Abstract
Purpose
The causal relationships between circulating adipokines and idiopathic pulmonary fibrosis (IPF) are yet to be established. We performed a two-sample Mendelian randomization (MR) study to investigate the causal roles of adipokines on IPF risk.
Methods
We analyzed the summary data from genome-wide association studies (GWAS), including adiponectin, leptin, resistin and monocyte chemoattractant protein-1 (MCP-1) and IPF. The inverse-variance weighted (IVW) method was considered as the major method and the MR-Egger, weighted median, simple mode and weighted mode were utilized as complementary methods. We also performed the sensitivity analyses, including heterogeneity test, horizontal pleiotropy test and leave-one-out analysis.
Results
The selected number of single nucleotide polymorphisms (SNPs) was 13 for adiponectin, 6 for leptin,12 for resistin, and 6 for MCP-1, respectively. The results showed a causal effect of the circulating adiponectin levels on the risk of IPF (OR 0.645, 95% CI 0.457–0.911, P = 0.013). However, we did not observe significant associations of genetic changes in serum leptin (OR 1.018, 95% CI 0.442–2.346, P = 0.967), resistin (OR 1.002, 95% CI 0.712–1.408, P = 0.993), and MCP-1 (OR 1.358, 95% CI 0.891–2.068, P = 0.155) with risk of developing IPF. There was no evidence of heterogeneity or horizontal pleiotropy. The sensitivity analyses confirmed that our results were stable and reliable.
Conclusions
The increase in serum adiponectin was associated causally with a decreased risk of developing IPF. There is no evidence to support a causal association between leptin, resistin or MCP-1 with risk of IPF. Further studies are needed to confirm our findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, fibrotic interstitial lung disease without a recognized etiology that occurs primarily in men over the age of 65. It is characterized by cough, exertional dyspnea, restrictive pulmonary dysfunction and histological pattern of usual interstitial pneumonia (UIP). The prevalence, rate of hospital admissions and mortality of IPF are increasing during last decades worldwide, which suggests a huge burden of disease. Although important progress has been made regarding the pharmacologic therapies for IPF, the prognosis is poor with an estimated median survival time of 2 to 5 years from diagnosis [1,2,3]. Thus, the identification of genetic or environmental risk factors for developing IPF and the early prevention are of extreme importance.
Adipokines, a category of peptide hormone mainly produced by adipose tissues, are often considered to be related to obesity-associated diseases. They participate in the mediation and regulation of immunological, inflammatory and metabolic processes [4]. Furthermore, evidence showed that adipokines might play a critical role in epithelial-mesenchymal transition (EMT) and fibrogenesis in the lung [5]. The associations between adipokines and IPF have been previously investigated in observational studies. It is reported that serum adipokine levels could be useful for predicting the prognosis of IPF [6]. Cao et al. found that plasma leptin levels were significantly higher in patients with acute exacerbation of IPF than in those with stable IPF or healthy controls [7]. In addition, Enomoto et al. demonstrated that the higher adiponectin/leptin ratio might be associated with the lower body mass index (BMI) and PaO2, the higher C-reactive protein, and a poor prognosis in patients with IPF [8]. However, in previous studies, the sample sizes were often small or median and the conclusions are not consistent, or controversial. In addition, the nature and direction of causal relationship between them are yet to be established. It remains unclear whether aberrant adipokines cause IPF or IPF contributes to changed adipokine levels from previous cross-sectional results. New approach is warranted to clarify the causal effect of circulating adipokine levels on IPF, which might help better understanding the causes and risk factors of disease, providing early prevention or diagnosis, and finally improving prognosis.
Mendelian randomization (MR) exploits genetic variants, which are randomly distributed in a population, as instruments to enable reliable causal inferences. It is not affected by environmental confounding factors and could minimize reverse causation, and thus known as “nature’s RCT” [9]. Genome-wide association studies (GWAS) have identified a large number of genetic associations for many diseases and disease-related traits. The availability of summary statistics from GWAS makes it possible to determine whether there are causal effects between underlying risk factors and common diseases in a cost-effective manner [10]. Herein, we performed a two-sample MR study to investigate the causal roles of common circulating adipokines, including adiponectin, leptin, resistin and monocyte chemoattractant protein-1 (MCP-1), on IPF risk.
Methods
Study Design and Data Source
The analytic and reporting process followed the Strengthening the Reporting of Observational Studies in Epidemiology Statement using MR guidelines (STROBE-MR) [11]. The present two-sample MR study relied only on publicly available de-identified summary statistics from relevant published GWAS. The ethical approval and informed consent were obtained in all original studies. Additional ethical approval was not required for our study.
The summary-level data related to the adiponectin level were obtained from a meta-analysis of GWAS in 39,883 individuals of European ancestry [12]. The summary-level data of leptin level originated from an exome-based analysis in up to 57,232 individuals of diverse ancestries of whom 50,321 were of European descent [13]. Moreover, data on resistin and monocyte chemoattractant protein-1 (MCP-1) were both taken from a genome-wide meta-analysis that included up to 21,758 European ancestries [14]. The numbers of included SNPs in these studies varied from 231,001 to 13,138,697.
The GWAS data of outcome variable IPF were from FinnGen biobank (FinnGen release 5), including genotype data of 1028 patients and 196,986 controls of European ancestry [15]. There was little overlap between the populations included in exposure datasets and outcome datasets, and the sample was almost entirely of European ancestry including males and females, which both guarantee the validity of the findings.
The summary data of all GWAS analyses can be downloaded from the open-access GWAS dataset MRC Integrative Epidemiology Unit (MRC-IEU) at https://gwas.mrcieu.ac.uk/ (adiponectin GWAS ID: ieu-a-1; leptin GWAS ID: ebi-a-GCST90007307; resistin GWAS ID: ebi-a-GCST90012034; MCP-1 GWAS ID: ebi-a-GCST90012007; IPF GWAS ID: finn-b-IPF).
Selection of Instrumental Variables
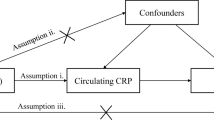
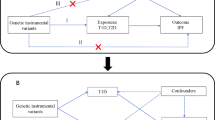
To assess the causal association between exposure (adipokines) and outcome (IPF), it must satisfy three critical assumptions for instrumental variables (IVs): (1) IVs are powerfully related to serum adipokines at a genome-wide significant level; (2) The selected IVs must be independent of any confounders of the association between exposure and outcome; (3) IVs affect IPF only through the serum adipokine levels, not via other pathways. [16]
IVs are generally genetic variations, among which single nucleotide polymorphisms (SNPs) are the most commonly used. First, SNPs were identified to be strongly associated with exposures with P values < 5 × 10 −8. Then, SNPs with R2 > 0.001 and within 10,000 kb distance were considered to be in strong linkage disequilibrium (LD) structure and were subsequently excluded from the analysis. Besides, the F statistic, calculated as R2/(1-R2) * (n-2), was used to evaluate the strength of the IV–exposure correlation. It reflects both magnitude and precision of the SNPs’ effects on exposures. The R2 refers to the cumulative explained variance of selected SNP on circulating adipokine levels. The n is the sample size. We deleted those SNPs with F-statistics less than 10 to avoid the bias caused by weak IVs. [17]
By matching SNPs, we harmonized GWAS summary data between the exposure and the outcome, and corrected the palindromic variants to ensure allelic consistency and alignment of effect.
Statistical Analysis
The wald ratio for each SNP was calculated and then combined using the inverse-variance weighted (IVW) method which required all selected SNPs to be valid IVs. It was considered as the major analytic approach because it provided the most accurate assessments. The MR-Egger, weighted median, simple mode and weighted mode were utilized as complementary methods. [18, 19] MR-Egger method allowed all SNPs to be voided, while the weighted median method allowed up to 50% of invalid SNPs. The odds ratios (ORs) and 95% confidence intervals (95% CIs) were used to describe the MR results of relationships between exposures and outcome.
Then, we performed the sensitivity analyses to test the robustness of the associations and to identify potential bias. The heterogeneity among IVs was examined using Cochran’s Q-test. Cochran’s Q-test P value < 0.05 indicated heterogeneity. The horizontal pleiotropy of IVs was tested using MR-Egger regression and MR pleiotropy residual sum and outlier (MR-PRESSO) global test [20]. If outliers were detected through MR-PRESSO, they would be removed and then the remaining SNPs would be re-analyzed. In addition, the leave-one-out analysis based on IVW point estimation was performed by removing one SNP at a time to explore the stability of results.
The statistical analysis was performed in R 4.2.1 software (R Foundation for Statistical Computing) utilizing the R package TwoSampleMR (version 0.5.6). A two-tailed P < 0.05 was considered as statistically significant.
Results
Selection of Single Nucleotide Polymorphisms
Initially, we obtained 14 SNPs that were significantly related to adiponectin levels. A total of 13 SNPs were selected as IVs after rs1597466 was removed due to a F-statistics of 9.35. Six SNPs associated with circulating leptin levels were extracted and analyzed. In addition, we excluded the rs73008259 because it had no appropriate corresponding data in IPF GWAS and then utilized remaining 12 SNPs for analysis of resistin. Six SNPs related to MCP-1 were utilized. No SNP was removed from the present study due to being palindromic with intermediate allele frequencies. The detailed information of the SNPs was summarized in Supplementary material 1 Table S1.
Mendelian Randomization Analyses
The results showed a causal effect of the circulating adiponectin level on the risk of IPF (IVW OR 0.645, 95% CI 0.457–0.911, P = 0.013; MR-Egger OR 0.587, 95% CI 0.365–0.944, P = 0.05; weighted medianOR 0.560, 95% CI 0.355–0.883, P = 0.012; weighted mode OR 0.566, 95% CI 0.360–0.889, P = 0.03). Although non-significant result was found via the simple mode method (OR 0.559, 95% CI 0.288–1.085, P = 0.111), the direction of β value or estimated OR was consistent with those calculated by the other four methods. However, we did not observe significant associations of genetic changes in serum leptin (IVW OR 1.018, 95% CI 0.442–2.346, P = 0.967), resistin (IVW OR 1.002, 95% CI 0.712–1.408, P = 0.993), and MCP-1 (IVW OR 1.358, 95% CI 0.891–2.068, P = 0.155) with risk of developing IPF (Fig 1). We obtained similar results in the replicated analyses with other methods.
As shown in the scatter plot, the risk of IPF decreases with the increasing circulating adiponectin level (Fig 2A). The results of the other three adipokines are shown in Fig 2B–D. The causal effects of each SNP on IPF among these adipokines were displayed in the forest plots (Fig 3A–D). The funnel plots did not show noticeable asymmetries (Supplementary material 2 Figure S1 A–D).
Sensitivity Analyses
There was no evidence of heterogeneity across estimates of included SNPs for those four adipokines (P values > 0.05). No significant horizontal pleiotropy existed among IVs with P values > 0.05 in both MR-egger-intercept and MR-PRESSO Global Test. The intercept in MR-Egger regression did not differ from zero significantly, suggesting that IVs are unlikely to affect IPF risk through pathways other than adipokines. Besides, no outlier was detected and thus, no further correction or analysis is needed. The detailed results of sensitivity analyses are all shown in Table 1. The leave-one-out sensitivity analysis showed stable results (Supplementary material 2 Figure S2 A–D). Thus, the results of MR analyses were deemed to be stable and reliable in our study.
Discussion
To our knowledge, the current study is the first attempt to clarify the causal effects of serum adipokines on IPF through MR analyses. Our results could reflect the effects of long-term circulating adipokine levels, which are genetically controlled, on IPF. These conclusions are realistic and not susceptible to short-term or environmental confounding factors. Ma et al. conducted a two-sample MR analysis to assess the causal effects of different indicators of obesity on IPF risk. Only increased BMI was associated with higher risk of IPF (OR = 1.51, 95% CI 1.22–1.87) [21]. However, the increased BMI represents the overall adiposity which is regulated by multiple factors. In another recent MR study, Xiao et al. reported that the circulating leptin (OR = 2.26, 95% CI 1.41–3.64) and adiponectin (OR = 0.81,95% CI 0.68–0.98) level was a risk factor and protective factor, respectively, of pulmonary embolism which is a common co-morbidity of IPF [22]. However, none of previous study investigated the association of adipokines with IPF using MR analysis.
Although the exact mechanisms by which adipokines contribute to IPF are unknown, current data suggest that a persistent elevation of adipokines might increase susceptibility to IPF via the aberrant innate and adaptive immune responses and the chronic inflammation. [23,24,25] Adiponectin is the most abundant adipokine and is negatively correlated with adiposity. It exhibits intense anti-insulin, anti-inflammatory, anti-apoptotic, and anti-fibrotic properties and serves as an essential messenger facilitating interactions between adipose tissues and multiple systemic organs [26]. It has been previously reported that adiponectin protects against paraquat-induced pulmonary fibrosis via suppression of lung fibroblast activation in a dose-dependent manner. [27], A recent study from Wu et al. indicated that adiponectin-mediated fatty acid metabolism greatly suppressed lipid peroxide accumulation fibroblast proliferation, and fibrosis but activated autophagy [28]. Another study suggested that the antifibrotic effects of adiponectin might be mediated via inhibiting the NF-κB signaling pathway [29]. Moreover, previous studies suggested that adiponectin could alleviate fibrosis in various organs, such as kidney and intestine [30, 31]. The present study further demonstrated that adiponectin is a protective factor in IPF causally, providing a basis for further in-depth investigation to explore its clinical applicability.
Leptin primarily regulates the neuroendocrine, metabolism and energy homeostasis and plays a role in fetal growth, angiogenesis, lipolysis, etc [32]. In contrast with adiponectin, leptin has been reported to be a pro-inflammatory and pro-fibrotic factor. It accelerates the EMT of alveolar epithelial cells and progression of pulmonary fibrosis through inhibiting autophagy via PI3K/Akt/mTOR pathway. [33] Resistin has been shown to play a pivotal role in glucose metabolism, insulin resistance and proinflammatory immune responses. Thus, it is involved in various metabolic, inflammatory, and autoimmune diseases [34]. High serum resistin level is also found to correlate with lung function impairment and pulmonary fibrosis via mediating immune responses [35]. MCP-1, also known as chemokine (CC-motif) ligand 2 (CCL2), could attract or enhance the expression of inflammatory cytokines in the pathogenesis of numerous diseases, including neuroinflammatory diseases, autoimmune diseases and cardiovascular diseases [36]. It has also been identified as a critical proinflammatory chemokine and inflammatory mediator in the fibrogenesis process [37]. However, Pulito-Cueto et al. reported that serum MCP-1 level was increased significantly in rheumatoid arthritis-related interstitial lung disease patients rather than in IPF patients [38]. Our results showed that the above three adipokines are not significantly associated with IPF although elevated ORs were observed.
Our MR study assessed the validity of each MR assumption adequately. For the relevance and independence assumptions, we carefully checked the populations and IVs to strengthen the causal inference of our analysis. The consistency of results across different MR statistical methods revealed the robustness of our conclusions. In present study, we did not manually scan selected SNPs for potential secondary phenotypes in the PhenoScanner. It is noticeable that the number of SNPs after harmonization in our study is not large enough. The pathogenesis and etiology of IPF are complex and obscure. Thus, substantial bias might be brought out if the SNPs which were probably related to other traits were excluded. In this condition, the analysis with all selected IVs was thought to be credible according to previous similar researches and viewpoints [39, 40]. Although the exclusion-restriction assumption cannot be fully tested, this can be partly verified via multiple approaches of sensitivity analyses. We did not observe any evidence of heterogeneity or horizontal pleiotropy. Therefore, the possibility of violation of MR assumptions in our study is considered to be low. We also attempted to investigate the causal associations of more adipokines, such as plasminogen activator inhibitor-1 and leptin receptor, with IPF risk. However, there were not enough SNPs as IVs to support MR analyses. The causal effects of them on IPF could be investigated in future when sufficient genetic data become available. Besides, Kulkarni et al. showed that IPF patients with BMI decrements had worse outcomes. They reported that leptin and adiponectin levels are related to BMI, but neither adipokine was prognostic per se [41]. Our study only identified one adipokine correlated with risk of developing IPF due to limited data. More studies are warranted to investigate the associations of adipokines with prognosis of IPF.
Our results strengthened the evidence for the causal effects of adipokines on IPF and might have some clinical implications for raising the interest in adipokines as a potential preventive target for IPF. However, there are some limitations in the study. First, the results were mainly based on populations of European ancestry, limiting the generalization to other ethnicities. Second, the limited number of SNPs associated with adipokines were selected in our study, indicating limited proportion of variance in exposures explained by the IVs and probably low statistical power. Then, although we did not find the evidence of horizontal pleiotropy, there might be residual bias considering that the accurate functions of most SNPs remain to be unknown until now. Last, we failed to stratify our analysis by covariates of interest, including age and gender, due to lack of individual data.
Conclusions
Overall, our study showed that the increase in serum adiponectin was associated causally with a decreased risk of developing IPF. However, there is no evidence to support a causal association between leptin, resistin or MCP-1 with risk of IPF. The results expand our knowledge of novel etiology of IPF and also provide new insights into the understanding of its pathogenesis. Further studies with updated data from large genetic studies are needed to confirm our results.
Data Availability
Publicly available datasets were analyzed in this study. All GWAS data used in this study are available in the IEU open GWAS project (https://gwas.mrcieu.ac.uk/). All data generated or analyzed during this study are included in this published article.
Abbreviations
- IPF:
-
Idiopathic pulmonary fibrosis
- MR:
-
Mendelian randomization
- GWAS:
-
Genome-wide association studies
- MCP-1:
-
Monocyte chemoattractant protein-1
- IVW:
-
Inverse-variance weighted
- MR-PRESSO:
-
MR pleiotropy residual sum and outlier
- SNP:
-
Single nucleotide polymorphism
- OR:
-
Odds ratio
- 95%CI:
-
95% Confidence interval
- EMT:
-
Epithelial-mesenchymal transition
- BMI:
-
Body mass index
- IV:
-
Instrumental variable
References
Richeldi L, Collard HR, Jones MG (2017) Idiopathic pulmonary fibrosis. Lancet 389(10082):1941–1952. https://doi.org/10.1016/S0140-6736(17)30866-8
Martinez FJ, Collard HR, Pardo A et al (2017) Idiopathic pulmonary fibrosis. Nat Rev Dis Primers 3:17074. https://doi.org/10.1038/nrdp.2017.74
Lederer DJ, Martinez FJ (2018) Idiopathic pulmonary fibrosis. N Engl J Med 378(19):1811–1823. https://doi.org/10.1056/NEJMra1705751
Fasshauer M, Blüher M (2015) Adipokines in health and disease. Trends Pharmacol Sci 36(7):461–470. https://doi.org/10.1016/j.tips.2015.04.014
Jain M, Budinger GR, Lo A et al (2011) Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-γ. Am J Respir Crit Care Med 183(11):1490–1498. https://doi.org/10.1164/rccm.201009-1409OC
d’Alessandro M, Bergantini L, Refini RM et al (2020) Adiponectin and leptin levels in idiopathic pulmonary fibrosis: a new method for BAL and serum assessment. Immunobiology 225(5):151997. https://doi.org/10.1016/j.imbio.2020.151997
Cao M, Swigris JJ, Wang X et al (2016) Plasma Leptin is elevated in acute exacerbation of idiopathic pulmonary fibrosis. Mediators Inflamm 2016:6940480. https://doi.org/10.1155/2016/6940480
Enomoto N, Oyama Y, Yasui H et al (2019) Analysis of serum adiponectin and leptin in patients with acute exacerbation of idiopathic pulmonary fibrosis. Sci Rep 9(1):10484. https://doi.org/10.1038/s41598-019-46990-3
Emdin CA, Khera AV, Kathiresan S (2017) Mendelian randomization. JAMA 318(19):1925–1926. https://doi.org/10.1001/jama.2017.17219
Boehm FJ, Zhou X (2022) Statistical methods for Mendelian randomization in genome-wide association studies: a review. Comput Struct Biotechnol J 20:2338–2351. https://doi.org/10.1016/j.csbj.2022.05.015
Skrivankova VW, Richmond RC, Woolf BAR et al (2021) Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA 326(16):1614–1621. https://doi.org/10.1001/jama.2021.18236
Dastani Z, Hivert MF, Timpson N et al (2012) Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet 8(3):e1002607. https://doi.org/10.1371/journal.pgen.1002607
Yaghootkar H, Zhang Y, Spracklen CN et al (2020) Genetic studies of leptin concentrations implicate leptin in the regulation of early adiposity. Diabetes 69(12):2806–2818. https://doi.org/10.2337/db20-0070
Folkersen L, Gustafsson S, Wang Q et al (2020) Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nat Metab 2(10):1135–1148. https://doi.org/10.1038/s42255-020-00287-2
Dhindsa RS, Mattsson J, Nag A et al (2021) Identification of a missense variant in SPDL1 associated with idiopathic pulmonary fibrosis. Commun Biol 4(1):392. https://doi.org/10.1038/s42003-021-01910-y
Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey SG (2008) Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27(8):1133–1163. https://doi.org/10.1002/sim.3034
Burgess S, Thompson SG, CRP CHD genetics collaboration (2011) Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol 40(3):755–764. https://doi.org/10.1093/ije/dyr036
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44(2):512–525. https://doi.org/10.1093/ije/dyv080
Bowden J, Davey Smith G, Haycock PC, Burgess S (2016) Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol 40(4):304–314. https://doi.org/10.1002/gepi.21965
Verbanck M, Chen CY, Neale B, Do R (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50(5):693–698. https://doi.org/10.1038/s41588-018-0099-7
Ma Y, Feng C, Tang H et al (2022) Management of BMI Is a potential new approach for the prevention of idiopathic pulmonary fibrosis. Front Genet 13:821029. https://doi.org/10.3389/fgene.2022.821029
Xiao W, Li J, Feng T, Jin L (2023) Circulating adipokine concentrations and the risk of venous thromboembolism: a Mendelian randomization and mediation analysis. Front Genet 14:1113111. https://doi.org/10.3389/fgene.2023.1113111
Guo X, Sunil C, Qian G (2022) Obesity and the development of lung fibrosis. Front Pharmacol 12:812166. https://doi.org/10.3389/fphar.2021.812166
Heukels P, Moor CC, von der Thüsen JH, Wijsenbeek MS, Kool M (2019) Inflammation and immunity in IPF pathogenesis and treatment. Respir Med 147:79–91. https://doi.org/10.1016/j.rmed.2018.12.015
Nie YJ, Wu SH, Xuan YH, Yan G (2022) Role of IL-17 family cytokines in the progression of IPF from inflammation to fibrosis. Mil Med Res 9(1):21. https://doi.org/10.1186/s40779-022-00382-3
Fang H, Judd RL (2018) Adiponectin regulation and function. Compr Physiol 8(3):1031–1063. https://doi.org/10.1002/cphy.c170046
Yao R, Cao Y, He YR, Lau WB, Zeng Z, Liang ZA (2015) Adiponectin attenuates lung fibroblasts activation and pulmonary fibrosis induced by paraquat. PloS One 10(5):e0125169. https://doi.org/10.1371/journal.pone.0125169
Wu W, Zhang G, Qiu L, Liu X, Zhou S, Wu J (2022) Contribution of Adiponectin/Carnitine Palmityl Transferase 1A-mediated fatty acid metabolism during the development of idiopathic pulmonary fibrosis. Oxid Med Cell Longev 2022:5265616. https://doi.org/10.1155/2022/5265616
Wang X, Yang J, Wu L et al (2022) Adiponectin inhibits the activation of lung fibroblasts and pulmonary fibrosis by regulating the nuclear factor kappa B (NF-κB) pathway. Bioengineered 13(4):10098–10110. https://doi.org/10.1080/21655979.2022.2063652
Jing H, Tang S, Lin S et al (2020) Adiponectin in renal fibrosis. Aging (Albany NY) 12(5):4660–4672. https://doi.org/10.18632/aging.102811
Xie M, Xiong Z, Yin S et al (2022) Adiponectin alleviates intestinal fibrosis by enhancing AMP-activated protein kinase phosphorylation. Dig Dis Sci 67(6):2232–2243. https://doi.org/10.1007/s10620-021-07015-0
Perakakis N, Farr OM, Mantzoros CS (2021) Leptin in leanness and obesity: JACC state-of-the-art review. J Am Coll Cardiol 77(6):745–760. https://doi.org/10.1016/j.jacc.2020.11.069
Gui X, Chen H, Cai H, Sun L, Gu L (2018) Leptin promotes pulmonary fibrosis development by inhibiting autophagy via PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun 498(3):660–666. https://doi.org/10.1016/j.bbrc.2018.03.039
Acquarone E, Monacelli F, Borghi R, Nencioni A, Odetti P (2019) Resistin: a reappraisal. Mech Ageing Dev 178:46–63. https://doi.org/10.1016/j.mad.2019.01.004
Lin Q, Johns RA (2020) Resistin family proteins in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 319(3):L422–L434. https://doi.org/10.1152/ajplung.00040.2020
Singh S, Anshita D, Ravichandiran V (2021) MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol 101(Pt B):107598. https://doi.org/10.1016/j.intimp.2021.107598
Inoshima I, Kuwano K, Hamada N et al (2004) Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 286(5):L1038–L1044. https://doi.org/10.1152/ajplung.00167.2003
Pulito-Cueto V, Remuzgo-Martínez S, Genre F et al (2022) Elevated VCAM-1, MCP-1 and ADMA serum levels related to pulmonary fibrosis of interstitial lung disease associated with rheumatoid arthritis. Front Mol Biosci 9:1056121. https://doi.org/10.3389/fmolb.2022.1056121
Grinde KE, Arbet J, Green A et al (2017) Illustrating, quantifying, and correcting for bias in post-hoc analysis of gene-based rare variant tests of association. Front Genet. 8:117. https://doi.org/10.3389/fgene.2017.00117
Fan J, Zhu J, Sun L, Li Y, Wang T, Li Y (2021) Causal association of adipokines with osteoarthritis: a Mendelian randomization study. Rheumatology (Oxford) 60(6):2808–2815. https://doi.org/10.1093/rheumatology/keaa719
Kulkarni T, Yuan K, Tran-Nguyen TK et al (2019) Decrements of body mass index are associated with poor outcomes of idiopathic pulmonary fibrosis patients. PloS one 14(10):e0221905. https://doi.org/10.1371/journal.pone.0221905
Acknowledgements
The authors thank all investigators and participants from the open GWAS summary datasets. Special thanks to the IEU open GWAS project developed by the MRC Integrative Epidemiology Unit (IEU) at the University of Bristol. Thank them for extracting relevant GWAS summary-level data from published articles, UK Biobank, and FinnGen biobank.
Funding
This work was supported by the Science and Technology Department of Sichuan Province (2023NSFSC1459, 2022NSFSC1313), the West China Hospital of Sichuan University Postdoctoral Science Foundation (2023HXBH043).
Author information
Authors and Affiliations
Contributions
DH and LG gave the study concept and design; all authors acquired, analyzed, and interpreted the data, and critically revised the manuscript for important intellectual content; DH drafted the manuscript; DH carried out the statistical analysis; YS and ZL supervised the study; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical Approval
The present study relied only on publicly available de-identified summary statistics from relevant published GWASs. The ethical approval and informed consent were obtained in all original studies. Additional ethical approval was not required for our study.
Consent for Publication
Consent for publication was provided by all authors.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, D., Gong, L., Wu, Z. et al. Genetic Association of Circulating Adipokines with Risk of Idiopathic Pulmonary Fibrosis: A Two-Sample Mendelian Randomization Study. Lung 201, 355–362 (2023). https://doi.org/10.1007/s00408-023-00640-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-023-00640-8