Abstract
Despite many pharmacological and psychosocial treatment options, schizophrenia remains a debilitating disorder. Thus, new treatment strategies rooted in the pathophysiology of the disorder are needed. Recently, vagus nerve stimulation (VNS) has been proposed as a potential treatment option for various neuropsychiatric disorders including schizophrenia. The objective of this study was to investigate for the first time the feasibility, safety and efficacy of transcutaneous VNS in stable schizophrenia. A bicentric randomized, sham-controlled, double-blind trial was conducted from 2010 to 2012. Twenty schizophrenia patients were randomly assigned to one of two treatment groups. The first group (active tVNS) received daily active stimulation of the left auricle for 26 weeks. The second group (sham tVNS) received daily sham stimulation for 12 weeks followed by 14 weeks of active stimulation. Primary outcome was defined as change in the Positive and Negative Symptom Scale total score between baseline and week 12. Various other secondary measures were assessed to investigate safety and efficacy. The intervention was well tolerated with no relevant adverse effects. We could not observe a statistically significant difference in the improvement of schizophrenia psychopathology during the observation period. Neither psychopathological and neurocognitive measures nor safety measures showed significant differences between study groups. Application of tVNS was well tolerated, but did not improve schizophrenia symptoms in our 26-week trial. While unsatisfactory compliance questions the feasibility of patient-controlled neurostimulation in schizophrenia, the overall pattern of symptom change might warrant further investigations in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite all available antipsychotics being effective for the treatment of positive symptoms [20, 33], schizophrenia remains a debilitating disorder with a poor long-term outcome and prognosis. One reason is the unsatisfactory efficacy of antipsychotic treatment on affective, negative and cognitive symptoms. One should bear in mind that these symptom domains are the main predictors for reduced quality of life and poor functional outcomes [8, 27]. Thus, the development of new biological treatment options for these symptom domains, which are rooted in the pathophysiology of schizophrenia, is one of the major challenges in psychiatry. In this context, neurostimulation approaches have been proposed to offer such novel biological treatment options.
Different meta-analyses indicate that repetitive transcranial magnetic stimulation (rTMS) is a promising add-on treatment for positive and negative symptoms in schizophrenia [12, 42, 48, 49], but the efficacy for negative and affective symptoms was recently questioned by a large controlled multicentric trial [53]. In addition, one single-center trial showed that transcranial direct current stimulation might be a promising option for persistent auditory hallucinations [7]. Aside from these modern neurostimulation techniques, electroconvulsive therapy (ECT) is still a possible treatment option in treatment-refractory schizophrenia [41, 43]. Compared to transcranial brain stimulation techniques, stimulation of the vagus nerve provides a different approach to stimulate the human brain. Invasive vagus nerve stimulation (VNS) has been established as an adjunctive treatment for medically refractory epilepsy [13] and is also approved for major depression [35, 52]. It has also been assumed that VNS should have a beneficial effect on cognitive symptoms in epilepsy and other neuropsychiatric disorders [6, 44]. The exact modes of action behind the efficacy of VNS in epilepsy and depression are not well understood, but it is thought that VNS acts via innervation of the nucleus tractus solitaries, with further projections to limbic and cortical structures and to forebrain structures [38]. One positron emission tomography (PET) study in 10 partial epilepsy patients showed that invasive VNS induces widespread changes in the blood flow of different cortical and subcortical regions including both hippocampi, the amygdala and the insula [24]. A recently published rodent study using the methylozoxymethanol acetate model of schizophrenia demonstrated that 2-week invasive VNS resulted in a reversal of ventral hippocampal hyperactivity and aberrant mesolimbic neuron function and improved the behavioral correlate of positive symptoms [40].
Based on the reported efficacy of VNS for depressive and cognitive symptoms, and the biological modulations in cortico-subcortical networks that are also involved in the pathophysiology of schizophrenia, one could hypothesize that VNS could be a potential add-on treatment option in schizophrenia. However, VNS needs a surgical procedure for the implantation of the stimulating coil and the generator (with potential burdensome complications), and thus, the risk/benefit assessment for this intervention in schizophrenia is currently not warranted. However, to overcome the risks associated with invasive VNS, devices for the noninvasive transcutaneous stimulation of the afferent auricular branch of the vagus nerve have been developed. Animal studies indicate that both invasive VNS and noninvasive VNS have significant anticonvulsive effects and this has been confirmed by first pilot trials in patients with pharmacoresistant epilepsy [21, 50]. Functional MRI studies have demonstrated that transcutaneous VNS significantly decreases blood oxygenation levels in various cortical and subcortical areas, particularly limbic structures [11, 29]. A recently published monocentric clinical trial indicated that transcutaneous VNS is effective for the treatment of major depression [22], but failed to improve chronic tinnitus in another monocentric pilot trial [31]. Based on the expertise gathered from the animal, physiological and clinical studies discussed here, our goal was to determine, for the first time, the feasibility and efficacy of transcutaneous VNS for the treatment of schizophrenia.
Materials and methods
Subjects
We enrolled 25 schizophrenia patients from two university hospital centers in Goettingen and Munich for this bicentric randomized, sham-controlled, double-blind clinical investigation. The inclusion criteria were a diagnosis of schizophrenia according to ICD-10 criteria (F20.xx, confirmed by the Mini-International Neuropsychiatric Interview Plus interview [47]) with a disease duration ≥12 months, age 18–75 years and the appliance of the transcutaneous VNS medical device according to the manual. The exclusion criteria were a PANSSTotal score [26] improvement ≥20 % within the period of 2–4 weeks from screening to baseline, pregnancy, asthma, relevant neurological or medical conditions, significant psychiatric comorbidity, abuse of drugs or alcohol in the 4 weeks prior to enrollment, history of traumatic brain injury, invasive and noninvasive methods of treatment (e.g., cancer surgery), indication of structural basal ganglia or brainstem damage, any other implanted medical device, malformations of the pinna and all other disorders of the pinna or meatus, and any further circumstances that, at the discretion of the investigator, would prevent the subject’s inclusion in the clinical study. After a complete description of the study, written informed consent was obtained from all participants.
Study design
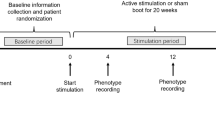
From 2010 to 2012, schizophrenia patients underwent a pretreatment assessment 14–28 days before the baseline visits at day 0. Eligible patients entered a 12-week double-blind tVNS intervention (active vs sham). Patients randomly allocated to the active group received daily active stimulation of the left auricle by tVNS for 26 weeks. Patients randomized to the sham group received daily sham stimulation of the left auricle by tVNS for 12 weeks, but were then switched to active tVNS for the following 14 weeks, i.e., until week 26 (see Fig. 1). The local ethics committees approved the protocol, which was conducted in accordance with the Declaration of Helsinki. Study monitoring for safety and GCP aspects, as well as statistical analyses, was performed by the Institut fuer anwendungsorientierte Forschung und klinische Studien GmbH (http://www.ifs-goettingen.de/). The study was registered at www.clinicaltrials.gov (NCT: 01176721).
Trial plan. After a screening period, patients with schizophrenia underwent a pretreatment assessment before the baseline visit on day 0. Eligible patients entered a 12-week double-blind parallel-group transcutaneous vagus nerve stimulation intervention (active vs sham). After 12 weeks, patients randomized to sham tVNS were switched to active tVNS and patients initially randomized to active tVNS continued this stimulation
tVNS parameters (active and sham tVNS)
tVNS was performed via stimulation of the left auricular branch of the vagus nerve through two titan electrodes connected to a stimulating device (CM02, Cerbomed, Erlangen, Germany). The place of stimulation was the outer ear canal. The tVNS device used in this trial was configured to provide stimulus intensities in the range of 0.1–10 mA. Active tVNS was programmed to stimulate with a pulse train of 30 s followed by break of 180 s (duty cycle 14 %, 30–180–30–180–30–…), a pulse width of 250 µs and a pulse frequency of 25 Hz. The stimulation intensity was adjusted individually for each patient at the beginning of the intervention and at every study visit. Patients had the possibility to adjust the stimulation intensity by themselves and were advised to use the highest stimulation intensity above the perception threshold which was tolerable. Patients started with a settling-in phase (3 × 1 h/day stimulation) followed by the adaption phases I (3 × 2 h/day stimulation) and II (3 × 3 h/day stimulation). The study phase was characterized by stimulation throughout the day (from morning to bedtime). Patients were able to decide for how long they use the stimulator, but advised to use the stimulator the whole day during the study phase. Sham tVNS was characterized by sham stimulation (no active stimulation) from a similarly designed stimulator. However, at the beginning of the study, sham stimulators were programmed to allow for the primary adjustment of the individual threshold and throughout the study patients were also allowed to adjust their individual stimulation parameters. For safety reasons, patients were instructed not to use the tVNS device during the night. The tVNS device was equipped with an internal memory that recorded the contact quality to the skin, all changes in the stimulation intensity and the periods of stimulation. The internal memory was read out at each visit and stored on a study computer using the individualized patient ID.
Randomization
Randomization was performed using a software-generated (SAS 9.2) 1:1 randomization schedule.
Baseline assessment and efficacy measures
This study was initiated to test the feasibility and safety of tVNS in schizophrenia. The primary outcome measure was change in the PANSS total score [26] after 12 weeks of intervention in both study groups. In both centers, raters were trained on PANSS by reviewing standardized videotaped interviews. The secondary outcomes reported here are change in positive and negative PANSS subscores and change in Beck Depression Inventory (BDI) scores [4]. Explorative data were collected to assess any changes in the Scale for the Assessment of Negative Symptoms (SANS, global and total scores) [2], in different depressive scores [Calgary Depression Rating Scale (CDSS), Montgomery and Åsberg Depression Rating Scale (MADRS), Hamilton Depression Rating Scale (HAMD-21)] [1, 17, 37], in the Personal and Social Performance Scale (PSP) [45], in Fagerstroem scores [14] and in Subjective Well-Being under Neuroleptics scale (SWN-K) [10]. Further explorative data were assessed for various neurocognitive domains. Complex visual scanning, motor speed and the ability to shift strategies were tested with the Trail-Making-Test A and B [51]. The “Wortschatztest” was used to test verbal intelligence [46], and the “Regensburger Wortflüssigkeitstest” was used to test verbal fluency [3]. Furthermore, visuospatial short-term memory and implicit visuospatial learning were investigated with the Corsi Block Tapping test [5], and the German version of the Rey Auditory Verbal Learning Test (Verbaler Lern- und Merkfähigkeitstest, VLMT) was used to investigate verbal short-term and long-term memory [23].
Safety measures
All patients received an ECG, and standard laboratory and clinical assessments to guarantee a high level of care. ECG was recorded until day 28 based on the assumption that cardiac events associated with the intervention would appear at the beginning of treatment. The proinflammatory cytokines IL-6 and TNF-alpha were measured before and after stimulation by commercially available ELISA kids. Standardized assessment of motor side effects was carried out with the St. Hans Rating Scale for extrapyramidal syndromes (SHRS) [16], and overall side effects were assessed with the UKU Side Effect Rating scale [34]. Spontaneous side effects, adverse events (AE) and serious AE (SAE) were documented according to ICH/GCP regulations.
Statistics
A statistical analysis plan was implemented before unblinding the study participants. This plan contained all planned statistical and explorative analyses. Deviations from the described analytic strategy were not permitted. All statistical analyses were performed with SAS (SAS Institute, Cary, NC, USA). Level of significance was set at p < 0.05. The primary outcome analysis was performed in the intention-to-treat (ITT) population, defined as all randomized patients with at least one documented visit following baseline [modified (mITT)]. For the mITT population, primary outcomes were analyzed with a general linear model analysis of variance [repeated-measures (RM)-ANOVA] using a restricted maximum likelihood (REML) estimation for missing data. The between-subjects factor was group (active vs sham) and the within-subject factor was time of visit (baseline vs week 12). The statistic analyzed for significance was the interaction between group and time of visit, indicating whether or not the change in outcome variables over time differed between groups. In a secondary analysis, the ANOVA was used to analyze all other outcome variables and the data from week 12 to week 26. The planned ANOVA controlled by center and gender was not performed due to an unexpectedly small sample size.
Results
Study subjects
We screened 25 patients until the recruitment objective was reached. Twenty patients were either randomized to active (n = 10) or sham (n = 10) tVNS, and 19 patients entered the interventional study phase. Fifteen patients reached the endpoint at 12 weeks (see Fig. 2 for dropout analysis and CONSORT diagram). At baseline, analyses could not detect a statically significant difference in any of the sociodemographic or clinical measures between study groups (see Table 1).
CONSORT Flow Diagram. A total of 20 patients were randomized. Ten patients were allocated to active tVNS, and 10 patients were allocated to sham tVNS. One patient in the sham group met the exclusion criteria and was withdrawn from the study. During the first 12 weeks, 2 patients in the active and 2 patients in the sham group left the study. In the subsequent 14 weeks, 1 patient in the active and 2 patients in the sham group discontinued the study
Efficacy outcome measures
PANSS total score and responder analysis
In the intention-to-treat analysis, PANSS total scores decreased by 8.7 (±3.6) in the active and by 3.2 (±3.6) in the sham group between baseline and week 12, with no differences between study groups (LSMeans difference = 5.5 ± 5.3; df = 11.4; p = 0.32). Exploratory analysis from week 12 to week 26 showed that PANSS total scores were reduced by 8.5 (±3.5) in the active tVNS and by 5.1 (±3.7) in the sham tVNS group (switched to active after week 12) with no significant differences between groups (LSMeans difference = 3.4 ± 4.1; df = 13.3, p = 0.52). From baseline to week 26, the RM-ANOVA comparing all patients initially randomized to verum tVNS to those who received sham tVNS showed a significant time × group interaction (p = 0.0263). Active tVNS reduced PANSS total scores by 17.2 (±2.8) and sham tVNS (switched to active tVNS at the end of week 12) by 8.8 (±2.8), with a significant difference between groups (LSMeans difference = 8.8 ± 4.1; df = 12.8, p = 0.049). However, both study groups showed numeric differences in PANSS total scores at baseline (LSMeans: sham tVNS = 56.3; active tVNS = 65.2; p = 0.14)), possibly contributing to the observed group differences from baseline to week 26. Despite the indirect dependency of the differences, an additional minor post hoc analysis to account for the crossover design was performed comparing sham tVNS (baseline to week 12) and active tVNS (patients receiving active tVNS from baseline to week 12 and week 12 to week 26, as well as patients in the previous sham group receiving active tVNS from week 12 to week 26). An independent t test was used for simplicity reasons and did not reveal a significant difference between PANSS total changes in the mITT population (please see Table 2 and Fig. 3). From baseline to week 12, analyses identified 3 responders and 5 nonresponders (response rate 37.5 %) in the sham tVNS group, and 2 responders and 7 nonresponders (response rate 22.2 %) in the active tVNS group, with no significant difference between groups (response difference 15.3 %; 95 % CI −27.9; 58.4 %).
Scores for severity of symptoms during the study. Data are presented as mean ± SEM in black for active tVNS and in white for sham tVNS. The change over time in PANSS scores: a PANSS total score, b PANSS positive score, c PANSS negative score and d PANSS general score. The change in different depression rating scores over time: e BDI score, f CDSS score, g MADRS score and h HAMD-21 score
Secondary outcome measures
PANSS subscores and SANS
For PANSS subscores, no significant differences between groups were observed but both groups showed an improvement between baseline and week 12 (PANSS positive LSMeans difference = 0.5 ± 1.4; df = 14.8; p = 0.73; PANSS negative LSMeans difference = 4.1 ± 3.0; df = 14.4; p = 0.19; PANSS general LSMeans difference = 0.7 ± 2.3; df = 11.5; p = 0.77). For SANS global ratings and total scores, no significant differences between groups were identified between baseline and week 12 (SANS global rating: LSMeans difference = −0.5 ± 1.6; df = 14.5; p = 0.74; SANS total scores: LSMeans difference = 2.8 ± 9.8; df = 12.8; p = 0.78). Please see Table 2 for further analyses.
Depression rating scales
All depression rating scales showed no significant differences between groups from baseline to week 12 (BDI: LSMeans difference = −1.5 ± 2.5; df = 14.3 p = 0.58; CDSS: LSMeans difference = −1.7 ± 1.0; df = 13.6; p = 0.11; MADRS: LSMeans difference = 1.0 ± 2.1; df = 13.1; p = 0.63; HAMD-21: LSMeans difference = −1.6 ± 2.6; df = 15.3; p = 0.54). Please see Table 2 for further analyses.
Other rating scales
The values and statistics of PSP, SWN-K and FTNA are displayed in Table 2. No significant group differences were observed for any of the other rating scales.
Neurocognition
No significant group differences in any of the neurocognitive assessments (Wortschatztest at baseline and VLMT, TMT, RWT Corsi Block Tapping Test over time) were observed (data not shown).
Safety measures and side effects
During the intervention, 3 SAEs (2 times hospitalization, appendectomy) were reported, but none were associated with the use of the medical product. In each study group, one patient has been hospitalized and in the sham tVNS group and one further patient had an appendectomy. The SAE rates were 22.2 % in the sham and 10 % in the active tVNS group (Fisher’s exact: p = 0.58).
Nineteen AEs were reported, of which 5 were associated with the use of the medical product. Eleven AEs (including 4 local skin irritations or pain; 1 before and 3 after switching to active stimulation) were reported in the sham, and 8 AEs (including 2 local skin irritations or pain) were reported in the active tVNS group. AE rates were 66.7 % in the sham and 60 % in the active tVNS group (Fisher’s exact: p = 1.00). In three patients (all in the active tVNS group), action was required due to the UKU ratings (2 × increased frequency of clinical examinations; 1 × stop of intervention).
One patient was withdrawn from the study at the screening visit, and two patients received close monitoring due to side effects. Laboratory measures including Na+, K+, creatinine; ASAT, ALAT, gamma-GT, cholesterin, triglycerides, HbA1C, TSH, prolactin and total blood count showed no significant differences between groups nor any changes over time (baseline to week 12). For creatine kinase, descriptive statistics indicate a decrease in the sham group and an increase in the active group without being significant or leaving the reference range. In both groups, fasting glucose levels decreased over time without reaching the threshold for statistical significance. IL-6 and TNF-alpha were rated as normal in both groups, and no significant changes were observed for both proinflammatory cytokines between groups. Blood pressure did not differ over time or between groups. Pulse and heart rate were higher in the sham group, and these differences were pronounced at the end of the study. ECGs were recorded for the first 4 weeks. No differences in ECG could be observed (normal/pathology with relevance/pathology without relevance/missing) over time (see Table 3). The percentage of normal evaluation was higher in the active tVNS group based on descriptive statistics. In summary, no severe adverse effects were observed during the whole study period.
Study discontinuation (dropouts)
Three patients allocated to active tVNS, and three patients allocated to sham tVNS discontinued the study before week 12. One patient in the sham tVNS group had a baseline improvement of >20 % and was excluded from analysis according to the protocol. The reasons for study discontinuation are displayed in the CONSORT Flow Diagram.
Compliance
Compliance was tested on the basis of the data recorded from the internal memory (log files). A sufficient stimulation procedure according to the protocol was assumed if a patient used the stimulator for at least 4 h/day and for at least 80 days during the first 12 study weeks. From the ITT population, only 9 patients (53 %) fulfilled these criteria, indicating a protocol violation in the remaining patients. The stimulation data (stimulation intervals, stimulation hours, active days from baseline to week 12) is displayed in Table 4
.
Discussion
We performed the first pilot study to investigate the feasibility, safety and efficacy of noninvasive transcutaneous vagus nerve stimulation (tVNS) for the treatment of schizophrenia. The intervention was well tolerated with no major side effects. Both groups showed an improvement in various outcome domains over time, but the limited compliance illustrates the problems arising from patient-controlled neurostimulation interventions in schizophrenia. Regarding efficacy, the comparison of active with sham tVNS applied to the left auricular branch of the vagus nerve did not offer a general benefit for the primary and secondary outcome measures during the intervention periods.
The efficacy of invasive and transcutaneous VNS has not been previously investigated in schizophrenia patients, although other neurostimulation techniques have been suggested as potential add-on treatments. Apart from reports of low-frequency rTMS for the treatment of auditory hallucinations [9, 32] and from evidence of electroconvulsive therapy for severe treatment-resistant patients [41], the results for all other symptom domains (e.g., negative symptoms, depressive symptoms) are not satisfying [18, 19, 53]. Thus, our results with tVNS are in line with various alternative studies in the field using other neurostimulation techniques. However, in cases of negative findings from a pilot study, it is necessary to consider different explanations that may account for the lack of group differences.
At baseline, our patients suffered from a mild psychopathology as indicated by average PANSS total values of ≤65. One recent meta-analysis from six randomized controlled schizophrenia trials comparing antipsychotics to placebo indicated that the expected benefit of antipsychotic drugs is related to initial symptom severity [15]. Thus, we can speculate that the mild psychopathology at baseline might counteract a potential effect of active tVNS. In our study, patients randomized to the active group had higher baseline PANSS values (65.22 ± 12.95) compared to those who received sham tVNS (56.52 ± 10.70). In the first 12 weeks, active tVNS reduced the total PANSS by 8.7 points, whereas sham tVNS resulted in a decrease of only 3.2 points. At week 12, the sham tVNS group was also switched to active tVNS and both group showed a reduction of 5.1 and, respectively, 8.5 points in PANSS total scores from week 12 to week 26. This differing pattern is revealed by a significant “time × group interaction” (p = 0.0263). Therefore, the change in symptoms over time in both study groups cannot only be explained by the setting of a clinical trial, and one can assume that the inclusion of patients with moderate-to-severe psychopathology and longer observation intervals in a two-arm study with no switching would have resulted in significant differences between active and sham tVNS.
A further major reason for the negative finding is the unsatisfactory compliance of our schizophrenia patients. As indicated in the log files, only half of our mITT population used the stimulation device according to the protocol requirements during the first 12 study weeks. This might explain the lacking efficacy of our intervention and also raises questions about the feasibility of self-stimulated tVNS in schizophrenia patients. Schizophrenia patients are characterized by significant noncompliance, which results in 40–60 % discontinuing their antipsychotic treatment [36, 39]. The noncompliance rate in our study is in this range. Due to the small sample size, the group of patients who received protocol-adherent stimulation (9 patients: 6 in Goettingen, 3 in Munich) was only analyzed with descriptive statistics. However, this descriptive analysis of our per-protocol population did not show other results than the ITT analysis (data not shown). Aside from noncompliance, poor illness insight is another characteristic of schizophrenia. Both these factors and the results presented here suggest that patient-controlled neurostimulation is associated with specific challenges in schizophrenia.
One could only speculate whether other stimulation parameters or longer stimulation intervals would have been more effective. The decision to use the given protocol was based on previous experience from fMRI studies conducted on healthy subjects [11, 28], and it would appear that an adjustment of the parameters based on the pathophysiological changes in schizophrenia might have resulted in greater efficacy. In this context, one fMRI study indicates that the regional cerebral blood flow in the left temporal lobe of schizophrenia patients discriminates rTMS responders from nonresponders [25]. It could be hypothesized that only schizophrenia patients with demonstrable activation deficits in cortical and subcortical tVNS target areas would respond to active stimulation. Regarding the duration of the intervention, it is intriguing that active tVNS showed, in both study periods, similar improvements in the total PANSS scores, indicating an additive effect over time. Thus, future studies may have to explore whether longer stimulation periods or different stimulation protocols are more effective in the improvement of our target symptoms. A further limitation refers to the fact that ECG recording was limited to the first 4 weeks of treatment. Therefore, we cannot generalize our observation of no cardiac events for the whole study period. However, other tVNS studies (e.g., for the treatment of tinnitus) showed that tVNS is not associated with cardiac events for longer study periods (e.g., 24 weeks) [30, 31].
Interestingly, one animal study (schizophrenia rodent model) showed that invasive VNS for 2 weeks normalized aberrant hippocampal and downstream alterations in VTA dopamine [40]. Obviously, the translation of animal findings to the clinical setting is associated with many problems, but these preclinical findings are promising since they provide a basis for a pathophysiology-based treatment. However, the procedure described in the animal model is related to invasive VNS and the question as to whether invasive VNS could be effective in schizophrenia patients remains open. On the basis of the present negative findings and the situation that invasive VNS is associated with surgery, we are reluctant to suggest such an intervention as a treatment strategy. Regarding safety, our pilot study showed that active VNS is generally well tolerated and associated neither with stressful symptoms nor with relevant adverse effects. Our reported tolerability is in line with previous reports using tVNS for epilepsy, major depression and tinnitus [22, 31, 50].
The results of this pilot study indicate that tVNS is not a beneficial add-on treatment in stable schizophrenia patients over 3 months. Nonadherence and the initial low disease severity may account for this negative finding. Future studies should make specific arrangements to increase the therapeutic adherence (e.g., reminder via phone calls or via medical apps). However, the pattern of symptom change over time could suggest that long stimulation periods (>3 months) might be possibly effective in those patients who are compliant to the intervention. Further research is also needed to disentangle the biological underpinnings of tVNS in schizophrenia to allow for targeted interventions. Finally, the potential of tVNS in the context of combined approaches (e.g., tVNS combined with psychotherapy or with cognitive-enhancing interventions) should be explored in future clinical trials.
References
Addington D, Addington J, Maticka-Tyndale E (1993) Assessing depression in schizophrenia: the calgary depression scale. Br J Psychiatry (Suppl 22):39–44
Andreasen NC (1983) Scale for the assessment of negative symptoms. University of Iowa Press, Iowa City
Aschenbrenner S, Tucha O, Lange KW (2000) Regensburger wortflüssigkeits-test (rwt): Handanweisung. In: Hogrefe, Verlag für Psychologie., Göttingen
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Berch DB, Krikorian R, Huha EM (1998) The corsi block-tapping task: methodological and theoretical considerations. Brain Cogn 38:317–338
Boon P, Moors I, De Herdt V, Vonck K (2006) Vagus nerve stimulation and cognition. Seizure 15:259–263
Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF, Saoud M, Mechri A, Poulet E (2012) Examining transcranial direct-current stimulation (tdcs) as a treatment for hallucinations in schizophrenia. Am J Psychiatry 169:719–724
Buchanan RW (2007) Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull 33:1013–1022
Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, Himelhoch S, Fang B, Peterson E, Aquino PR, Keller W, Schizophrenia Patient Outcomes Research T (2010) The 2009 schizophrenia port psychopharmacological treatment recommendations and summary statements. Schizophr Bull 36:71–93
de Haan L, Weisfelt M, Dingemans PM, Linszen DH, Wouters L (2002) Psychometric properties of the subjective well-being under neuroleptics scale and the subjective deficit syndrome scale. Psychopharmacology 162:24–28
Dietrich S, Smith J, Scherzinger C, Hofmann-Preiss K, Freitag T, Eisenkolb A, Ringler R (2008) A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional mri. Biomed Technik Biomed Eng 53:104–111
Dlabac-de Lange JJ, Knegtering R, Aleman A (2010) Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: review and meta-analysis. J Clin Psychiatry 71:411–418
Englot DJ, Chang EF, Auguste KI (2011) Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg 115:1248–1255
Fagerstrom KO (1978) Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 3:235–241
Furukawa TA, Levine SZ, Tanaka S, Goldberg Y, Samara M, Davis JM, Cipriani A, Leucht S (2015) Initial severity of schizophrenia and efficacy of antipsychotics: participant-level meta-analysis of 6 placebo-controlled studies. JAMA Psychiatry 72:14–21
Gerlach J, Korsgaard S, Clemmesen P, Lauersen AM, Magelund G, Noring U, Povlsen UJ, Bech P, Casey DE (1993) The st. Hans rating scale for extrapyramidal syndromes: reliability and validity. Acta Psychiatr Scand 87:244–252
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Moller HJ (2012) World federation of societies of biological psychiatry (wfsbp) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry 13:318–378
Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Moller HJ, Schizophrenia WTFoTGf (2015) World federation of societies of biological psychiatry (wfsbp) guidelines for biological treatment of schizophrenia part 3: update 2015 management of special circumstances: depression, suicidality, substance use disorders and pregnancy and lactation. World J Biol Psychiatry 16:142–170
Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Thibaut F, Moller HJ, World Federation of Societies of Biological Psychiatry Task Force on Treatment Guidelines for S (2012) World federation of societies of biological psychiatry (wfsbp) guidelines for biological treatment of schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry 13:318–378
He W, Jing X, Wang X, Rong P, Li L, Shi H, Shang H, Wang Y, Zhang J, Zhu B (2013) Transcutaneous auricular vagus nerve stimulation as a complementary therapy for pediatric epilepsy: a pilot trial. Epilepsy Behav E&B 28:343–346
Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K, Kornhuber J, Kraus T (2013) Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm 120:821–827
Helmstaedter C, Lendt M, Lux S (2000) Testhandbuch verbaler lern- und merkfähigkeitstest. Hogrefe Verlag, Göttingen
Henry TR, Bakay RA, Pennell PB, Epstein CM, Votaw JR (2004) Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: ii. Prolonged effects at high and low levels of stimulation. Epilepsia 45:1064–1070
Homan P, Kindler J, Hauf M, Hubl D, Dierks T (2012) Cerebral blood flow identifies responders to transcranial magnetic stimulation in auditory verbal hallucinations. Transl Psychiatry 2:e189
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (panss) for schizophrenia. Schizophr Bull 13:261–276
Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR (2006) The nimh-matrics consensus statement on negative symptoms. Schizophr Bull 32:214–219
Kraus T, Hosl K, Kiess O, Schanze A, Kornhuber J, Forster C (2007) Bold fmri deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm 114:1485–1493
Kraus T, Kiess O, Hosl K, Terekhin P, Kornhuber J, Forster C (2013) Cns bold fmri effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal—a pilot study. Brain Stimul 6:798–804
Kreuzer PM, Landgrebe M, Husser O, Resch M, Schecklmann M, Geisreiter F, Poeppl TB, Prasser SJ, Hajak G, Langguth B (2012) Transcutaneous vagus nerve stimulation: retrospective assessment of cardiac safety in a pilot study. Front Psychiatry 3:70
Kreuzer PM, Landgrebe M, Resch M, Husser O, Schecklmann M, Geisreiter F, Poeppl TB, Prasser SJ, Hajak G, Rupprecht R, Langguth B (2014) Feasibility, safety and efficacy of transcutaneous vagus nerve stimulation in chronic tinnitus: an open pilot study. Brain Stimul 7:740–747
Lefaucheur JP, Andre-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, Cantello RM, Cincotta M, de Carvalho M, De Ridder D, Devanne H, Di Lazzaro V, Filipovic SR, Hummel FC, Jaaskelainen SK, Kimiskidis VK, Koch G, Langguth B, Nyffeler T, Oliviero A, Padberg F, Poulet E, Rossi S, Rossini PM, Rothwell JC, Schonfeldt-Lecuona C, Siebner HR, Slotema CW, Stagg CJ, Valls-Sole J, Ziemann U, Paulus W, Garcia-Larrea L (2014) Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 125:2150–2206. doi:10.1016/j.clinph.2014.05.021
Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lassig B, Salanti G, Davis JM (2013) Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962
Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K (1987) The uku side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334:1–100
Martin JL, Martin-Sanchez E (2012) Systematic review and meta-analysis of vagus nerve stimulation in the treatment of depression: variable results based on study designs. Eur Psychiatry 27:147–155
Mojtabai R, Lavelle J, Gibson PJ, Sohler NL, Craig TJ, Carlson GA, Bromet EJ (2002) Gaps in use of antipsychotics after discharge by first-admission patients with schizophrenia, 1989 to 1996. Psychiatr Serv 53:337–339
Montgomery SA, Asberg M (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389
Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK (2006) Vns therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 31:1345–1355
Osterberg L, Blaschke T (2005) Adherence to medication. N Engl J Med 353:487–497
Perez SM, Carreno FR, Frazer A, Lodge DJ (2014) Vagal nerve stimulation reverses aberrant dopamine system function in the methylazoxymethanol acetate rodent model of schizophrenia. J Neurosci 34:9261–9267
Petrides G, Malur C, Braga RJ, Bailine SH, Schooler NR, Malhotra AK, Kane JM, Sanghani S, Goldberg TE, John M, Mendelowitz A (2015) Electroconvulsive therapy augmentation in clozapine-resistant schizophrenia: a prospective, randomized study. Am J Psychiatry 172:52–58
Prikryl R, Kucerova HP (2013) Can repetitive transcranial magnetic stimulation be considered effective treatment option for negative symptoms of schizophrenia? J ECT 29:67–74
Rosenquist PB, Miller B, Pillai A (2014) The antipsychotic effects of ect: a review of possible mechanisms. J ECT 30:125–131
Sackeim HA, Keilp JG, Rush AJ, George MS, Marangell LB, Dormer JS, Burt T, Lisanby SH, Husain M, Cullum CM, Oliver N, Zboyan H (2001) The effects of vagus nerve stimulation on cognitive performance in patients with treatment-resistant depression. Neuropsychiatry Neuropsychol Behav Neurol 14:53–62
Schaub D, Juckel G (2011) PSP scale: german version of the personal and social performance scale: valid instrument for the assessment of psychosocial functioning in the treatment of schizophrenia. Der Nervenarzt 82:1178–1184
Schmidt KH (1992) Der wortschatztest (wst). Beltz Test GmbH, Weinheim
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The mini-international neuropsychiatric interview (m.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for dsm-iv and icd-10. J Clin Psychiatry 59(Suppl. 20):22–33 (quiz 34-57)
Shi C, Yu X, Cheung EF, Shum DH, Chan RC (2013) Revisiting the therapeutic effect of rtms on negative symptoms in schizophrenia: a meta-analysis. Psychiatry Res 215:505–513
Slotema CW, Blom JD, van Lutterveld R, Hoek HW, Sommer IE (2014) Review of the efficacy of transcranial magnetic stimulation for auditory verbal hallucinations. Biol Psychiatry 76:101–110
Stefan H, Kreiselmeyer G, Kerling F, Kurzbuch K, Rauch C, Heers M, Kasper BS, Hammen T, Rzonsa M, Pauli E, Ellrich J, Graf W, Hopfengartner R (2012) Transcutaneous vagus nerve stimulation (t-vns) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia 53:e115–e118
Tombaugh TN (2004) Trail making test a and b: normative data stratified by age and education. Arch Clin Neuropsychol 19:203–214
Wani A, Trevino K, Marnell P, Husain MM (2013) Advances in brain stimulation for depression. Ann Clin Psychiatry 25:217–224
Wobrock T, Guse B, Cordes J, Wolwer W, Winterer G, Gaebel W, Langguth B, Landgrebe M, Eichhammer P, Frank E, Hajak G, Ohmann C, Verde PE, Rietschel M, Ahmed R, Honer WG, Malchow B, Schneider-Axmann T, Falkai P, Hasan A (2015) Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: a sham-controlled, randomized multicenter trial. Biol Psychiatry 77:979–988
Acknowledgments
We would like to thank Ms Louise Marshall for English editing.
Conflict of interest
The study was sponsored by CerboMed GmbH, Erlangen, Germany. The authors deny any conflict of interest in relation to the subject of this report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Alkomiet Hasan and Claus Wolff-Menzler have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hasan, A., Wolff-Menzler, C., Pfeiffer, S. et al. Transcutaneous noninvasive vagus nerve stimulation (tVNS) in the treatment of schizophrenia: a bicentric randomized controlled pilot study. Eur Arch Psychiatry Clin Neurosci 265, 589–600 (2015). https://doi.org/10.1007/s00406-015-0618-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-015-0618-9