These data build upon our previous findings with BAL fluid from pwCF, wherein we found a specific association between mucoid P. aeruginosa infection and elevated proinflammatory cytokines, whereas nonmucoid P. aeruginosa infection correlated with reduced IL-6 levels [10]. While we had formerly seen that mucoid P. aeruginosa modulates the inflammatory microenvironment in patients with stable CF pulmonary disease, here we demonstrate that this host-bacterial axis likely persists despite organ failure.
The observed correlations between P. aeruginosa infection and inflammation within the explants suggest that cytokines merit further study as long-term biomarkers of disease in the CF lung microenvironment. Given the severity of parenchymal damage in the native CF lung undergoing explantation, it could be theorized that the immune response may be burned out in the advanced stages of illness. This “exhausted inflammation” model does apply to some chronic pulmonary diseases (e.g., hypersensitivity pneumonitis), wherein acute inflammation ultimately gives way to a profibrotic phenotype [20]. However, our study supports prior observations that the CF lung contains abundant inflammatory cytokines, effectors, and cells, even late in disease progression [20, 21].
More work is needed to define correlates of cytokine burden to patient-centered metrics, including pulmonary function and imaging findings of parenchymal/airway disease. In this study, we observed only a limited correlation between cytokine concentration and tissue damage (IL-1β was directly associated with luminal inflammation, Supplementary Table 4), but we look forward to repeating these analyses in a larger cohort. Additionally, we were unable to distinguish between intracellular and extracellular cytokine burden because homogenization of explanted lung tissue may have caused cell lysis, although filtration would have removed any in-tact cells and cell debris. Future studies could utilize immunohistochemistry with cytokine-specific staining of formalin-fixed lung tissue, which may localize cytokines within and outside of different cell types (e.g., epithelial and inflammatory cells).
When stratified by type of P. aeruginosa variant infection (i.e., No P. aeruginosa, nonmucoid only, and mucoid or mixed P. aeruginosa infection) there were no statistically significant differences in cytokine concentrations between the mucoid or mixed P. aeruginosa group and the no P. aeruginosa infection group; similarly, there were no differences between the nonmucoid and the no P. aeruginosa groups (Fig. 2). This may be due to our limited sample size, which might not have been large enough to detect statistical significance. An alternative explanation is that there are non-Pseudomonal factors, including other bacterial, fungal, and viral pathogens within the CF lung that were not isolated or studied here, but do modulate the inflammatory microenvironment. For instance, both Staphylococcus aureus and Burkholderia cenocepacia are known to persist in advanced CF pulmonary disease and have direct impacts on inflammation and clinical outcomes [22,23,24,25]. While out-of-scope for this study, investigating multi-species interactions within CF lung explants could shed light upon progression of pulmonary disease in pwCF. Future studies could capitalize on differential staining techniques including Fluorescence in Situ Hybridization (FISH) and/or immunohistochemistry to study the colocalization of microbial species with one another and immune cells in lobar explant biopsies.
Intriguingly, our work suggests the possibility of differential effects of mucoid and nonmucoid P. aeruginosa variants upon inflammation and tissue damage. As above, our correlative analysis indicated that within mixed-variant infection, mucoid constituents are associated with increased proinflammatory cytokines. In terms of histopathology, the mucoid or mixed-variant P. aeruginosa group also had statistically greater bronchiolar epithelial infiltrate compared to the no P. aeruginosa infection group (Fig. 5A); in this grouped analysis, there was no difference in histopathology between the mucoid or mixed group and the nonmucoid infection only group. However, surprisingly, via linear regression, nonmucoid variants were directly correlated with bronchiolar epithelial infiltrate, whereas mucoid variants did not correlate with any markers of tissue damage (Supplementary Table 3). These data imply that in mixed-variant populations, nonmucoid variants may influence immune cell infiltration.
Of note, total P. aeruginosa CFUs correlated with increased bronchiolar epithelial infiltrate as well as luminal inflammation, suggesting that bacterial burden may be an independent determinant of histopathology and could confound our assessment of variant-specific effects. However, for the majority of 5 tissue damage indicators as well as the histopathology composite, there was no correlation with either mucoid or nonmucoid variant infection, in terms of CFUs (i.e., variant burden) or relative percentage (Fig. 5 and Supplementary Table 3). This may be because patients needing transplantation are likely to have diffuse parenchymal/airway damage, making it difficult to resolve lobar differences in histopathology within our small cohort size. As such, it is possible that the correlation between nonmucoid infection and bronchiolar epithelial infiltrate is stochastic. However, if this does represent a true biological finding and can be reproduced in future, large-cohort analyses, it may point toward different ways that mucoid and nonmucoid variants impact CF lung disease, particularly in mixed populations.
It is telling that despite our limited cohort, there were only three specimens (~10% of all explant biopsies) wherein mucoid variants existed in isolation without nonmucoid counterparts (Supplementary Table 2). We have previously demonstrated that mixed populations of both mucoid and nonmucoid variants produce and share communal resources, facilitating more effective evasion of the host immune response [11]. As such, the persistence of mucoid P. aeruginosa may partially depend upon the presence of nonmucoid partners and vice versa. Mucoid variants are still thought to represent the more chronically adapted P. aeruginosa variants, and their staying power within the lung hinges upon evasion of immune detection. This is partially accomplished through downregulation of pathogen-associated molecular patterns (PAMPs) such as flagella after mucoid conversion [26]. Our findings here might suggest that mucoid variants still affect cytokine signaling late in disease, but regional immune cell influx into the tissues (i.e., bronchiolar epithelial infiltrate) is associated with the less patho-adapted and therefore, more immunogenic nonmucoid variants. In consortia, then, it is possible that while mucoid variants stimulate cytokine production and facilitate persistence of diverse P. aeruginosa communities, nonmucoid variants attract the attention of immune cells and drive local tissue damage.
The posited mechanism here is speculative, though deserving of attention in controlled animal studies and future human lung explant research. To our knowledge, there is no study that has coinfected a CF animal model with mixed inoculum of both mucoid and nonmucoid variants to understand differential immune modulation and histopathology. Additionally, since mucoid P. aeruginosa variants exhibit heightened antibiotic resistance, predict the loss of pulmonary function, and alter the inflammatory cytokine response, novel therapies are needed to selectively target these bacterial isolates [5, 6]. However, targeting nonmucoid variants may be just as important, not only because these variants contribute to the survival of their mucoid partners, but also because they associate with inflammatory cell influx, which could contribute to loss of tissue/organ function.
There are several important limitations of our study, many of which are alluded to above. First, our study is limited by a small cohort, so associations between P. aeruginosa variants, cytokine concentration, and histopathology must be recapitulated via a larger sample size. Mechanistic questions, including the differential effects of P. aeruginosa variants upon immune modulation and histopathology needs further exploration via appropriately controlled animal models of CF lung disease. Furthermore, we observed direct correlations between total P. aeruginosa burden and cytokines as well as histopathology, suggesting the presence of a confounding variable when studying the effects of colony morphotypes. However, in our linear regression analysis, we control for this by examining correlations between nonmucoid and mucoid CFUs and our variables of interest. If bacterial burden were the major deterministic factor, we would expect direct correlations between both nonmucoid and mucoid CFUs and cytokines/histopathology; on the contrary, we observed differential trends based on the variants present (including inverse correlations between nonmucoid variants and proinflammatory cytokines) (Table 2).
In this study, we took two types of biopsies from each lung explants: superficial (parenchymal) and deep (airway) biopsies. As acknowledged above, there were 5 parenchymal biopsies that did not include a representation of larger airways (bronchi or bronchioles), so they could only be scored for alveolar involvement. As such, both types of biopsies may represent distinct anatomical niches within the bronchial tree that could affect the isolated microbial populations, cytokine burden, and histopathology. For instance, in CF explant 1, none of the parenchymal samples had P. aeruginosa isolates, while 3/5 airway biopsies had only nonmucoid variants, 1/5 had only mucoid variants, and 1/5 had mixed variants (Supplementary Table 2). Given our limited sample size, we did not compare parenchymal and airway specimens across our readouts of infection, cytokines, and tissue damage, but this will be addressed in future studies.
To our knowledge, this work still represents the first application of a detailed histopathology scoring system to assess human CF lung tissue. The quantitative elements of this method (e.g., use of imaging software to measure percentage area affected by intraluminal infiltrate and alveolar involvement) as well as blinding of the scorer are strengths that may enable reproducibility. However, this scoring system will require further validation in a larger cohort of CF explants. It will also be helpful to include more than one “non-CF lung” for comparison, ideally taken from multiple non-CF disease states (e.g., chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis) to assess if our scoring system can identify histopathology that is CF-specific. Furthermore, we would propose that our scoring system could feasibly be tested and applied in animal models of CF lung disease, as the five indicators of tissue damage posited here are not specific to human disease [18]. Future studies should combine H&E staining with immunohistochemistry to define specific immune cell populations that correlate with infection. It would also be helpful to further support the effectiveness of this scoring scheme by examining correlations between histopathology and computed tomography (CT) findings of CF lung disease (e.g., bronchiectasis).
Additionally, although we focused upon P. aeruginosa infection only, examining the colocalization of various microbial species and immune cells within lung explants would further expand our understanding of host–pathogen dynamics in CF. While we phenotypically identified mucoid and nonmucoid variants here by colony morphology, we did not pursue further genotypic/phenotypic analysis of these isolates by in vitro studies. We did not observe reversion of the isolated mucoid colonies to a nonmucoid phenotype on PIA; this is partially because we completed our colony counts within 48 h of growth, after which we have noticed that the mucoid phenotype does become unstable in vitro. Future work should also seek to minimize the time between explantation, processing, and plating of tissue on growth medium (2–4 h in this study) to ensure the most optimal recovery of bacterial populations and phenotypic variants.
Nevertheless, reversion of mucoid variants can occur within the CF lung as well and we did not pursue genotypic testing to specifically identify nonmucoid revertants. To clarify, nonmucoid P. aeruginosa variants in the CF lung can be either “nonmucoid progenitors,” that initially colonize the airway and later acquire mutations to become mucoid variants; or they can be “nonmucoid revertants,” which are mucoid variants that have acquired secondary mutations, losing the ability to synthesize alginate, and therefore transition back to a nonmucoid phenotype [27]. We intend to pursue further in vitro and in vivo investigations focused upon the impacts of progenitor and revertant nonmucoid P. aeruginosa on inflammation and tissue damage. Other specific nonmucoid variants not analyzed here, including the rugose small colony variants (RSCVs), should also be part of these future studies.
Data Availability
No datasets were generated or analyzed during the current study.
Abbreviations
- CF:
-
Cystic fibrosis
- PwCF:
-
People with cystic fibrosis
- BAL:
-
Bronchoalveolar lavage
References
Blanchard AC, Waters VJ (2019) Microbiology of cystic fibrosis airway disease. Semin Respir Crit Care Med 40:727–736. https://doi.org/10.1055/s-0039-1698464
Jurado-Martín I, Sainz-Mejías M, McClean S (2021) Pseudomonas aeruginosa: an audacious pathogen with an adaptable arsenal of virulence factors. Int J Mol Sci 22:1–37
Govan JR, Deretic V (1996) Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60:539–574. https://doi.org/10.1111/j.1365-2672.2007.03706.x
Pritt B, O’Brien L, Winn W (2007) Mucoid Pseudomonas in cystic fibrosis. Am J Clin Pathol 128:32–34. https://doi.org/10.1309/KJRPC7DD5TR9NTDM
Li Z, Kosorok MR, Farrell PM et al (2005) Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 293:581–588
Talwalkar JS, Murray TS (2016) The Approach to Pseudomonas aeruginosa in Cystic Fibrosis. Clin Chest Med 37:69–81. https://doi.org/10.1016/j.ccm.2015.10.004
Clark ST, Diaz Caballero J, Cheang M et al (2015) Phenotypic diversity within a Pseudomonas aeruginosa population infecting an adult with cystic fibrosis. Sci Rep 5:10932. https://doi.org/10.1038/srep10932
Høiby N, Ciofu O, Bjarnsholt T (2010) Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol 5:1663–1674. https://doi.org/10.2217/fmb.10.125
Tai AS, Sherrard LJ, Kidd TJ et al (2017) Antibiotic perturbation of mixed-strain Pseudomonas aeruginosa infection in patients with cystic fibrosis. BMC Pulm Med 17:1–10. https://doi.org/10.1186/s12890-017-0482-7
Malhotra S, Hayes D, Wozniak DJ (2019) Mucoid Pseudomonas aeruginosa and regional inflammation in the cystic fibrosis lung. J Cyst Fibros 18:796–803. https://doi.org/10.1016/j.jcf.2019.04.009
Malhotra S, Limoli DH, English AE et al (2018) Mixed communities of mucoid and nonmucoid pseudomonas aeruginosa exhibit enhanced resistance to host antimicrobials. mBio. 9:1–15. https://doi.org/10.1128/mBio
Goddard M (2011) Histopathology of bronchiectasis. Bronchiectasis. https://doi.org/10.1183/1025448x.10003310
Dasenbrook EC, Lu L, Donnola S et al (2013) Normalized T1 magnetic resonance imaging for assessment of regional lung function in adult cystic fibrosis patients—a cross-sectional study. PLoS ONE 8:1–7. https://doi.org/10.1371/journal.pone.0073286
Li Z, Sanders DB, Rock MJ et al (2012) Regional differences in the evolution of lung disease in children with cystic fibrosis. Pediatr Pulmonol 47:635–640. https://doi.org/10.1002/ppul.21604
Wyckoff TJO, Thomas B, Hassett DJ, Wozniak DJ (2002) Static growth of mucoid Pseudomonas aeruginosa selects for non-mucoid variants that have acquired flagellum-dependent motility. Microbiology (N Y) 148:3423–3430. https://doi.org/10.1099/00221287-148-11-3423
Ciofu O, Lee B, Johannesson M et al (2008) Investigation of the algT operon sequence in mucoid and non-mucoid Pseudomonas aeruginosa isolates from 115 Scandinavian patients with cystic fibrosis and in 88 in vitro non-mucoid revertants. Microbiology (N Y) 154:103–113. https://doi.org/10.1099/mic.0.2007/010421-0
Govan JRW, Fyfe JAM, McMillan C (1979) The instability of mucoid pseudomonas aeruginosa: fluctuation test and improved stability of the mucoid form in shaken culture. J Gen Microbiol 110:229–232
Dubin PJ, Kolls JK (2007) IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 292:L519–L528. https://doi.org/10.1152/ajplung.00312.2006
Ribeiro CMP, Higgs MG, Muhlebach MS et al (2023) Revisiting host-pathogen interactions in cystic fibrosis lungs in the era of CFTR modulators. Int J Mol Sci 24(5):5010
Gifford AH, Matsuoka M, Ghoda LY et al (2012) Chronic inflammation and lung fibrosis: pleotropic syndromes but limited distinct phenotypes. Mucosal Immunol 5:480–484
Lammertyn EJ, Vandermeulen E, Bellon H et al (2017) End-stage cystic fibrosis lung disease is characterised by a diverse inflammatory pattern: an immunohistochemical analysis. Respir Res 18:1–9. https://doi.org/10.1186/s12931-016-0489-2
Sajjan U, Corey à M, Humar{ A, et al Immunolocalisation of Burkholderia cepacia in the lungs of cystic ®brosis patients
Daccò V, Alicandro G, Consales A et al (2023) Cepacia syndrome in cystic fibrosis: a systematic review of the literature and possible new perspectives in treatment. Pediatr Pulmonol 58:1337–1343
Ahlgren HG, Benedetti A, Landry JS et al (2015) Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulm Med. https://doi.org/10.1186/s12890-015-0062-7
Goss CH, Muhlebach MS (2011) Review: staphylococcus aureus and MRSA in cystic fibrosis. J Cyst Fibros 10:298–306
Garrett ES, Perlegas D, Wozniak DJ (1999) Negative control of flagellum synthesis in Pseudomonas aeruginosa is modulated by the alternative sigma factor AlgT (AlgU). J Bacteriol 181:7401–7404
Malhotra S, Hayes D, Wozniak DJ (2019) Cystic fibrosis and pseudomonas aeruginosa: the host-microbe interface. Clin Microbiol Rev 32(3):10–128
Acknowledgements
This study was supported by a TL1 fellowship awarded to S.M. by the Center for Clinical and Translational Science (CCTS), The Ohio State University College of Medicine (TL1TR001069).
Funding
This study was supported by a TL1 fellowship awarded to S.M. by the Center for Clinical and Translational Science (CCTS), The Ohio State University College of Medicine (TL1TR001069).
Author information
Authors and Affiliations
Contributions
S.M. contributed to study design, specimen acquisition and processing, data analysis and interpretation, and writing the manuscript. C.Y. contributed to study design, data analysis and interpretation, and writing the manuscript. K.L.N. contributed to data analysis and writing the manuscript. D.J.W. contributed to study design, data analysis and interpretation, and writing the manuscript. D.H. contributed to study design, specimen acquisition and processing, data analysis and interpretation, and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the local Institutional Review Board (IRB07-00396).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malhotra, S., Yang, C., Nicholson, K.L. et al. Pseudomonas aeruginosa Infection and Inflammation in Cystic Fibrosis: A Pilot Study With Lung Explants and a Novel Histopathology Scoring System. Lung (2024). https://doi.org/10.1007/s00408-024-00733-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00408-024-00733-y