Abstract
Introduction
Alterations in body composition are commonly present in chronic obstructive pulmonary disease (COPD). The hypothesis of this study is that COPD patients would achieve clinical benefits after pulmonary rehabilitation (PR) independent of muscle mass depletion or body weight.
Methods
We conducted a retrospective cohort study using single-frequency bioelectrical impedance analysis (BIA) for assessment of fat-free mass (FFM) depletion (muscle depletion). Patients were stratified into three categories based on (1) obesity BMI ≥ 30 kg/m2, (2) non-obesity BMI < 30 kg/m2, and (3) combined cachexia (BMI < 21 kg/m2 and FFM index < 16 kg/m2) and muscle atrophy (BMI ≥ 21 kg/m2 and FFMI < 16 kg/m2). PR outcomes were defined as the improvement in exercise capacity (maximal exercise capacity, 6-min walk, constant workload cycle exercise duration) and quality of life determined by Chronic Respiratory Questionnaire after PR.
Results
We studied 72 patients with available FFM measured by BIA. Patients were predominantly elderly man (N = 71; 98%), with a mean age of 72 years with COPD GOLD stage I–IV. The groups were balanced in terms of age, comorbidities, baseline FEV1, exercise capacity, and quality of life. The absolute changes in patients with muscle depletion or obesity compared to those without muscle depletion or obesity were not statistically different as was the percentage of patients reaching the minimal clinically important difference (MCID) after PR.
Conclusion
A comprehensive PR program in COPD patients improved exercise tolerance and quality of life independent of muscle mass depletion or obesity. Similarly, muscle depletion or obesity had no effect on the percentage of patients achieving the MCID for measures of quality of life and exercise tolerance after PR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alterations in body composition (depleted or obese) are commonly present in chronic respiratory diseases. This has been observed in numerous clinical studies especially in individuals with chronic obstructive pulmonary disease (COPD). Measurement of body weight or body mass index (BMI) may not accurately reflect changes in body composition in chronic respiratory disease such as COPD. Body weight consists of fat mass and fat-free mass (FFM), which includes water and body cell mass (bones, organs, muscle). An estimation of body cell mass can be performed by measurement of FFM in clinically stable patients [1]. Cachexia and/or muscle depletion are characterized by FFM depletion, which can be estimated using skinfold anthropometry, bioelectrical impedance analysis [2], or dual-energy X-ray absorptiometry (DEXA; which determines lean non-fat, non-bone mass) [3]. FFM measurement by bioelectrical impedance analysis is easy to perform and has shown significant correlations to reference methods such as magnetic resonance imaging [4] or deuterium dilution [5]. FFM depletion or skeletal muscle wasting is commonly found in underweight patients; however, it may also occur in normal-weight, overweight, or even obese patients [6, 7].
The association between low body mass index and worsening prognosis is a common clinical observation in COPD patients [8,9,10]. On the opposite side of the weight spectrum, despite a paradoxical potential mortality benefit [11], obese patients with COPD have greater symptom burden and reduced exercise tolerance compared to normal-weight patients with a similar degree of airflow obstruction. Pulmonary rehabilitation is a comprehensive non-pharmacological treatment including exercise, nutritional improvement, psychological counseling, education on the nature of disease progression, prevention of exacerbating factors, and breathing strategies, which leads to improvement in important clinical outcomes such as quality of life, dyspnea, and exercise capacity in COPD patients [12]. We hypothesize that COPD patients will achieve the same clinical benefits after pulmonary rehabilitation independent of fat-free muscle mass depletion or obesity. Thus, the objective of this study is to assess the influence of FFM depletion assessed by bioelectrical impedance analysis and body mass index on pulmonary rehabilitation outcomes in COPD patients.
Methods
Patients
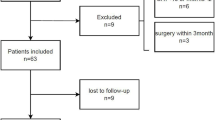
This is a single-center, retrospective cohort study of COPD patients who completed pulmonary rehabilitation at the Veterans Affairs Western New York Healthcare System from January 2001 to December 2011. The Western New York Veterans Affairs institutional review board approved this study. The diagnosis of COPD was established by clinical history consistent with emphysema or chronic bronchitis, history of cigarette smoking greater than 20 pack years, and pulmonary function tests that demonstrated irreversible airflow obstruction [13]. Patients must have had at least 3 months of successful smoking cessation before they become eligible for outpatient pulmonary rehabilitation. They also received education on COPD, disease progression, use of inhalers, breathing strategies, coping strategies, and healthy diet modification by a nutritionist, but did not receive individualized nutritional and psychological counseling. Patients who were diagnosed with other pulmonary diseases (asthma, interstitial lung disease), lost to follow-up, repeated pulmonary rehabilitation and patients who had contraindications for bioelectrical impedance analysis measurement, e.g., pacemaker or defibrillator placement, were excluded from this study.
Body Composition Measurement
Bioelectrical impedance analysis is a simple and non-invasive method of FFM measurement. Whole-body single-frequency bioelectrical impedance (Quantum X, RJL systems, Clinton Township, Mi) was used for assessment of FFM using the disease-specific equations described for patients with COPD [5]. All measurements were performed in the morning between 9 and 10 am. BMI calculation was defined as the weight in kilograms divided by the height in meters squared (weight/height2). Measurement of muscle mass depletion by BIA method is an independent predictor of mortality in COPD patients [14], and it has been shown to significantly correlate with reference methods such as magnetic resonance imaging [4] or deuterium dilution [5]. BIA measurement is practical in outpatient clinics since it is portable and accepted by the European Working Group on Sarcopenia in Older People (EWGSOP) for assessment of fat-free muscle mass [15].
Several studies have defined and quantified FFM depletion. Patients can be considered FFM depleted based on the FFM index (FFM/height2) with values below 16 kg/m2 for men and below 15 kg/m2 for women [14, 16]. Patients were stratified by body composition into three different categories based on BMI and FFM depletion: (1) obesity was defined as BMI ≥ 30 kg/m2, (2) non-obesity was defined as BMI < 30 kg/m2, and (3) cachexia and muscle atrophy were defined as BMI < 21 kg/m2 and FFM index < 16 in men or < 15 in women; and BMI ≥ 21 kg/m2 and FFM index < 16 in men or < 15 in women, respectively.
On the basis of BMI, patients were also stratified into four categories: (1) underweight (BMI < 21 kg/m2), (2) normal weight and overweight (BMI 21–30 kg/m2), (3) obese (BMI > 30–35 kg/m2), and (4) severely obese (BMI > 35 kg/m2) [1]. We used a BMI < 21 kg/m2 to define underweight because this cutoff has been determined to predict poor outcome in COPD [17].
Pulmonary Rehabilitation Program
The program consists of a 1.5-h session three times a week for a total of 24 sessions throughout an 8-week period. If patients missed a session, it was added to the end so all subjects completed 24 sessions. Patients exercised during each rehabilitation session on a treadmill and a stationary cycle ergometer. They also performed stretching exercises and light floor exercises with and without weights.
Pulmonary Rehabilitation Outcomes
Quality of life was assessed by the Chronic Respiratory Questionnaire (CRQ). This questionnaire has 20 questions in 4 domains, which includes 5 questions in dyspnea domain, 4 questions in fatigue domain, 7 questions in emotional domain, and 4 questions in mastery domain. A positive response to pulmonary rehabilitation is considered if the absolute difference after training and before training exceeds the minimal clinically important difference (MCID). The MCID for CRQ scores is 0.5 units per question. Thus, patients who had a greater than 2.5, 2.0, 3.5, and 2.0 unit increase in dyspnea, fatigue, emotion, and mastery domain, respectively, after pulmonary rehabilitation were considered responders [18]. Exercise tolerance was assessed by 6-min walk test (6MWT), a maximal symptom-limited incremental cycle ergometer test (MIET), and a constant workload cycle endurance time (CWET) at 70% of the maximal work capacity obtained during the incremental test. The MCID for 6MWT, maximal incremental exercise test (MIET), and constant workload exercise time (CWET) were considered to be 26 m [19], 4 watts [19], and 170 s [20], respectively. Patients who exceeded these thresholds were considered responders for that particular outcome. We assessed the impact of body composition on both improvement in pulmonary rehabilitation outcomes and the impact of body composition on the percentage of patients reaching the MCID for each pulmonary rehabilitation outcome.
Statistical Analysis
Statistical analysis was performed using MedCalc Statistical Software version 15.8 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2015). The impact of body composition or BMI on outcomes was compared by one-way ANOVA. When the ANOVA was positive, Student–Newman–Kuels post hoc analysis was used to determine which comparisons were responsible. Data were displayed as mean ± standard deviation unless otherwise stated. The effect of body composition or BMI on the percentage of subjects reaching MCID for each outcome after rehabilitation was compared by Chi-squared test. Statistical tests were two-sided and statistical significance was indicated by a P value of less than 0.05.
Results
Baseline characteristics of each body composition group are demonstrated in Table 1. Seventy-two patients (71 men; 98%) with stage I–IV COPD (one patient with stage I) had completed the assessment of body composition before pulmonary rehabilitation. Three patients had BMI < 21 kg/m2 and FFM index < 16 kg/m2 (cachexia), while there were no patients with BMI < 21 kg/m2 and FFM index ≥ 16 kg/m2. Sixty-nine patients had BMI ≥ 21 kg/m2, five of which had BMI ≥ 21 kg/m2 and FFM index < 16 kg/m2 (muscle atrophy or sarcopenia), while 64 patients had BMI ≥ 21 kg/m2 and FFM index > 16 kg/m2. Eight out of 72 patients were considered to have FFM depletion (11.11%), of which 5 patients (62%) had a BMI ≥ 21 kg/m2. Five of the 69 patients (7%) with BMI ≥ 21 kg/m2 were FFM depleted. The groups were well balanced in terms of age, comorbidities as assessed by the modified Charlson index, baseline FEV1, all domains of the Chronic Respiratory Questionnaire score, and baseline MIET and CWET. The group with FFM depletion had lower mean BMI, FFM by definition, FFM index by definition, and diffusion capacity of the lungs for carbon monoxide (DLCO). The absolute changes after pulmonary rehabilitation in patients who were FFM depleted and non-FFM depleted with regard to the total CRQ score, individual domain scores, and exercise outcomes were not statistically different (Table 2). The percentage of patients reaching the MCID after pulmonary rehabilitation showed no difference between FFM-depleted and non-FFM-depleted patients for all outcomes (Table 3).
Baseline demographic data stratified by body weight only are shown in Table 4. There were a total of 164 patients with prevalent clinical diagnosis of stage I–IV COPD who completed pulmonary rehabilitation. The FFM index was not available for review in 92 patients. We stratified the body weight subgroups as 6.09% underweight, 61.58% normal weight and overweight, 22.56% obese, and 9.75% morbidly obese. The groups were well balanced in age, modified Charlson index, baseline FEV1, all domains of the Chronic Respiratory Questionnaire score (except lower dyspnea domain in underweight subgroup), and exercise tolerance. The absolute changes after pulmonary rehabilitation across all subgroups with regard to the total CRQ score, its individual domains, and exercise tolerances were not statistically different (Table 5). The percentage of patients reaching MCID after pulmonary rehabilitation showed no difference between the four subgroups (Table 6).
Discussion
The prevalence of COPD patients with muscle depletion or who were underweight that we found (11%) was not dramatically different from what had been found in prior studies (10–15%) of patients being evaluated for pulmonary rehabilitation [21,22,23,24,25]. The number of patients categorized as muscle depleted (8 of 72) or underweight (10 of 164) were not balanced across GOLD subgroups. Of these patients, 62.5% (5 out of 8 patients) would not have been identified by measurement of body weight alone. The prevalence of muscle depletion/sarcopenia in patients who are not underweight is 7.2% in our study, which is in keeping with a previous study that found a prevalence of 9.6% in both male and female gender using the same definition of muscle depletion/sarcopenia (BMI ≥ 21 and FFM index < 16 kg/m2 in men and < 15 kg/m2 in women) [14]. Our findings cannot be generalized to female patients with COPD since the studied population comprised veteran patients, and the majority of the patients were male. There were only five female patients in the entire cohort.
The data on the significance of COPD classification and muscle mass depletion were heterogeneous. A previous study reported that muscle mass depletion subgroup, defined as BMI < 21 kg/m2, and FFM index < 16 in males or FFM index < 15 in females, was more prevalent in COPD GOLD stage IV compared to GOLD stages II–III [14]. In contrast, our study had only 3 of 8 patients with GOLD stage IV in the muscle mass depletion subgroup. Studies with a larger COPD population with muscle mass depletion is required to further address this issue.
Although muscle depletion is usually associated with lower body weight, studies in the past including ours have consistently demonstrated that muscle depletion can occur in a substantial proportion of weight-stable COPD patients [14, 21]. These patients have significant physical and respiratory impairment compared to underweight or normal-weight individuals with preserved muscle mass. Autopsy studies in malnourished COPD and non-COPD patients demonstrated a significant reduction of diaphragmatic muscle mass [26, 27]. The respiratory muscle strength in COPD patients is further reduced because of lung hyperinflation [28, 29]. The identification of muscle mass depletion in stable COPD patients could assist in selection of patients who might particularly benefit from exercise and nutritional therapy.
Our study demonstrates the improvement in exercise tolerance (6MWT, MIET, CWET) and quality of life as measured by a disease-specific questionnaire independent of muscle depletion or total body weight. Improvement in exercise tolerance and each domain of Chronic Respiratory Questionnaire were unequivocally similar in both muscle-depleted and non-depleted subgroups (Table 2) as was the percentage of patients who were classified as responders in our study (Table 3).
Previous studies hypothesized that FFM-depleted COPD patients may represent a group less likely to improve after pulmonary rehabilitation since exercise can promote systemic inflammation and oxidative stress in muscle-depleted COPD patients [30, 31], which might counterbalance the favorable effects of exercise training. However, despite these concerns, muscle-depleted patients in our cohort responded similarly to exercise training compared to those without muscle depletion. In a recent study, patients with sarcopenia had similar responses to pulmonary rehabilitation as those without sarcopenia in terms of quality of life (St. George’s questionnaire) and exercise performance (incremental shuttle walk) [24]. Our study extends these findings by showing similar effects in muscle-depleted patients for additional exercise performance outcomes: 6-min walk test (6MWT), maximal incremental exercise test (MIET), and constant workload endurance time (CWET). We did not specifically look at the rate of COPD exacerbation after pulmonary rehabilitation because the number of underweight or muscle depletion patients was too low to meaningfully look at whether exacerbation rates or time to first exacerbation after pulmonary rehabilitation was altered differentially in this patient subgroup compared to the general pulmonary rehabilitation population. Peripheral muscle and respiratory muscle performance were not evaluated in our study. However, previous studies found that there was no statistical difference in response for handgrip and maximum quadriceps voluntary contraction after pulmonary rehabilitation between patients with and without sarcopenia [24]. Future studies aiming to evaluate peripheral muscle performance, such as quadriceps strength or quadriceps fatigability, and respiratory muscle performance such as maximal inspiratory muscle strength may be warranted.
Patients in our cohort performed aerobic exercise on a treadmill and a stationary cycle ergometer in addition to the stretching and light floor exercises. Patients with muscle depletion have weaker muscles than those without muscle depletion, and the quadriceps muscle, the main locomotor muscle, is particularly affected. [32]. Quadriceps strength is an independent determinant of exercise capacity in patients with COPD [24]. In those with weak muscles, the addition of strength training to aerobic training in unselected COPD patients is associated with increased muscle mass and muscle strength. However, it has been challenging to show that the addition of strength training to endurance training leads to additive benefits in patients with COPD [33,34,35]. Patients with muscle depletion have not been specifically targeted and this may be a subgroup that might particularly benefit from the addition of strength training to aerobic exercise. The current data on the response to pulmonary rehabilitation in muscle-depleted or underweight patients is quite limited so we feel that our study is an important addition to the currently sparse data available on the response to pulmonary rehabilitation of this patient subgroup. Future studies could integrate other components of pulmonary rehabilitation program such as muscle strength training, individualized nutritional or psychological counseling to evaluate response to pulmonary rehabilitation specifically in the muscle depletion subgroup.
In subgroups stratified by body weight or BMI, we did not find a significant relationship between obesity and baseline clinical characteristics in contrast to some previous studies that reported a lower 6-min walk distance in obese patients [36, 37]. However, the baseline 6-min walk distance was decreased in our obese patients compared to the non-obese patients; this difference was potentially clinically relevant (40 m which is above the MCID) but did not reach statistical significance. A reduced exercise capacity in morbidly obese patients has been attributed to the need for more energy output to move the total body mass [38]. Our study showed that obesity also did not affect the number of patients reaching the MCID after pulmonary rehabilitation, for exercise tolerance (6MWT, MIET, CWET) or quality of life in all four domains of CRQ scores consistent with previous studies [37, 39]. The lack of effect of obesity on pulmonary rehabilitation outcomes has been a consistent finding in the literature, and our study extends this by examining additional exercise outcome variables (maximal exercise capacity and constant workload cycle exercise to exhaustion).
All patients were given an exercise prescription upon completion of the pulmonary rehabilitation program and could use the rehabilitation center in the afternoon free of charge (our rehabilitation programs run in the morning). Some patients made themselves available for these opportunities and some did not. Long-term outcomes are markedly dependent on whether the patient continues to exercise after pulmonary rehabilitation is completed [40]. With our modest number of muscle-depleted or underweight patients, it would be hard to come to definitive conclusions about any differences in the loss of benefits from pulmonary rehabilitation over time between this particular subgroup and the rest of the patients. We do not feel that the lack of long-term follow-up is a major limitation in this study.
Conclusion
A comprehensive pulmonary rehabilitation program in COPD patients improves exercise tolerance and quality of life independent of muscle depletion and body weight. Similarly, muscle depletion and body weight had no effect on the percentage of patients achieving the MCID for measures of quality of life and exercise tolerance after pulmonary rehabilitation. Physicians should consider pulmonary rehabilitation referral as a standard treatment in COPD patients regardless of their muscle mass or body weight.
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- FFM:
-
Fat-free mass
- FFMI:
-
Fat-free mass index
- BMI:
-
Body mass index
- BIA:
-
Bioelectrical impedance analysis
- Kg:
-
Kilogram
- M:
-
Meter
- MIET:
-
Maximal incremental exercise test
- 6MWT:
-
6-min walk test
- CWET:
-
Constant workload cycle endurance time at 70% of the maximal work capacity obtained during the incremental exercise test
- CRQ:
-
Chronic Respiratory Questionnaire
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- FEV1:
-
Forced expiratory volume in 1 s
- MCID:
-
Minimal clinically important difference
- DEXA:
-
Dual-energy X-ray absorptiometry
- ANOVA:
-
Analysis of variance
- DLCO:
-
Diffusing capacity of the lung for carbon monoxide
- PRP:
-
Pulmonary rehabilitation program
References
Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbeau J et al (2006) American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 173(12):1390–1413
Schols AM, Fredrix EW, Soeters PB, Westerterp KR, Wouters EF (1991) Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 54(6):983–987
Engelen MP, Schols AM, Heidendal GA, Wouters EF (1998) Dual-energy X-ray absorptiometry in the clinical evaluation of body composition and bone mineral density in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 68(6):1298–1303
Janssen I, Heymsfield SB, Baumgartner RN, Ross R (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 89(2):465–471
Schols AM, Wouters EF, Soeters PB, Westerterp KR (1991) Body composition by bioelectrical-impedance analysis compared with deuterium dilution and skinfold anthropometry in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 53(2):421–424
Hughes RL, Katz H, Sahgal V, Campbell JA, Hartz R, Shields TW (1983) Fiber size and energy metabolites in five separate muscles from patients with chronic obstructive lung diseases. Respiration 44(5):321–328
Gosker HR, Engelen MP, van Mameren H, van Dijk PJ, van der Vusse GJ, Wouters EF et al (2002) Muscle fiber type IIX atrophy is involved in the loss of fat-free mass in chronic obstructive pulmonary disease. Am J Clin Nutr 76(1):113–119
Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP (1999) Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160(6):1856–1861
Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG (1996) Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 153(3):961–966
Schols AM, Slangen J, Volovics L, Wouters EF (1998) Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 157(6 Pt 1):1791–1797
Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W (2012) Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS ONE 7(8):e43892
Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA et al (2007) Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest 131(5 Suppl):4s–42s
Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS (2001) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 163(5):1256–1276
Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF (2005) Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 82(1):53–59
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F et al (2010) Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 39(4):412–423
VanItallie TB, Yang MU, Heymsfield SB, Funk RC, Boileau RA (1990) Height-normalized indices of the body’s fat-free mass and fat mass: potentially useful indicators of nutritional status. Am J Clin Nutr 52(6):953–959
Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA et al (2004) The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 350(10):1005–1012
Chauvin A, Rupley L, Meyers K, Johnson K, Eason J (2008) Outcomes in cardiopulmonary physical therapy: chronic respiratory disease questionnaire (CRQ). Cardiopulm Phys Ther J 19(2):61–67
Puhan MA, Chandra D, Mosenifar Z, Ries A, Make B, Hansel NN et al (2011) The minimal important difference of exercise tests in severe COPD. Eur Respir J 37(4):784–790
Laviolette L, Bourbeau J, Bernard S, Lacasse Y, Pepin V, Breton MJ et al (2008) Assessing the impact of pulmonary rehabilitation on functional status in COPD. Thorax 63(2):115–121
Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF (1993) Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis 147(5):1151–1156
Steiner MC, Barton RL, Singh SJ, Morgan MD (2002) Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J 19(4):626–631
Engelen MP, Schols AM, Baken WC, Wesseling GJ, Wouters EF (1994) Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J 7(10):1793–1797
Jones SE, Maddocks M, Kon SS, Canavan JL, Nolan CM, Clark AL et al (2015) Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 70(3):213–218
Legrand D, Vaes B, Mathei C, Swine C, Degryse JM (2013) The prevalence of sarcopenia in very old individuals according to the European consensus definition: insights from the BELFRAIL study. Age Ageing 42(6):727–734
Thurlbeck WM (1978) Diaphragm and body weight in emphysema. Thorax 33(4):483–487
Arora NS, Rochester DF (1982) Respiratory muscle strength and maximal voluntary ventilation in undernourished patients. Am Rev Respir Dis 126(1):5–8
Rochester DF, Braun NM (1985) Determinants of maximal inspiratory pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis 132(1):42–47
Derenne JP, Macklem PT, Roussos C (1978) The respiratory muscles: mechanics, control, and pathophysiology. Am Rev Respir Dis 118(1):119–133
Van Helvoort HA, Heijdra YF, Thijs HM, Vina J, Wanten GJ, Dekhuijzen PN (2006) Exercise-induced systemic effects in muscle-wasted patients with COPD. Med Sci Sports Exerc 38(9):1543–1552
Couillard A, Maltais F, Saey D, Debigare R, Michaud A, Koechlin C et al (2003) Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167(12):1664–1669
Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigare R et al (2014) An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 189(9):e15–e62
Bernard S, Whittom F, Leblanc P, Jobin J, Belleau R, Berube C et al (1999) Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159(3):896–901
Berton DC, Silveira L, Da Costa CC, De Souza RM, Winter CD, Zimermann Teixeira PJ (2013) Effectiveness of pulmonary rehabilitation in exercise capacity and quality of life in chronic obstructive pulmonary disease patients with and without global fat-free mass depletion. Arch Phys Med Rehabil 94(8):1607–1614
Mador MJ, Bozkanat E, Aggarwal A, Shaffer M, Kufel TJ (2004) Endurance and strength training in patients with COPD. Chest 125(6):2036–2045
Enright PL, McBurnie MA, Bittner V, Tracy RP, McNamara R, Arnold A et al (2003) The 6-min walk test: a quick measure of functional status in elderly adults. Chest 123(2):387–398
Ramachandran K, McCusker C, Connors M, Zuwallack R, Lahiri B (2008) The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chronic Respir Dis 5(4):205–209
Seres L, Lopez-Ayerbe J, Coll R, Rodriguez O, Manresa JM, Marrugat J et al (2003) Cardiopulmonary function and exercise capacity in patients with morbid obesity. Rev Esp Cardiol 56(6):594–600
Garrod R, Marshall J, Barley E, Jones PW (2006) Predictors of success and failure in pulmonary rehabilitation. Eur Respir J 27(4):788–794
Beauchamp MK, Evans R, Janaudis-Ferreira T, Goldstein RS, Brooks D (2013) Systematic review of supervised exercise programs after pulmonary rehabilitation in individuals with COPD. Chest 144(4):1124–1133
Acknowledgements
Pichapong Tunsupon MD and M. Jeffery Mador MD made substantial contribution to the concept and design, acquisition, analysis, and interpretation of data, and approved the manuscript for submission. This study was approved by the Western New York Veterans Administration Health Care System Institutional Review Board.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that that they have no conflict of interest.
Informed Consent
Since the study involved retrospective abstraction of patient data from the medical record, our Institutional Review Board approved a waiver of the informed consent.
Rights and permissions
About this article
Cite this article
Tunsupon, P., Mador, M.J. The Influence of Body Composition on Pulmonary Rehabilitation Outcomes in Chronic Obstructive Pulmonary Disease Patients. Lung 195, 729–738 (2017). https://doi.org/10.1007/s00408-017-0053-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-017-0053-y