Abstract
Patients suffering from bipolar affective disorder show deficits in working memory functions. In a previous functional magnetic resonance imaging study, we observed an abnormal hyperactivity of the amygdala in bipolar patients during articulatory rehearsal in verbal working memory. In the present study, we investigated the dynamic neurofunctional interactions between the right amygdala and the brain systems that underlie verbal working memory in both bipolar patients and healthy controls. In total, 18 euthymic bipolar patients and 18 healthy controls performed a modified version of the Sternberg item-recognition (working memory) task. We used the psychophysiological interaction approach in order to assess functional connectivity between the right amygdala and the brain regions involved in verbal working memory. In healthy subjects, we found significant negative functional interactions between the right amygdala and multiple cortical brain areas involved in verbal working memory. In comparison with the healthy control subjects, bipolar patients exhibited significantly reduced functional interactions of the right amygdala particularly with the right-hemispheric, i.e., ipsilateral, cortical regions supporting verbal working memory. Together with our previous finding of amygdala hyperactivity in bipolar patients during verbal rehearsal, the present results suggest that a disturbed right-hemispheric “cognitive–emotional” interaction between the amygdala and cortical brain regions underlying working memory may be responsible for amygdala hyperactivation and affects verbal working memory (deficits) in bipolar patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bipolar disorder is characterized by recurrent episodes of mania and depression which suggests that mood instability and an impaired regulation of emotional states may be the core of the disorder [1]. Research into the neurobiological basis of this illness indicates structural differences of emotion processing brain regions such as the amygdala [2, 3]. Furthermore, functional magnetic resonance imaging (fMRI) studies investigating the pathophysiological basis of bipolar disorder revealed quite consistently hyperactivation of brain regions subserving affective processing both in symptomatic (depressive or manic) and in asymptomatic (euthymic) bipolar patients [4–9]. Additionally, evidence from numerous neuropsychological studies converges to suggest that deficits in cognitive control processes, including attention and working memory, are highly prevalent in bipolar patients [10–15].
There are two at least partially dissociable brain systems which underlie verbal working memory in humans. A left-lateralized network of brain regions is involved in the articulatory rehearsal of phonological information, including Broca’s area, the left lateral and medial premotor cortex, the intraparietal cortex, and the contralateral (right) cerebellum. Responsible for the non-articulatory maintenance of phonological information is a more bilateral system which comprises the anterior middle frontal gyrus, the inferior parietal lobule, deep frontal opercular cortex, medial frontal cortices, and the cerebellum [16, 17].
Anatomical connections between the amygdala as a central part of the emotion processing brain network and cortical areas have been found in the macaque monkey. These anatomical connections comprise the cingulate and prefrontal cortex, parahippocampal gyrus, and the insula [18–21]. In a path analysis of a large human data set, Stein and collaborators found that the amygdala functions as a hub of connections and have direct and indirect bidirectional interactions with the parahippocampal gyrus, hypothalamus, subgenual cingulate cortex, orbitofrontal cortex, posterior cingulate, insula, and supragenual cingulate cortex [22]. The anatomical connections of the amygdala indicate that this brain structure is strategically placed to receive highly processed information from the cortex and to influence motor systems, autonomic systems, some of the cortical areas from which it receives inputs, and other limbic areas [23–25]. On a neurofunctional level, amygdala activity has been found to be regulated by several prefrontal brain regions involved in cognitive control and emotion regulation [26]. Furthermore, there is a growing body of evidence showing the influence of the amygdala on cortical areas, for instance, on cortical sensory processing systems [e.g., 27, 28 and for review see also 29]. Widespread efferent projections as well as bidirectional direct and indirect pathways from the amygdala to the cortex have been proposed and may facilitate perception and attention processes and in addition may influence encoding processes.
In a previous study, we found a pathological activation of the right amygdala in bipolar patients in contrast to healthy controls during an articulatory working memory task. Moreover, the bipolar patients showed hyperactivations of right-hemispheric areas relevant for verbal working memory, while performing the articulatory rehearsal task [30]. Here, we present the results of a functional connectivity analysis of bipolar patients and healthy controls performing articulatory rehearsal. Based on the findings of our previous fMRI study, the right amygdala was selected as seed region for the functional connectivity analysis.
We aimed to investigate functional interactions between the right amygdala and cortical brain areas during articulatory rehearsal in bipolar patients in comparison with healthy controls.
Materials and methods
Subjects
We analyzed data from a sample of 18 euthymic patients with bipolar affective disorder and 18 healthy comparison subjects. Patients were recruited from the outpatients departments of the Central Institute of Mental Health in Mannheim and the Saarland University Hospital in Homburg. Healthy control subjects were recruited from the hospital staff, medical students, and the community. Written informed consent was obtained from all subjects, and the study was approved by the local ethics committee.
Patients (44 % female) and healthy control subjects (61 % female) were matched with respect to age (patients 38.2 ± 9.9 years; control subjects 33.9 ± 11.5 years) and education (patients 14.4 ± 3.1 years, control subjects 15.8 ± 2.2 years). All subjects were Caucasian and right-handers as assessed by the Edinburgh Handedness Inventory. The diagnosis of bipolar I disorder was confirmed by using the German version of the Structured Clinical Interview (SCID-I) for DSM-IV. Exclusion criteria were substance abuse or dependence, other current comorbid psychiatric disorders, acute suicidal tendency, and a history of neurological illness or severe brain injury. Severity of psychopathology was evaluated by using the Young Mania Rating Scale (YMRS) and the Hamilton Depression Scale (HAMD) or the Montgomery Asberg Depression Rating Scale (MADRS). The euthymic state was defined as scores of <7 on these scales. All patients were in a remitted state for at least 1 month. Patients had to be either free of medication or no changes in medication type and dosage of medication had been made for at least 2 weeks prior to the experiment. Most of the investigated patients were taking psychotropic medication at the time of the study: 12 patients were receiving mood stabilizers (five lithium, five valproic acid, two carbamazepine, and two lamotrigine), four were taking neuroleptics (three atypical, one typical), six were receiving antidepressants (three SSRIs, three mirtazapine, two venlafaxine), and four were taking benzodiazepines. Three bipolar patients were without any medication at the time of the study.
Experimental design
Subjects performed an established variant of a verbal delayed matching to sample task, which reliably activates one of the two established different brain systems that make up the dual architecture of verbal working memory in humans [16, 17, 31]. Four different target letters were visually presented for 2 s, followed by a delay of 4 s during which a fixation cross was displayed. Then, a probe letter was presented for 1.5 s, followed by a 1.5-s blank screen. Within this 3 s response, window subjects had to indicate via button press whether or not the probe letter matched one of the four target letters presented before. Overall the experiment consisted of two runs, each composed of 2 × 6 alternating 30-s blocks of one variant of the verbal working memory task and its corresponding control condition (see Fig. 1). Blocks consisted of three trials of the same task type (3 × 9 s), and a 3-s cue at the beginning of each block indicated whether memory tasks or judgment tasks had to be performed in the upcoming block. Subjects were instructed to intensely use (sub)articulatory rehearsal (sometimes referred to as “inner speech”) to remember the letters presented. Performance of this articulatory rehearsal task reliably activates a left-lateralized network of brain regions including Broca’s area, left lateral and medial premotor cortex, intraparietal cortex, and the contralateral (right) cerebellum [16, 17]. A letter-case judgment task performed on the single probe letters served as a well-matched comparison condition and allowed to dissociate activations related to working memory from more general activations emanating from other (e.g., sensory or motor) components of the task. For more detailed information, see [16, 17, 32].
FMRI data acquisition
All stimuli were visually presented on a screen as white stimuli on black background, except for the task cues, which were presented in yellow color. Subjects underwent fMRI at 1.5 T (Siemens Vision; voxel size 3.6 × 3.6 × 4 mm3, interscan interval 2,500 ms, TE 50 ms, distance factor 12 %, flip angle 90°, field of view 230 mm, 64 × 64 matrix). A total of 271 functional image volumes were acquired, consisting of 26 axial slices parallel to the AC–PC plane (slice acquisition in ascending order). Functional imaging was synchronized with stimulus presentation by means of ERTS (Experimental run time system, version 3.11, BeriSoft cooperation, Frankfurt am Main, Germany). Additionally, a high-resolution, T1-weighted 3D anatomical set (MPRAGE sequence, TE 4.42 ms, TR 11.9 ms, flip angle 15°, field of view 256 × 256 mm2, voxel size 1 × 1 × 1 mm3, 176 consecutive slices) was collected for each subject. All participants were measured from late afternoon until early evening.
Data preprocessing
Demographic and behavioral data were analyzed using SPSS (version 15.0). The analysis of between-group differences in these variables was conducted by means of one-way ANOVA. Functional imaging data were processed using the SPM2 software package (www.fil.ion.ucl.ac.uk/spm/spm2.html). The first five volumes were discarded. Preprocessing comprised coregistration, corrections for motion artifacts, time differences in slice acquisition, global signal intensity variation, and low-frequency fluctuations (high-pass filter with 128-s cutoff), normalization into standard stereotactic space, and spatial smoothing with a Gaussian kernel (FWHM = 12 mm). Participants that exceeded 2.5 mm of movement were excluded, resulting in elimination of two out of 38 participants (one patient and one control person). Each individual’s time series data were regressed on six motion parameters.
Psychophysiological interaction analysis (PPI)
As our previous study had revealed a pathological activation of the right amygdala in patients with bipolar disorder during a working memory task [30], this brain structure was chosen as seed region for the functional connectivity analysis (seed coordinates 28 0 −24; MNI activation maximum from the second level analysis of our prior study).
To analyze functional connectivity, we used the psychophysiological interactions (PPI) approach [33] which allows a detailed examination of process-specific functional interactions between brain regions. PPI analysis requires two independent factors, i.e., one regressor representing the signal time course in a given volume of interest (VOI) and one regressor representing the psychological variable of interest. The third regressor is the product of the first and second regressor and represents the PPI. The physiological factor was determined by extracting the blood oxygenation level-dependent (BOLD) signal from spheres created around the mean seed coordinates 28 0 −24 (see above). The spheres had a radius of 3 mm, taking the estimated average amygdala volume into consideration. The time series of all voxels in the VOI were averaged, and the resulting activation time course served as the physiological variable. As psychological factor we used the subtraction contrast of the articulatory rehearsal working memory task and the letter-case judgement control task. The product of the physiological and the psychological factor was calculated for each subject, creating the PPI term. Based on our a priori hypothesis of a functional interaction of the amygdala with brain areas involved in verbal working memory, we created and used a mask of the brain areas which were activated during the verbal working memory task in healthy control subjects (p < 0.05, uncorrected). For the within-group analysis, single-subject data were entered into a random-effects model (one-sample t test) in order to determine the brain regions showing significant positive or negative correlation with the seed region in the patient and the control group, respectively [34]. Within-group connectivity maps were thresholded at p < 0.05, FDR-corrected. For the between-group analysis, we directly compared the connectivity patterns between both groups in a random-effects model (two-sample t tests, p = 0.001, uncorrected) in order to evaluate whether observed differences in the patterns of functional connectivity displayed by the patients and controls are statistically significant.
Results
Within-group analysis
One-way ANOVA revealed a slight trend for reduced performance rates of bipolar patients in the articulatory rehearsal task compared with the healthy controls (86.7 % vs. 91.1 %, p < 0.1). The response times were not significantly different between groups (F (1.34) = 0.82, p = 0.37).
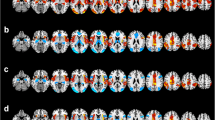
Healthy controls and bipolar patients showed a differential pattern of functional connectivity during performance of the (sub)articulatory rehearsal task. In healthy controls, a significant negative functional interaction was found between the right amygdala and bilateral brain regions which are involved in verbal working memory. The right amygdala showed negative functional connectivity with the bilateral precentral gyrus, the left inferior frontal gyrus (Broca’s area), the right frontal eye field, the left inferior parietal lobule, the bilateral intraparietal cortex, the left temporopolar cortex, the (pre)supplementary motor area [(pre)-SMA)], the bilateral cerebellum, the vermis cerebelli, and the ventral pallidum in the healthy controls (see Fig. 2; Table 1 for details).
Brain regions showing negative psychophysiological interactions of the right amygdala under articulatory rehearsal in (a) healthy control subjects and (b) euthymic bipolar patients. For illustration purposes, statistical effects are shown at a significance level of p < 0.001, uncorrected, masked with the within-group effects in bipolar patients at a significance level of p < 0.05, FDR-corrected, on voxel level and shown on the rendered surface of the standard MNI Template (see Table 1 for coordinates and significance levels)
In contrast, in the patients with bipolar disorder, substantially less negative functional connectivity was found for the right amygdala. Only the left hemisphere [left inferior frontal gyrus (Broca area), left inferior parietal lobule, and left intraparietal cortex] showed negative functional connections with the amygdala, whereas the negative interaction with the right-hemispheric precentral gyrus, frontal eye field and intraparietal cortex and the (pre)-SMA area was not found in bipolar patients (see Fig. 2; Table 1 for details).
Between-group analysis
The direct comparison between the group of healthy controls and bipolar patients (control group > bipolar patients) confirmed a statistically significant difference in negative functional connectivity of the right amygdala with ipsilateral cortical areas. Compared with controls, bipolar patients showed a significantly reduced negative functional connectivity between the right amygdala and the right precentral gyrus [T-value 3.38, stereotactic (MNI) coordinates (48 4 48)], the right frontal eye field [3.35 (36 0 52)], the right intraparietal cortex [3.38 (32 -52 36)], and the (pre)-SMA [3.36 (0 20 52)]; p < 0.001, uncorrected. In all other regions, no significant differences were found. These results are illustrated in Fig. 3.
Reduced negative functional interactions of the right amygdala in bipolar patients in contrast to healthy control subjects under articulatory rehearsal. For illustration purposes, the statistical threshold was lowered to p < 0.005, uncorrected, masked with the within-group effects in bipolar patients at a significance level of p < 0.05, uncorrected, on voxel level. Findings were overlaid onto the rendered surface of the standard MNI Template
Discussion
In a previous fMRI study comparing euthymic bipolar patients with healthy controls, we had found pathological hyperactivation of the right amygdala as well as of right-hemispheric cortical areas relevant for working memory (right precentral gyrus, right intraparietal cortex, right frontal eye field, and right cerebellum) in bipolar patients while performing a verbal working memory task [30]. In the functional connectivity study presented here, the covariance in activity between the seed region (amygdala) and areas relevant for working memory regions during rehearsal was significantly lower than that during the control task. In other words, we found a disrupted negative functional interaction between the right amygdala and exactly these cortical areas [right precentral gyrus, right intraparietal cortex, right frontal eye field, and additionally the (pre)-SMA and the temporopolar cortex] in bipolar patients.
These findings support our hypothesis that in bipolar patients, the ipsilateral hemispherical hyperactivation is a compensatory, but failing attempt to suppress the pathological activation of the right amygdala during a working memory task [30]. As the inhibitory coupling between these cortical areas and the amygdala appears to be impaired in bipolar patients, the activation of the amygdala is not suppressed as it is the case in healthy subjects. The hyperactivation of the right amygdala would therefore be related to a dysfunction of cortical working memory areas in bipolar patients, particularly of their connections to the ipsilateral amygdala.
However, converging evidence complements the notion that the amygdala automatically becomes activated in response to trigger features such that the amygdala itself produces attentional and affective responses [for review see 35]. Interestingly, animal research suggests an involvement of the amygdala in working memory tasks even unrelated to the presence of emotional stimuli [36]. On a structural level, efferent projections as well as bidirectional direct and indirect pathways from the amygdala to the cortex have been proposed and may not only facilitate perception and attention processes, but in addition may influence working memory processes such as encoding of information [27, 35]. One might therefore also hypothesize that in our patient group, a dysfunctional amygdala function and dysfunctional bottom-up processes may lead to the dysfunction of working memory in the patient group.
It has to be noted that results of PPI analyses per se are non-directional. Therefore, the direction of functional connectivity in the present study is subject to interpretation. As the tested paradigm specifically activates cortical working memory areas [16, 17], and healthy controls did not show any amygdala activation during working memory tasks [30], an inhibition of the right amygdala through these activated cortical areas is the most probable explanation. Of note, a recent study using dynamic causal modeling found a bidirectional interaction between prefrontal cortices and the amygdala during an emotional associative learning task in healthy participants [37]. Effective connectivity in that study was stronger top–down from the prefrontal cortices to the amygdala than the other way, thus supporting our hypothesis of inhibition of the amygdala by cortical areas in healthy controls.
Banks and collaborators examined, performing a PPI analysis of fMRI data of healthy subjects, the connectivity of the amygdala with cortical areas. Participants performed a task involving active, voluntary regulation of negative emotion by cognitive reappraisal. They also demonstrated a significant coupling between the amygdala and specific areas of the frontal cortex (dorsolateral, dorsal medial, anterior cingulate, and orbital) specifically during emotion regulation [38]. Several studies examining patients with an affective disorder revealed disturbances in the connectivity between cortical areas and the amygdala. For example, Moses-Kolko and collaborators showed a significantly diminished dorsomedial prefrontal cortical amygdala effective connectivity in response to negative emotional faces in women with postpartum depression [39]. In bipolar patients, Versace and colleagues reported disturbances in the connectivity of the left and right amygdala with the orbitofrontal cortex in response to emotional faces. Sad stimuli evoked an abnormally elevated and happy stimuli an abnormally reduced functional connectivity between the amygdala and the orbitofrontal cortex in contrast to healthy controls [40]. In a group of either depressed or manic unmedicated bipolar patients, Anand and his colleagues found in a resting state fMRI study in bipolar patients a decreased pregenual anterior cingulate cortex connectivity with the left and right amygdala [41]. There is also evidence for negative functional connectivity between cortical areas and the amygdala: Rosenkranz and collaborators performed in vivo recordings in rats and found that the stimulation of the prefrontal cortex inhibited the amygdala [42]. In humans, Chepenik and his colleagues detected a negative correlation between activity in the left ventral prefrontal cortex (vPFC) and the left amygdala using low-frequency resting state fMRI. This functional negative connectivity appeared to be decreased in bipolar patients [43]. In an fMRI study with manic bipolar patients, Foland and collaborators showed, also using the PPI approach, a significantly reduced ventrolateral prefrontal cortex (VLPFC) regulation of the amygdala response during an emotion-labeling task. They conclude that a reduction in inhibitory frontal activity in these patients may lead to an increased reactivity of the amygdala [44]. To summarize, the connections between the amygdala and cortical region are extensive and appear to be disturbed in patients with affective disorders [39–41, 43]. Negative functional coupling between the amygdala and cortical areas was detected in rats and in healthy humans and—in line with the presented results—appears to be disturbed in bipolar patients [42–44].
There is evidence that the suppression of amygdala activity during a working memory task as performed in our study is necessary to suppress emotions which could distract from the cognitive task at hand [45, 46]. In particular, the amygdala has been found to be responsible for the effects of emotional interference on cognitive processing [47]. In an fMRI study, Melcher and his colleagues could show that the induction of negative emotion selectively impaired behavioral performance in a stroop and an oddball interference task. Connectivity analyses revealed a negative coupling between lateral PFC on the one hand and amygdala and OFC on the other hand [48].
Medication such as lithium and lamotrigine has been reported to possibly influence neuroimaging results [e.g., 49, 50]. Most of the investigated patients were taking psychotropic medication at the time of the study. However, in the present study, medication was quite variable. Only a few patients received antidepressant (SSRIs) or antipsychotics. The fact that the medication was quite variable makes it unlikely that group differences in brain connectivity may have resulted from a systematic effect of one specific drug. Recently, Hafeman et al. [51] reviewed the effects of medication on neuroimaging findings. They concluded that medication appears to normalize neuroimaging effects, meaning that medicated individuals with bipolar disorder were more similar to healthy subjects. Similarly, medication might rather have normalized disturbed connectivity in the present study. However, the effect of medication itself on brain connectivity could not be addressed in this study. Further studies are needed to directly investigate the effect of medication on (disturbed) brain connectivity in bipolar patients.
Historically, emotion and cognition have been viewed as separated entities. To understand disorders of complex behavior comprehension of the contribution of emotion and cognition to the control of behavior in terms of systems, neuroscience view is needed. Our findings indicate a task-dependent disturbed “cognitive–emotional” interaction in euthymic bipolar patients. The findings might enhance our understanding of neural processes associated with bipolar disorder. In addition, the results point to the specific role of the amygdala and its interactions in bipolar disorder with specific cognitive processes. The shown abnormality during a circuit-specific working memory task (articulatory rehearsal) appears to be a trait marker in bipolar disorders that can be observed even in the euthymic state and that seems to be largely independent of medication. Further study of this circuitry is warranted, and future studies should address whether disturbance in these circuits might contribute to relapse of illness.
The present study has some limitations which have to be discussed. Given that we wanted to be able to compare our results we used the same analyzing software and parameters as used for the previous study. In the previous study, analyzing parameters were carefully chosen according to scanning parameters [30]. But, a resulting limiting factor might be that for data analysis an older version of the SPM software package (SPM2) was used. Although this software version also includes standard methods for motion correction, slice time correction, some new features have been developed for new versions of SPM. However, SPM supporting team from Wellcome Trust Centre for Neuroimaging (http://www.fil.ion.ucl.ac.uk/spm/software) recommends using a single SMP version for one given data set. We therefore used one version for analysis of the complete data set.
In addition, applied smoothing kernel of FWHM of 12 mm might be rather high. In general, spatial smoothing with a large enough, kernel might eliminate artifacts such as ring-artifacts and “side lobes” that distort the image, but at a cost in image resolution. On the other hand, too little spatial smoothing leaves the ringing artifacts and side lobes caused by k-space truncation intact, resulting in a decrease in signal-to-noise ratio and statistical power [52]. Interestingly, increasing smoothing kernel size has been hypothesized to possibly shift activation foci to areas with higher gray matter density [53]. Specifically, subcortical structures, including small and irregular gray matter structure as well as variable white matter boundaries, might therefore be difficult to detect. Further white matter (WM) and cerebrospinal fluid (CSF) were not excluded from the analysis. Excluding these brain tissues may lead to reduction of artifacts [54, 55]. However, based on our a priori hypothesis of a functional interaction of the amygdala with brain areas involved in verbal working memory, we used a mask of the brain areas which have been shown to be reliably activated during the verbal working memory task. Finally, head motion is specifically difficult to handle in connectivity data. Two participants (one patient and one healthy control participant) had to be excluded from data analysis as motion exceeded 2.5 mm during the scanning. Further, motion parameters have been included in the model. Yet in general, motion might affect connectivity data and likewise this might also be the case in our data set. Summing up, to be able to compare our present result to previous results, analysis software and parameters have not been changed. This may be a limitation of the present study. However, analyzing parameters have been carefully chosen in our previous fMRI study [30]. Furthermore, the shown brain activation of the original work and the findings of the presented study show a pattern of activity in meaningful brain areas which have consistently been associated with working memory processes [e.g., 16, 17 and 32]. The reported results are therefore unlikely to be artifacts due to analyzing parameters or relatively old analyzing software.
Conclusion
Together with our previous finding of amygdala hyperactivity in bipolar patients during verbal rehearsal, the present results suggest that a disturbed right-hemispheric “cognitive–emotional” interaction between the amygdala and cortical brain regions underlying working memory may be responsible for amygdala hyperactivation and verbal working memory deficits in bipolar patients.
References
Phillips ML, Vieta E (2007) Identifying functional neuroimaging biomarkers of bipolar disorder: toward DSM-V. Schizophr Bull 33:893–904. doi:10.1093/schbul/sbm060
Wang F, Kalmar JH, He Y et al (2009) Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 66:516–521. doi:10.1016/j.biopsych.2009.03.023
Usher J, Leucht S, Falkai P, Scherk H (2010) Correlation between amygdala volume and age in bipolar disorder—a systematic review and meta-analysis of structural MRI studies. Psychiatry Res 182:1–8. doi:10.1016/j.pscychresns.2009.09.004
Yurgelun-Todd DA, Gruber SA, Kanayama G et al (2000) fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord 2:237–248
Lawrence NS, Williams AM, Surguladze S et al (2004) Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 55:578–587. doi:10.1016/j.biopsych.2003.11.017
Malhi GS, Lagopoulos J, Ward PB et al (2004) Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci 19:741–754
Rich BA, Vinton DT, Roberson-Nay R et al (2006) Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA 103:8900–8905. doi:10.1073/pnas.0603246103
Pavuluri MN, O’Connor MM, Harral E, Sweeney JA (2007) Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry 62:158–167. doi:10.1016/j.biopsych.2006.07.011
Bermpohl F et al (2009) A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disord 11(1):70–75. doi:10.1111/j.1399-5618.2008.00648.x
Blumberg HP, Martin A, Kaufman J et al (2003) Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry 160:1345–1347
Blumberg HP, Leung H-C, Skudlarski P et al (2003) A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry 60:601–609. doi:10.1001/archpsyc.60.6.601
Gruber SA, Rogowska J, Yurgelun-Todd DA (2004) Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. J Affect Disord 82:191–201. doi:10.1016/j.jad.2003.10.010
MacQueen GM, Hajek T, Alda M (2005) The phenotypes of bipolar disorder: relevance for genetic investigations. Mol Psychiatry 10:811–826. doi:10.1038/sj.mp.4001701
Kronhaus DM, Lawrence NS, Williams AM et al (2006) Stroop performance in bipolar disorder: further evidence for abnormalities in the ventral prefrontal cortex. Bipolar Disord 8:28–39. doi:10.1111/j.1399-5618.2006.00282.x
Melcher T, Wolter S, Falck S et al (2013) Common and disease-specific dysfunctions of brain systems underlying attentional and executive control in schizophrenia and bipolar disorder. Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-013-0445-9
Gruber O, von Cramon DY (2001) Domain-specific distribution of working memory processes along human prefrontal and parietal cortices: a functional magnetic resonance imaging study. Neurosci Lett 297:29–32
Gruber O, von Cramon DY (2003) The functional neuroanatomy of human working memory revisited. Evidence from 3-T fMRI studies using classical domain-specific interference tasks. NeuroImage 19:797–809
Amaral DG, Price JL (1984) Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230:465–496. doi:10.1002/cne.902300402
Barbas H, De Olmos J (1990) Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol 300:549–571. doi:10.1002/cne.903000409
Stefanacci L, Suzuki WA, Amaral DG (1996) Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. J Comp Neurol 375:552–582. doi:10.1002/(SICI)1096-9861(19961125)375:4<552:AID-CNE2>3.0.CO;2-0
Ghashghaei HT, Barbas H (2002) Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115:1261–1279
Stein JL, Wiedholz LM, Bassett DS et al (2007) A validated network of effective amygdala connectivity. NeuroImage 36:736–745. doi:10.1016/j.neuroimage.2007.03.022
Rolls ET (2005) Emotion explained. University Press, Oxford
Dannlowski U et al (2007) Amygdala reactivity predicts automatic negative evaluations for facial emotions. Psychiatry Res 154(1):13–20
Kim MJ, Loucks RA, Palmer AL et al (2011) The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 223:403–410. doi:10.1016/j.bbr.2011.04.025
Ochsner KN, Ray RD, Cooper JC et al (2004) For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage 23:483–499. doi:10.1016/j.neuroimage.2004.06.030
Weinberger NA, Metherate R, McKenna T et al (1990) Neural adaptive information processing: a preliminary model of receptive-field plasticity in auditory cortex during Pavlovian conditioning. In: Gabriel M, Moore J (eds) Learning and computational neuroscience: foundations of adaptive networks. The MIT Press, Cambridge, pp 91–138
Pare D, Quirk GJ, Ledoux JE (2004) New vistas on amygdala networks in conditioned fear. J Neurophysiol 92:1–9
Phelps EA, LeDoux JE (2005) Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48:175–187
Gruber O, Tost H, Henseler I et al (2010) Pathological amygdala activation during working memory performance: evidence for a pathophysiological trait marker in bipolar affective disorder. Hum Brain Mapp 31:115–125. doi:10.1002/hbm.20849
Trost S, Gruber O (2012) Evidence for a double dissociation of articulatory rehearsal and non-articulatory maintenance of phonological information in human verbal working memory. Neuropsychobiology 65:133–140. doi:10.1159/000332335
Gruber O (2001) Effects of domain-specific interference on brain activation associated with verbal working memory task performance. Cereb Cortex 11(11):1047–1055. doi:10.1093/cercor/11.11.1047
Friston KJ, Buechel C, Fink GR et al (1997) Psychophysiological and modulatory interactions in neuroimaging. NeuroImage 6:218–229. doi:10.1006/nimg.1997.0291
Penny WD, Holmes AJ (2003) Random-effects analysis. Academic Press, San Diego
Pessoa L (2008) On the relationship between emotion and cognition. Nat Rev Neurosci 9:148–158. doi:10.1038/nrn2317
McIntyre CK, Marriott LK, Gold PE (2003) Cooperation between memory systems: acetylcholine release in the amygdala correlates positively with good performance on a hippocampus-dependent task. Behav Neurosci 117:320–326
Curcić-Blake B, Swart M, Aleman A (2012) Bidirectional information flow in frontoamygdalar circuits in humans: a dynamic causal modeling study of emotional associative learning. Cereb Cortex 22(2):436–445. doi:10.1093/cercor/bhr124
Banks SJ, Eddy KT, Angstadt M et al (2007) Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci 2:303–312. doi:10.1093/scan/nsm029
Moses-Kolko EL, Perlman SB, Wisner KL et al (2010) Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry 167:1373–1380. doi:10.1176/appi.ajp.2010.09081235
Versace A, Thompson WK, Zhou D et al (2010) Abnormal left and right amygdala–orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry 67:422–431. doi:10.1016/j.biopsych.2009.11.025
Anand A, Li Y, Wang Y et al (2009) Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res 171:189–198. doi:10.1016/j.pscychresns.2008.03.012
Rosenkranz JA, Moore H, Grace AA (2003) The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci Off J Soc Neurosci 23:11054–11064
Chepenik LG, Raffo M, Hampson M et al (2010) Functional connectivity between ventral prefrontal cortex and amygdala at low frequency in the resting state in bipolar disorder. Psychiatry Res 182:207–210. doi:10.1016/j.pscychresns.2010.04.002
Foland LC, Altshuler LL, Bookheimer SY et al (2008) Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res 162:27–37. doi:10.1016/j.pscychresns.2007.04.007
Gray JR (2001) Emotional modulation of cognitive control: approach-withdrawal states double-dissociate spatial from verbal two-back task performance. J Exp Psychol Gen 130:436–452
Northoff G, Heinzel A, Bermpohl F et al (2004) Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional–cognitive interaction. Hum Brain Mapp 21:202–212. doi:10.1002/hbm.20002
Dolcos F, McCarthy G (2006) Brain systems mediating cognitive interference by emotional distraction. J Neurosci Off J Soc Neurosci 26:2072–2079. doi:10.1523/JNEUROSCI.5042-05.2006
Melcher T, Born C, Gruber O (2011) How negative affect influences neural control processes underlying the resolution of cognitive interference: an event-related fMRI study. Neurosci Res 70:415–427. doi:10.1016/j.neures.2011.05.007
Foland LC, Altshuler LL, Sugar CA, Lee AD et al (2008) Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. NeuroReport 19:221–224
Haldane M, Jogia J, Cobb A, Kozuch E, Kumari V, Frangou S (2008) Changes in brain activation during working memory and facial recognition tasks in patients with bipolar disorder with Lamotrigine monotherapy. Eur Neuropsychopharmacol 18:48–54
Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML (2012) Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord 14:375–410
Lindquist MA, Wager TD (2008) Spatial smoothing in fMRI using prolate spheroidal wave functions. Hum Brain Mapp 29:1276–1287
Sacchet MD, Knutson B (2012) Spatial smoothing systematically biases the localization of reward-related brain activity. Neuroimage 66C:270–277
Dagli MS, Ingeholm JE, Haxby JV (1999) Localization of cardiac-induced signal change in fMRI. Neuroimage 9:407–415
Windischberger C, Langenberger H, Sycha T, Tschernko EM, Fuchsjager-Mayerl G, Schmetterer L, Moser E (2002) On the origin of respiratory artifacts in BOLD-EPI of the human brain. Magn Reson Imaging 20:575–582
Acknowledgments
This work was partially supported by the Deutsche Forschungsgemeinschaft (DFG) via the Clinical Research Group 241 “Genotype-phenotype relationships and neurobiology of the longitudinal course of psychosis” (http://www.kfo241.de; Grant Number GR 1950/5-1) to O.G..
Conflicts of interest
O.G. was honorary speaker for the following companies: Astra Zeneca, Bristol Myers Squibb, Janssen Cilag, Lilly, Otsuka and Servier. He has been invited to scientific congresses by Astra Zeneca, Janssen Cilag and Pfizer and has received a research grant from Servier. O.G. reports that these potential conflicts have no relation to the subject of the present study. All other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Katharina Stegmayer and Juliana Usher contributed equally to this work.
Rights and permissions
About this article
Cite this article
Stegmayer, K., Usher, J., Trost, S. et al. Disturbed cortico–amygdalar functional connectivity as pathophysiological correlate of working memory deficits in bipolar affective disorder. Eur Arch Psychiatry Clin Neurosci 265, 303–311 (2015). https://doi.org/10.1007/s00406-014-0517-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-014-0517-5