Abstract
Major depression is associated with impairments in semantic verbal fluency (VF). However, the neural correlates underlying dysfunctional cognitive processing in depressed subjects during the production of semantic category members still remain unclear. In the current study, an overt and continuous semantic VF paradigm was used to examine these mechanisms in a representative sample of 33 patients diagnosed with a current episode of unipolar depression and 33 statistically matched healthy controls. Subjects articulated words in response to semantic category cues while brain activity was measured with functional magnetic resonance imaging (fMRI). Compared to controls, patients showed poorer task performance. On the neural level, a group by condition interaction analysis, corrected for task performance, revealed a reduced task-related deactivation in patients in the right parahippocampal gyrus, the right fusiform gyrus, and the right supplementary motor area. An additional and an increased task-related activation in patients were observed in the right precentral gyrus and the left cerebellum, respectively. These results indicate that a failure to suppress potentially interfering activity from inferior temporal regions involved in default-mode network functions and visual imagery, accompanied by an enhanced recruitment of areas implicated in speech initiation and higher-order language processes, may underlie dysfunctional cognitive processing during semantic VF in depression. The finding that patients with depression demonstrated both decreased performance and aberrant brain activation during the current semantic VF task demonstrates that this paradigm is a sensitive tool for assessing brain dysfunctions in clinical populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is one of the most common psychiatric disorders, affecting about 16 % of the population at some time in their lives [50]. According to present knowledge, MDD is related to dysfunctions within extended networks of cortical, subcortical, and limbic regions, including, for example, prefrontal cortex (PFC), anterior cingulate cortex (ACC), insula, amygdala, basal ganglia, thalamus, cerebellum as well as temporal regions (superior temporal gyrus and (para-)hippocampus), which may cause emotional and cognitive disturbances observed in this disorder (for reviews and meta-analyses see [18–20, 25, 75, 92, 102, 124]). Cognitive dysfunction is a prominent and persistent aspect of MDD, which has been demonstrated on a range of neuropsychological measures (for meta-analyses see [61, 109, 128, 138]). Impaired cognition severely deteriorates social and occupational functioning as well as quality of life, even in apparently remitted patients [6, 45, 74]. Deficits in the verbal fluency (VF) domain, which are among those with the largest effect sizes [138], have been shown to contribute independently of mood symptoms to poor functional outcome in depression [45]. Characterising the neural mechanisms underlying these dysfunctions is not only an important step towards a comprehensive understanding of this disorder but also relevant for the development of new treatment options [75].
Verbal fluency, which is assessed by the quantity of words produced in response to a stimulus (typically within 1 min), represents one of the most sensitive measures for detecting brain dysfunction [65]. As opposed to phonemic (lexical/letter/phonological) VF (PVF) tasks, which require subjects to generate as many words as possible that begin with a specific letter (e.g. initial letter P), semantic (category) VF (SVF) tasks afford to produce words that belong to a specific semantic category (e.g. animals). SVF is supposed to tap multiple cognitive processes including access to and retrieval from semantic memory, verbal working memory (WM), articulation of the output, sustained attention, and several executive processes such as efficient task initiation and flexibility [11, 41, 42, 48, 100, 126], yet it forms a distinct cognitive domain that is separable from other executive functions [24, 109]. Most behavioural studies that examined SVF in MDD reported poorer performance in patients as compared to healthy controls [30, 59, 80, 81, 96, 103, 117, 136]; comparatively, few did not find significant differences between groups [17, 35, 38]. Moreover, the findings of several meta-analyses of cognitive impairments in depression point to a substantially larger deficit in SVF as compared to PVF [109, 128, 138].
Neuroimaging studies using functional magnetic resonance imaging (fMRI) have recently shed light on the neural correlates underlying SVF in healthy subjects. Overt SVF as compared to rest produces robust activation in a widespread bilateral language network encompassing mainly (pre-)frontal and temporal cortical areas as well as the cerebellum [11, 40, 52, 78]. Regarding the neural correlates of VF in depression, a large number of neuroimaging studies examined PVF by means of near-infrared spectroscopy (NIRS) [43, 47, 71, 72, 82, 85, 93, 94, 121], fMRI [86, 87, 123], positron emission tomography (PET) [127], or single photon (computed) emission tomography (SPET/SPECT) [5, 89]. In these studies, aberrant brain activation, particularly hypoactivation in PFC, was frequently revealed in patients when compared to controls (for review see [53]).
By contrast, little has been done to determine the effect of depression on the neural correlates of SVF. To date, two neuroimaging studies exist on this subject, which yielded inconsistent results and examined small patient samples with specific characteristics [5, 89]. In a pilot SPET investigation, Philpot et al. [89] measured regional cerebral blood flow (rCBF) in 10 elderly (over the age of 65 years) depressed patients at rest and during a mixed semantic and phonemic VF paradigm with a time limit of 1 min per item. Patients’ performance was not significantly impaired, and during task execution, only right parietal rCBF was significantly lower in patients as compared to controls. Frontal lobe deficits were not found. For the evaluation of brain activations, however, the authors conducted region-of-interest (ROI)-based analyses and did not differentiate between PVF and SVF. Moreover, group comparisons of rCBF were carried out separately for rest and VF. Audenaert et al. [5] used a similar task with a new baseline condition to examine the functional neuroanatomy of SVF by means of SPECT in 10 depressed patients with a recent suicide attempt. In contrast to the results of Philpot et al. [89], the depressed group displayed significantly poorer SVF performance. SPECT results demonstrated blunted perfusion in the depressed group in the left inferior frontal gyrus (IFG), the right inferior parietal gyrus, and the bilateral ACC. However, patients in this study were significantly older than control subjects, and the group analysis was not corrected for task performance, raising the possibility that decreased prefrontal perfusion in patients reflected poorer task performance in these subjects. Therefore, the pathophysiological mechanisms during SVF performance that are related to depression per se still remain unclear. Further studies are required that examine the neural correlates of SVF in sufficiently large and representative patient samples and well-matched control groups by means of whole-brain analyses corrected for task performance.
Functional magnetic resonance imaging is ideally suited to assess brain function in depression [20]. However, previous VF studies that used this method, including those examining PVF in depression [86, 87, 123], have typically required silent and experimenter-paced single word generation. Since silent word generation tasks do not provide any performance measures, the results of these studies are difficult to interpret (for details see [7, 11]). Recently, paradigms that allow for overt and self-paced responses have successfully been applied in fMRI [11, 52, 77, 78]. It has been demonstrated that the use of a block design with relatively short task (overt speech) and rest (no speech) periods of about 10 s is suitable for reducing task-related motion artefacts [10, 11]. Moreover, shorter task periods may be important for the study of patients with VF deficits, since in the clinical observation, patients’ word production drops off considerably in the course of 1 min [11].
In the current fMRI study, we used such an overt and continuous SVF paradigm with relatively short word production periods to examine the neural correlates of SVF in 33 patients diagnosed with a current episode of unipolar depression and 33 matched healthy controls. To our knowledge, this is the first fMRI study on SVF in depression. In contrast to previous fMRI studies on PVF in depression [86, 87, 123], the present study allowed for overt and self-paced generation of multiple words.
We expected patients to produce fewer words than controls, in line with evidence from studies using standard behavioural tests [30, 59, 80, 81, 96, 103, 117, 136]. Considering the uncertain and preliminary evidence base regarding the neural correlates of SVF in depression, the current study sought to clarify whether SVF in depressed patients is related to aberrant PFC functioning, which is frequently suggested to underlie executive as well as PVF deficits observed in this disorder [20, 53, 124]. The second aim of the study was to assess whether patients show dysfunctional activation patterns in other brain regions associated with the pathophysiology of MDD, e.g. temporal areas such as superior temporal gyrus and (para-)hippocampus [25]. Based upon results of previous imaging studies on SVF in depression, we anticipated different activation patterns between groups in prefrontal areas, particularly in the left IFG and the ACC [5], as well as in the right parietal cortex [5, 89].
Materials and methods
Subjects
In total, 66 native German speakers were included in this study (for characteristics of study participants see Table 1). We recruited 33 inpatients diagnosed according to ICD-10 criteria with an acute moderate or severe depressive episode at the Department of Psychiatry and Psychotherapy, Philipps-University Marburg. Patients with a history of manic or psychotic episodes were excluded from the study as well as patients with current alcohol or drug abuse, or with a lifetime history of alcohol or drug dependence. Eleven patients had a single diagnosis of depression; fifteen had dysthymia as well. Patients with comorbid somatoform, anxiety, or personality disorders (narcissistic or dependent) were only included in the study, if these comorbidities were not relevant for current hospitalisation. Nine patients (two with “double depression”) were additionally diagnosed with one or two of these disorders. Four patients were drug naive; the other patients were treated with either one (n = 19) or a combination of two standard antidepressants (n = 10). Five patients additionally received one antipsychotic, and four patients were taking one mood stabiliser, respectively. In sum, this patient sample was representative of the population of depressed inpatients.
To minimise the influence of confounding factors, we used the optimal matching algorithm implemented in the MatchIt package [44] for R (version 2.13.1, http://www.r-project.org) to select 33 healthy controls (HC) from a pool of 112 previously recruited local volunteers. The groups were matched for sex, age, and (premorbid) verbal IQ that was assessed with the “Mehrfachwahl-Wortschatz-Intelligenztest” (MWT-B) [62]. In addition, patients and controls did not differ significantly in years of education or lateralisation quotient that was evaluated with the Edinburgh Handedness Inventory [88] (see Table 1).
We applied the Trail Making Test (TMT) parts A and B [97] as a screening instrument for executive dysfunction [65]. Whereas part A is supposed to measure primarily visual search and motor speed skills, part B is presumed to tap also higher cognitive functions such as mental flexibility [12]. We calculated the TMT B/A completion time ratio for each subject, because relative performance on parts B and A provides a measure of executive function that is rather independent of motor and visual scanning speed [4, 12]. Additionally, trait anxiety was assessed in all subjects with the trait version of the State-Trait Anxiety Inventory (STAI-T) [60].
To exclude psychiatric disorders in control subjects, the German version of the Structured Clinical Interview for DSM-IV (SKID-I) [135] was conducted. Furthermore, healthy subjects with psychiatric history in first-degree relatives or Beck-Depression-Inventory (BDI) [39] score ≥9 were excluded from the study. Exclusion criteria for all subjects were neurological disorders, serious head injury, mental retardation, severe somatic diseases, or any condition that might have an effect on cerebral metabolism or MR safety. Subjects with head movement exceeding 3 mm or 3° in any direction were also excluded.
After a complete description of the procedure, subjects provided written informed consent to participate in the study. The protocol was approved by the local ethics committee according to the latest version of the Declaration of Helsinki.
fMRI task and procedure
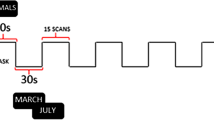
The SVF paradigm applied here has already been used successfully in a previous investigation of ours in healthy subjects [78]. In this paradigm, a block design was employed with 10 blocks for each of the two alternating conditions: word generation (WG) and baseline (BL). At the beginning of each WG block, an instruction slide with a German noun was shown for 3 s, followed by a fixation cross. From then on, subjects had to overtly name, within 12 s, as many members of the category the noun represented, e.g. say “dog”, “cat”, “eagle” […] after the word “animal” had been shown. The appearance of the word “silence” (presented for 3 s) indicated the beginning of the BL condition, in which the hash mark “#” was presented for 12 s. During this resting phase, participants were required to be silent. Subsequently, a new category name indicated the next WG block. The following 10 categories were applied in fixed order: animals, sports, clothes, professions, fruit, vehicles, furniture, flowers, hobbies, and spices.
In our previous study, analyses with a finite impulse response (FIR) model indicated that the timeframe of 12 s for WG was optimal with regard to the time course of the blood-oxygen-level-dependent (BOLD) signal [78]. Moreover, based on the number of produced words for the 10 WG blocks, a high internal consistency (Cronbach’s α = 0.82) has been identified for this task [78].
Stimuli were presented in white colour on a black background with Presentation software package (version 14.1.09.21.09, Neurobehavioral Systems Inc.). Subjects’ responses were recorded using a scanner-compatible microphone and Audacity software (version 1.2.6, Softonic International S.L.).
To familiarise participants with the task and the rules for WG (see “Behavioural data analysis”), a test session with two exemplary categories was conducted prior to the scanning procedure. These categories were not part of the fMRI investigation.
Behavioural data acquisition
The overt speech production in the scanner was recorded with a 40 dB noise-reducing microphone system (FOMRI-II, Optoacoustics Ltd.) allowing for online speech synchronisation. A dual adaptive filter system (for technical details see, e.g. [112]) subtracted the reference input (MRI noise) from the source input (speech signal) and filtered the speech production instantly while the overt output was recorded. The optic microphone was mounted on the head coil and wired to the sound filter box. The output port was directly wired to the audio in-line plug of the notebook sound card. All audio files were saved and afterwards transcribed into text files.
fMRI data acquisition
Imaging was performed on a 3 Tesla Tim Trio MR scanner (Siemens Medical Systems) at the Department of Psychiatry and Psychotherapy, Philipps-University Marburg. Functional data were acquired with a T2*-weighted echo-planar imaging (EPI) sequence sensitive to BOLD contrast (64 × 64 matrix, 224 mm × 224 mm FoV, 40 slices, 3.5 mm slice thickness, TR = 2.5 s, TE = 30 ms, flip angle = 90°). Slices covered the whole brain and were positioned transaxially parallel to the anterior–posterior commissural line (AC–PC). The initial three of the 130 collected functional images were excluded from further analysis to remove the influence of T1 stabilisation effects. To minimise head movements, subjects’ heads were fixated with foam pads.
Behavioural data analysis
All transcripts were analysed regarding the number of produced words (including errors) and checked for incorrect responses. The following answers were counted as errors: non-members of the given category, repetitions of words produced during the same block, grammatical variations of the previous word as well as words having the same stem as the preceding one. The total number of generated words, the total number of correctly generated words (correct responses), and the error rate (incorrect responses relative to the total number of generated words) were compared between patients and controls via one-sided independent samples t tests. All behavioural data analyses were carried out with SPSS 15.0 software (SPSS Inc.). A value of p < 0.05 (one-sided) was considered to be statistically significant.
fMRI data analysis
Preprocessing
Functional data were analysed using SPM8 (v4290) standard routines and templates (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm) running on MATLAB 7.7.0.471 (R2008b) (The MathWorks, Inc.). After slice-time correction (to the 20th slice), functional images were realigned and normalised to standard MNI (Montreal Neurological Institute) space (resulting voxel size: 2 mm × 2 mm × 2 mm). To increase the signal-to-noise ratio and to compensate for inter-subject anatomical variation, functional scans were spatially smoothed with an 8 mm full-width-at-half-maximum (FWHM) Gaussian kernel.
Single-subject analyses
On the first level, we used a block design to model single-subject BOLD responses during the 10 WG and the 10 BL blocks. The instruction periods (initial task instruction and category names) were included as separate condition of no interest to control for neural activations that were caused by reading the instructions. To account for within-subject differences in the amount of speech produced during the 10 WG blocks, the WG condition was modulated with the number of words generated by each subject within each block. Additionally, the six movement parameters of the realignment procedure were entered as regressors of no interest in the first-level analysis. Head movement parameters, which were carefully checked for each participant, were in an acceptable range not exceeding 3 mm or 3° in any direction, similar to previous reports [51, 56, 57, 70, 78]. To remove low-frequency drifts from time series, a high-pass filter with a cut-off period of 128 s was applied.
Group analyses
On the second level, parameter estimates of the WG and the BL condition were entered into an analysis of variance (ANOVA) using a full factorial design with group (MDD vs. HC) as between-subjects factor and condition (WG vs. BL) as within-subjects factor. Furthermore, we included the number of words produced as covariate to account for between-subjects differences in task performance and to exclude that differences in brain activation between groups reflect poorer performance in patients. Common areas of activation in patients and controls were assessed with a group conjunction analysis [(WG MDD > BL MDD) ∩ (WG HC > BL HC)].
Areas of differential activation in patients versus controls were identified with group by condition interaction analyses. An advantage of this procedure is that it takes into account potential differences between groups in BL activity (cf. [37]), which is important since patients with MDD frequently show altered BL activation when compared to HC [25, 58, 130]. This strategy also allows for a more precise analysis of activation patterns compared to subtractive designs (for details see [23, 34, 90]). For example, it provides the opportunity to evaluate whether hyperactivation in one group (e.g. MDD) reflects a greater task-related increase in activation compared to the other group (e.g. HC) or whether it is due to a weaker task-related decrease in activation in this group (here: MDD). We were interested in both, areas with greater activity during WG versus BL in patients as compared to controls, which were revealed by the interaction contrast [(WG MDD > BL MDD) > (WG HC > BL HC)], as well as in areas with greater activity during WG versus BL in controls as opposed to patients, which were assessed with the interaction contrast [(WG HC > BL HC) > (WG MDD > BL MDD)].
To correct for multiple comparisons within a search volume, we employed Monte Carlo simulation (for details see [107, 108, 118]). For a threshold at the voxel level at p = 0.001 and spatial properties as present in this study, 10,000 simulations resulted in an extent threshold of 43 resampled voxels. This procedure prevents a false-positive rate above 5 % due to multiple testing. The determined cluster threshold (based on the whole-brain volume) was applied for all contrasts.
For the anatomical localisation, the functional activations were assigned to probabilistic cytoarchitectonic areas with the SPM Anatomy Toolbox (version 1.6) [21]. Brain activations were plotted on the anatomical MRIcron (version 11 Nov. 2011, http://www.mccauslandcenter.sc.edu/mricrogl/) template.
Results
Behavioural results
Behavioural results are listed in Table 1. With a mean total number of 68.1 (SD = 12.3) words, patients produced significantly fewer words (including mistakes) during the 10 WG blocks than control subjects, who generated 73.2 (SD = 11.1) words on average (t 64 = −1.78; p = 0.04). A significant difference was also observed for the mean number of correct responses (MDD: M = 63.2, SD = 11.3; HC: M = 67.9, SD = 10.2; t 64 = −1.74; p = 0.043). The error rate of 7.09 % (SD = 4.07) in patients did not differ significantly from the error rate of 7.22 % (SD = 4.18) in controls (t 64 = −0.13; p = 0.447).
Regarding the TMT B/A ratio, groups did not differ significantly (see Table 1). To assess the association between executive functioning and performance on the SVF task, we correlated the TMT B/A ratio with the calculated SVF performance measures, separately for patients and controls. Neither in patients nor in controls was the TMT B/A ratio significantly correlated with the number of words produced (MDD: r = 0.07, p = 0.702; HC: r = −0.19, p = 0.283), the number of correct responses (MDD: r = 0.01, p = 0.975; HC: r = −0.21, p = 0.25), or the error rate (MDD: r = 0.24, p = 0.188; HC: r = 0.05, p = 0.788).
fMRI results
The conjunction analysis [(WG MDD > BL MDD) ∩ (WG HC > BL HC)] indicated that common activations of patients and controls were located in the bilateral cerebellum, the bilateral postcentral gyrus, the bilateral superior temporal gyrus, the left supplementary motor area (SMA), the left insula, and the left cuneus (Table 2).
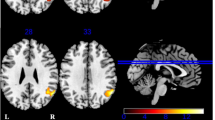
For the group by condition interaction contrast [(WG HC > BL HC) > (WG MDD > BL MDD)], no suprathreshold voxels were found. The opposite contrast [(WG MDD > BL MDD) > (WG HC > BL HC)] revealed five areas with greater activation during WG versus BL in patients as opposed to controls: the right precentral gyrus (PCG), the left cerebellum (Cb), the right SMA, the right fusiform gyrus (FG), and the right parahippocampal gyrus (PHG) (Table 3; Fig. 1). Including the TMT B/A ratio as additional covariate to control for variance due to individual differences in executive functioning did not alter the results.
Brain areas with significant group by condition interaction effect [(WG MDD > BL MDD) > (WG HC > BL HC)] (p < 0.001, corrected by Monte Carlo cluster simulations; cluster extent threshold = 43 voxels). Compared to controls, patients showed greater activity during word generation versus baseline in the right precentral gyrus (PCG), the left cerebellum (Cb), the right supplementary motor area (SMA), the right fusiform gyrus (FG), and the right parahippocampal gyrus (PHG). Bar graphs display the mean signal intensity during baseline and word generation for the whole PCG, Cb, SMA, FG, and PHG clusters in controls and patients. Values of the y axis differ between the graphs. L left, R right
To examine whether the significant clusters were part of the common SVF network and/or the SVF network of patients and/or controls, the interaction contrast was inclusively masked (p < 0.001) separately with the following contrasts: [(WG MDD > BL MDD) ∩ (WG HC > BL HC)], (WG MDD > BL MDD), and (WG HC > BL HC). From the clusters with significant group by condition interaction effect, only 61 voxels of the Cb cluster (x = −18, y = −54, z = −20) overlapped with the commonly activated SVF network and the SVF network of controls. However, 272 voxels of the PCG cluster (x = 46, y = −10, z = 56), 144 voxels of the Cb cluster (x = −16, y = −48, z = −24), 8 voxels of the SMA cluster (x = 6, y = −6, z = 58), and 3 voxels of the PHG cluster (x = 30, y = −6, z = −38) were part of the network activated during WG versus BL in patients.
To examine whether the significant clusters were part of the common BL network and/or the BL network of patients and/or controls, the interaction contrast was inclusively masked (p < 0.001) separately with the following contrasts: [(BL MDD > WG MDD) ∩ (BL HC > WG HC)], (BL MDD > WG MDD), and (BL HC > WG HC). These analyses revealed that the clusters with significant group by condition interaction effect did not overlap with the common BL network and the BL network of patients. However, 79 voxels of the SMA cluster (x = 6, y = −10, z = 56), 52 voxels of the FG cluster (x = 28, y = −42, z = −10), and 32 voxels of the PHG cluster (x = 28, y = 0, z = −34) were part of the network activated during BL versus WG in healthy subjects.
To further explore brain activation in the five areas with significant interaction effect (PCG, Cb, SMA, FG, and PHG), fMRI data were extracted as the first eigenvariate of the respective entire clusters. Post hoc paired t tests (two-tailed) revealed that in both groups, activity in the left Cb increased significantly during WG versus BL (MDD: t 32 = −8.04, p < 0.001; HC: t 32 = −4.12, p < 0.001). Activity in the right PCG did not change from BL to WG in controls (t 32 = −0.37, p = 0.713), but increased significantly in patients (t 32 = −4.11, p < 0.001). While activity in the right SMA, the right FG, and the right PHG decreased significantly from BL to WG in controls (SMA: t 32 = 3.41, p = 0.002; FG: t 32 = 2.88, p = 0.007; PHG: t 32 = 3, p = 0.005), activity in these areas did not change significantly in patients (SMA: t 32 = −1.5, p = 0.143; FG: t 32 = −1.84, p = 0.075; PHG: t 32 = −1.95, p = 0.06).
Discussion
This is, to our knowledge, the first fMRI study investigating the effect of depression on the neural mechanisms underlying the production of semantic category members. For this purpose, brain activity during an overt and continuous SVF paradigm was compared between 33 depressed patients and 33 well-matched controls. In line with previous imaging studies that contrasted overt word production with a resting condition [40, 52, 78], both groups engaged a widespread network of brain areas encompassing fronto-temporal regions, the cerebellum as well as the insular cortex, and the cuneus. In contrast to previous neuroimaging studies on VF in depression (e.g. [5, 86, 87, 127]), which used within-subject subtraction of BL from WG, we entered both conditions into a full factorial design to explore differential brain activation patterns in patients versus controls via group by condition interaction analyses. This strategy accounts for potential differences between groups in BL activity [25, 37] and, in contrast to subtractive designs in which for example hyperactivation in patients versus controls can also reflect a failure to deactivate (for details see [23, 34, 90]), enables a more precise analysis of activation patterns.
Behavioural results
As hypothesised, patients produced significantly fewer words during the 10 WG blocks (inclusive and exclusive errors) than control subjects. Since the error rate did not differ significantly between groups, depressed patients’ performance was affected with respect to the quantity but not regarding the quality of generated words. The finding of reduced word production confirms that unipolar depression is associated with impairments in SVF and is in line with much previous work in the field [5, 30, 59, 80, 81, 96, 103, 117, 136].
Furthermore, performance on the current SVF task was not associated with executive functioning as assessed with the TMT B/A ratio [4, 65], neither in patients nor in controls, which is in line with an understanding of SVF as a distinct cognitive domain that is separable from other executive functions [24, 109].
fMRI results
One aim of this study was to examine whether SVF performance in patients with MDD is related to PFC dysfunctions as suggested in an earlier study [5]. Particularly, aberrant activation was expected in PFC (IFG, ACC) [5] and right parietal cortex [5, 89]. However, the current results do not provide any evidence for dysfunctional activation of these regions in patients. Although the present study could not confirm these earlier reports, the absence of prefrontal deficits accords with the finding of Philpot et al. [89]. It might be speculated that attenuated activity in PFC may be eliminated when analyses are controlled for potential differences in SVF performance between groups, as in the current study, or when patients and controls are equally able to perform the task (cf. [89]). Moreover, previous studies examined only patients with suicidal tendency [5] or old age [89]. Results of these investigations may therefore be related to these specific patient characteristics, as acknowledged by the authors themselves [5].
The second aim of the present study was to detect potential dysfunctional activation patterns in other brain areas associated with the pathophysiology of MDD, e.g. in temporal regions such as superior temporal gyrus and (para-)hippocampus. The group by condition interaction analysis revealed that patients, compared to controls, showed greater activation during WG versus BL in clusters of the right PHG (~Brodmann area (BA) 36/20), the right FG (~BA 37), the right SMA (~BA 6), the right PCG (~BA 6/4), and the left Cb (lobules IV/V and VI), all of which are considered relevant to depression [18, 25, 26, 69, 130, 139]. In particular, patients showed a greater task-related increase in activity in the left Cb and an additional increase in activation in the right PCG when compared to controls. Moreover, while controls demonstrated a task-related suppression of activity in the PHG, FG, and SMA, depressed patients failed to do so. The data indicate that increased brain responses during SVF in patients were mainly due to decreased attenuation of brain regions not expected to be active during task performance. Enhanced and additional recruitment of brain areas likely supporting WG in these subjects also played a role. Note that the different activation patterns between groups did not reflect differences in task performance or speech output, since we controlled for the number of generated words in the second-level analysis. Therefore, the identified activation differences are supposed to reflect altered cognitive processing related to depression per se. Since the inclusion of TMT B/A ratio as additional covariate did also not alter the results, aberrant activation patterns during SVF in patients might be regarded as relatively independent from the processes that are tapped by this screening instrument for executive dysfunctions.
Functional alterations of PHG (e.g. [29, 120, 132]), FG (e.g. [16, 119, 130]), Cb (e.g. [32, 33, 66, 79]), and motor cortical areas such as SMA (e.g. [26, 129]) have been frequently reported in studies examining brain activity or connectivity in MDD during various tasks as well as at rest. Moreover, there is growing evidence for an involvement of PHG, FG, and Cb in the pathophysiology of this disorder [18, 19, 92, 130]. In the following, regions showing decreased task-related suppression or additional/increased task-related activation in patients are discussed with regard to their potential functional relevance for SVF performance.
Decreased task-related suppression in PHG, FG, and SMA
Activity in PHG and FG may be related to internal cognitive and affective processes in which subjects typically engage during rest such as recall and visual imagery of past experiences. PHG is an important hub of the default-mode network (DMN) [95] and mediates the connectivity between hippocampus and other cortical DMN hubs implicated in the pathophysiology of MDD, e.g. posterior cingulate cortex [13, 131]. The DMN represents a set of brain regions that typically increase activity during spontaneous cognition and task-independent self-referential thought such as autobiographical memory recall, mind wandering, considering social interactions, and thinking about the future [2, 13, 110]. Attenuation of DMN activity during various goal-directed behaviours [34, 95] is interpreted as a mechanism through which self-referential activity can be suppressed to optimise cognitive functioning [3]. In line with the default-mode interference hypothesis [110], reduced DMN suppression during task execution, as it has been reported for depressed subjects (e.g. [31, 105]), may lead to interference of self-referential introspection and internal emotional states with task-specific neural processing [3, 105, 133]. While controls showed a task-related deactivation of PHG in the current study, this was not the case in patients. Increased PHG activity in patients might therefore reflect a failure to suppress activity in an important node of the DMN during task execution, which might have led to detrimental effects on task-related cognitive processing. This assumption is in line with a growing body of evidence that implicates dysfunctions of DMN regions, including PHG, in cognitive impairments and symptoms associated with depression, e.g. in maladaptive depressive rumination, increased self-referential focus, and aberrant autobiographical memory recall [36, 64, 140].
Activity in the PHG has also been related to other processes of internal cognition such as visual mental imagery [27, 54, 55, 141], especially when the imagined scene is emotionally distressing [106]. Previous research suggests that, among other cortical areas, PHG as well as FG, another region in which we found a lack of deactivation in patients, is involved in visual mental imagery in general and especially with regard to scenes and faces, respectively [27, 28, 54, 55, 73, 83, 125, 141]. It has been proposed that PHG and FG may connect visual and long-term memory processes to support imagery of objects retrieved from long-term knowledge [28]. Although these processes likely are not unique to either the right or the left hemisphere, as activation of PHG and FG has been found bilaterally during visual imagery [27, 141], increased activity in the right FG during SVF may reflect, like the enhanced right PHG activity, interference from internal mental processes during task execution in patients. This interpretation is in line with the finding that large parts of the PHG and FG clusters were included within the neural network that was activated during rest as compared to WG in healthy subjects. It might further be speculated that the failure of patients to suppress activity in these areas during SVF reflects an inability to detach themselves from their internal emotional and cognitive states (e.g. ruminative thoughts), which may therefore occupy cognitive resources [109, 133].
The third region in which activity was suppressed during WG versus BL in controls but not in patients was the right SMA (BA 6). The SMA belongs to the supplementary motor complex (SMC), which is supposed to be important for linking action and cognition (for review see [76]). In the context of language, activity in the SMA is frequently related to the planning and execution of overt speech [1, 14, 91]. However, there is evidence that the SMC, especially the right-hemispheric medial BA 6, also contributes to processes that inhibit speech [137] and motor responses in general [76, 122]. In line with this function, the right SMA cluster was not part of the common SVF network in the current study and was even deactivated in controls during WG relative to BL. Moreover, a large part of the right SMA cluster was active during BL versus WG in controls. Greater activity in this area might therefore reflect a failure of depressed patients to reduce the influence of a region potentially involved in processes that inhibit speech during the current SVF task.
However, alternative explanations for increased SMA, PHG, and FG activity in patients have to be considered. One possibility regarding FG and PHG is that the activation differences in these areas reflected differences in retrieval strategies between groups. Since elements of the DMN may be differentially suppressed as a function of task demands [110], higher PHG and FG activity in patients might be explained by a retrieval strategy of patients that relied more on autobiographical and spatial information (e.g. visualising themselves in the zoo while producing items for the category “animals”) as those employed by controls (cf. [101, 104]). Although we cannot fully exclude this possibility, there seems to be no reason to suppose that groups differed systematically in their WG strategies.
A second alternative explanation for the activation differences would be an insufficient compensatory mechanism, assuming that patients recruited additional processing resources in SMA, FG, and PHG. According to such an interpretation, increased right SMA activity in patients might be interpreted as an additional need for patients to inhibit potentially inappropriate responses. Increased activity in PHG and FG might in this context reflect a supplementary recruitment of brain areas suggested to be involved in semantic processing [9]. These explanations, however, seem highly implausible since these regions did not show a significant task-related increase in activation in patients and even displayed significantly task-related deactivation in controls, implying that all three of these areas were not part of the SVF network in neither of the two groups.
Additional and increased task-related activation in PCG and Cb
The PCG cluster encompassed parts of the primary motor region BA 4 as well as parts of the premotor region BA 6, which both contribute to the initiation and execution of overt speech [46, 67, 91]. Furthermore, previous research has shown that a high rate of speech production is related to increased activity in motor regions such as primary motor cortex and cerebellum [98, 134]. Increased participation of (pre-)motor regions during WG might therefore represent an auxiliary mechanism in patients to maintain a certain level of performance. It also points to potential difficulties in speech initiation in patients, an assumption that is in line with psychomotor disturbances typically observed in depression such as slowed speech [68].
Like the PCG, the Cb typically increases activity during the production of speech [91] and plays an important role in speech motor control [67, 111, 113], specifically under time-critical conditions [134]. However, there is evidence for an important contribution of the Cb not only to articulatory but also to cognitive aspects of language [15, 84, 115]. In general, it has become apparent that beyond the motor domain, the Cb is involved in various higher cognitive functions including higher-order language processes, WM, and executive functions (for reviews and meta-analyses see [15, 49, 84, 113, 114, 116]). In the current study, increased activation in patients was found in left cerebellar lobules IV/V and VI, which are, according to a recent meta-analysis [49], related to language (left lobule VI) and verbal WM (left lobule IV/V). However, preceding research suggested that the anterior lobe including lobule IV is predominantly sensorimotor and engaged in motor control, whereas lobule VI (among others) contributes to higher-level processes [114, 116]. Therefore, the greater increase in left Cb activity during WG in patients might reflect enhanced recruitment of regions supporting higher-order language processes and verbal WM or speech motor control. Both these explanations seem possible since successful SVF performance involves articulatory as well as WM processes. Taken together, the additional and increased task-related responses of depressed patients in the right PCG and the left Cb likely reflect an enhanced need of these subjects to recruit brain areas supporting speech initiation and cognitive aspects of language production during the current SVF task. It might be speculated that the increased responses in these areas represent an insufficient compensatory mechanism for interfering activity in PHG, FG, and SMA.
Limitations
This investigation was limited by the inclusion of patients taking antidepressant medication. Due to the possibility of medication effects (for review see [8]), the generalisability to unmedicated patients is unclear. There are some reports of normalised brain activation, particularly in PFC, in depressed patients after successful antidepressant treatment [22, 63, 75, 99], raising the possibility that antidepressant therapy in the present patient sample led to the absence of activation differences in PFC between groups. However, in a recent meta-analysis, no effect of medication was found on SVF performance [109].
Conclusions
This is the first fMRI study providing evidence for effects of depression on the neural correlates underlying SVF. While healthy subjects down-regulated activity in the right PHG, the right FG, and the right SMA during periods of WG as compared to rest, patients with MDD failed to do so. Furthermore, patients showed additional and increased task-related activation of right PCG and left Cb, respectively. The data point to a failure of depressed patients to deactivate right-hemispheric brain regions involved in DMN-related functions, such as self-referential thought or visual imagery (PHG and FG), and speech inhibitory processes (SMA) during WG, which may lead to interference with task execution. Results further demonstrate enhanced task-related recruitment of areas supporting speech initiation and articulation (PCG/Cb) as well as higher-order language processes and verbal WM (Cb). Therefore, a failure to reduce potentially distracting activity from task-irrelevant regions combined with an insufficient compensational recruitment of task-relevant areas may underlie poorer SVF in MDD. In general, it might be speculated that depressed subjects are unable to completely detach from internal emotional and cognitive states, which may occupy cognitive resources during task execution and may thus lead to cognitive dysfunctions [109, 133].
The novel findings of the present investigation illustrate that studies examining the neural correlates of cognitive deficits in depression should not only focus on prefrontal brain areas, as was the case in most PVF studies (for review see [53]). We found, for example, dysfunctional activation patterns in PHG, FG, and Cb, which are increasingly recognised as relevant to depression [25, 119, 130]. Taken together, the findings of decreased performance as well as of aberrant brain activation during the current SVF task in patients with depression demonstrate that this paradigm represents a sensitive tool for detecting brain dysfunctions in clinical populations.
References
Alario FX, Chainay H, Lehericy S, Cohen L (2006) The role of the supplementary motor area (SMA) in word production. Brain Res 1076:129–143
Andrews-Hanna JR (2012) The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18:251–270
Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH (2012) The role of default network deactivation in cognition and disease. Trends Cogn Sci 16:584–592
Arbuthnott K, Frank J (2000) Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol 22:518–528
Audenaert K, Goethals I, Van Laere K, Lahorte P, Brans B, Versijpt J, Vervaet M, Beelaert L, Van Heeringen K, Dierckx R (2002) SPECT neuropsychological activation procedure with the verbal fluency test in attempted suicide patients. Nucl Med Commun 23:907–916
Austin MP, Mitchell P, Goodwin GM (2001) Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry 178:200–206
Basho S, Palmer ED, Rubio MA, Wulfeck B, Muller RA (2007) Effects of generation mode in fMRI adaptations of semantic fluency: paced production and overt speech. Neuropsychologia 45:1697–1706
Bellani M, Dusi N, Yeh PH, Soares JC, Brambilla P (2011) The effects of antidepressants on human brain as detected by imaging studies. Focus on major depression. Prog Neuropsychopharmacol Biol Psychiatry 35:1544–1552
Binder JR, Desai RH, Graves WW, Conant LL (2009) Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796
Birn RM, Cox RW, Bandettini PA (2004) Experimental designs and processing strategies for fMRI studies involving overt verbal responses. Neuroimage 23:1046–1058
Birn RM, Kenworthy L, Case L, Caravella R, Jones TB, Bandettini PA, Martin A (2010) Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage 49:1099–1107
Bowie CR, Harvey PD (2006) Administration and interpretation of the trail making test. Nat Protoc 1:2277–2281
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38
Christoffels IK, Formisano E, Schiller NO (2007) Neural correlates of verbal feedback processing: an fMRI study employing overt speech. Hum Brain Mapp 28:868–879
De Smet HJ, Paquier P, Verhoeven J, Mariën P (2013) The cerebellum: its role in language and related cognitive and affective functions. Brain Lang 27:334–342
Demenescu LR, Renken R, Kortekaas R, van Tol MJ, Marsman JB, van Buchem MA, van der Wee NJ, Veltman DJ, den Boer JA, Aleman A (2011) Neural correlates of perception of emotional facial expressions in out-patients with mild-to-moderate depression and anxiety. A multicenter fMRI study. Psychol Med 41:2253–2264
Den Hartog HM, Derix MM, Van Bemmel AL, Kremer B, Jolles J (2003) Cognitive functioning in young and middle-aged unmedicated out-patients with major depression: testing the effort and cognitive speed hypotheses. Psychol Med 33:1443–1451
Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H (2012) A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. Neuroimage 61:677–685
Drevets WC, Price JL, Furey ML (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118
Ebmeier K, Rose E, Steele D (2006) Cognitive impairment and fMRI in major depression. Neurotox Res 10:87–92
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335
Fales CL, Barch DM, Rundle MM, Mintun MA, Mathews J, Snyder AZ, Sheline YI (2009) Antidepressant treatment normalizes hypoactivity in dorsolateral prefrontal cortex during emotional interference processing in major depression. J Affect Disord 112:206–211
Fernández-Corcuera P, Salvador R, Monté GC, Salvador Sarró S, Goikolea JM, Amann B, Moro N, Sans-Sansa B, Ortiz-Gil J, Vieta E, Maristany T, McKenna PJ, Pomarol-Clotet E (2013) Bipolar depressed patients show both failure to activate and failure to de-activate during performance of a working memory task. J Affect Disord 148:170–178
Fisk JE, Sharp CA (2004) Age-related impairment in executive functioning: updating, inhibition, shifting, and access. J Clin Exp Neuropsychol 26:874–890
Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ (2008) A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp 29:683–695
Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP (2013) Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry 52:628–641
Ganis G, Thompson WL, Kosslyn SM (2004) Brain areas underlying visual mental imagery and visual perception: an fMRI study. Cogn Brain Res 20:226–241
Gardini S, Cornoldi C, De Beni R, Venneri A (2009) Cognitive and neuronal processes involved in sequential generation of general and specific mental images. Psychol Res 73:633–643
Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL (2011) Aberrant brain activation during a working memory task in psychotic major depression. Am J Psychiatry 168:173–182
Gohier B, Ferracci L, Surguladze SA, Lawrence E, El Hage W, Kefi MZ, Allain P, Garre JB, Le Gall D (2009) Cognitive inhibition and working memory in unipolar depression. J Affect Disord 116:100–105
Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G (2009) Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology 34:932–943
Guo WB, Liu F, Chen JD, Gao K, Xue ZM, Xu XJ, Wu RR, Tan CL, Sun XL, Liu ZN, Chen HF, Zhao JP (2012) Abnormal neural activity of brain regions in treatment-resistant and treatment-sensitive major depressive disorder: a resting-state fMRI study. J Psychiatr Res 46:1366–1373
Guo W, Liu F, Xue Z, Gao K, Liu Z, Xiao C, Chen H, Zhao J (2013) Abnormal resting-state cerebellar-cerebral functional connectivity in treatment-resistant depression and treatment sensitive depression. Prog Neuropsychopharmacol Biol Psychiatry 44:51–57
Gusnard DA, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694
Halvorsen M, Høifødt RS, Myrbakk IN, Wang CE, Sundet K, Eisemann M, Waterloo K (2012) Cognitive function in unipolar major depression: a comparison of currently depressed, previously depressed and never depressed individuals. J Clin Exp Neuropsychol 34:782–790
Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH (2011) Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry 70:327–333
Hanslmayr S, Backes H, Straub S, Popov T, Langguth B, Hajak G, Bäuml KH, Landgrebe M (2012) Enhanced resting-state oscillations in schizophrenia are associated with decreased synchronization during inattentional blindness. Hum Brain Mapp 34:2266–2275
Harvey PO, Le Bastard G, Pochon JB, Levy R, Allilaire JF, Dubois B, Fossati P (2004) Executive functions and updating of the contents of working memory in unipolar depression. J Psychiatr Res 38:567–576
Hautzinger M, Bailer M, Worall H, Keller F (1994) Beck-Depressions-Inventar (BDI). Testhandbuch. Hans Huber, Bern
Heim S, Eickhoff SB, Amunts K (2008) Specialisation in Broca’s region for semantic, phonological, and syntactic fluency? Neuroimage 40:1362–1368
Henry JD, Crawford JR (2004) A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology 18:284–295
Henry J, Crawford JR (2005) A meta-analytic review of verbal fluency deficits in depression. J Clin Exp Neuropsychol 27:78–101
Herrmann MJ, Ehlis AC, Fallgatter AJ (2004) Bilaterally reduced frontal activation during a verbal fluency task in depressed patients as measured by near-infrared spectroscopy. J Neuropsychiatry Clin Neurosci 16:170–175
Ho DE, Imai K, King G, Stuart EA (2011) MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw 42:1–28
Jaeger J, Berns S, Uzelac S, Davis-Conway S (2006) Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res 145:39–48
Jürgens U (2002) Neural pathways underlying vocal control. Neurosci Biobehav Rev 26:235–258
Kameyama M, Fukuda M, Yamagishi Y, Sato T, Uehara T, Ito M, Suto T, Mikuni M (2006) Frontal lobe function in bipolar disorder: a multichannel near-infrared spectroscopy study. Neuroimage 29:172–184
Katzev M, Tuscher O, Hennig J, Weiller C, Kaller CP (2013) Revisiting the functional specialization of left inferior frontal gyrus in phonological and semantic fluency: the crucial role of task demands and individual ability. J Neurosci 33:7837–7845
E KH, Chen SH, Ho MH, Desmond JE (2012) A meta-analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp. doi:10.1002/hbm.22194
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005) Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 62:593–602
Kircher T, Krug A, Markov V, Whitney C, Krach S, Zerres K, Eggermann T, Stöcker T, Shah NJ, Treutlein J, Nöthen MM, Becker T, Rietschel M (2009) Genetic variation in the schizophrenia-risk gene neuregulin 1 correlates with brain activation and impaired speech production in a verbal fluency task in healthy individuals. Hum Brain Mapp 30:3406–3416
Kircher T, Nagels A, Kirner-Veselinovic A, Krach S (2011) Neural correlates of rhyming versus lexical and semantic fluency. Brain Res 1391:71–80
Klumpp H, Deldin P (2010) Review of brain functioning in depression for semantic processing and verbal fluency. Int J Psychophysiol 75:77–85
Kosslyn SM, Thompson WL (2003) When is early visual cortex activated during visual mental imagery? Psychol Bull 129:723–746
Kreiman G, Koch C, Fried I (2000) Imagery neurons in the human brain. Nature 408:357–361
Krug A, Markov V, Sheldrick A, Krach S, Jansen A, Zerres K, Eggermann T, Stöcker T, Shah NJ, Kircher T (2009) The effect of the COMT val(158)met polymorphism on neural correlates of semantic verbal fluency. Eur Arch Psychiatry Clin Neurosci 259:459–465
Krug A, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stöcker T, Shah NJ, Nöthen MM, Georgi A, Strohmaier J, Rietschel M, Kircher T (2011) Genetic variation in G72 correlates with brain activation in the right middle temporal gyrus in a verbal fluency task in healthy individuals. Hum Brain Mapp 32:118–126
Kühn S, Gallinat J (2013) Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull 39:358–365
Lafont V, Medecin I, Robert PH, Beaulieu FE, Kazes M, Danion JM, Pringuey D, Darcourt G (1998) Initiation and supervisory processes in schizophrenia and depression. Schizophr Res 34:49–57
Laux L, Glanzmann P, Schaffner P, Spielberger CD (1981) Das State-Trait-Angstinventar. Theoretische Grundlagen und Handanweisung. Beltz Test GmbH, Weinheim
Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA (2012) A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord 140:113–124
Lehrl S, Triebig G, Fischer B (1995) Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 91:335–345
Lemogne C, Mayberg H, Bergouignan L, Volle E, Delaveau P, Léhericy S, Allilaire JF, Fossati P (2010) Self-referential processing and the prefrontal cortex over the course of depression: a pilot study. J Affect Disord 124:196–201
Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P (2012) Medial prefrontal cortex and the self in major depression. J Affect Disord 136:e1–e11
Lezak MD (1995) Neuropsychological assessment. Oxford University Press, Oxford
Liu L, Zeng LL, Li Y, Ma Q, Li B, Shen H, Hu D (2012) Altered cerebellar functional connectivity with intrinsic connectivity networks in adults with major depressive disorder. PLoS One 7:e39516
Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SN, Gerwig M, Habas C, Hagura N, Ivry RB, Marien P, Molinari M, Naito E, Nowak DA, Oulad Ben Taib N, Pelisson D, Tesche CD, Tilikete C, Timmann D (2012) Consensus paper: roles of the cerebellum in motor control: the diversity of ideas on cerebellar involvement in movement. Cerebellum 11:457–487
Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L (2010) Cognitive impairment in major depression. Eur J Pharmacol 626:83–86
Marchand WR, Lee JN, Johnson S, Thatcher J, Gale P, Wood N, Jeong EK (2012) Striatal and cortical midline circuits in major depression: implications for suicide and symptom expression. Prog Neuropsychopharmacol Biol Psychiatry 36:290–299
Markov V, Krug A, Krach S, Whitney C, Eggermann T, Zerres K, Stöcker T, Shah NJ, Nöthen MM, Treutlein J, Rietschel M, Kircher T (2009) Genetic variation in schizophrenia-risk-gene dysbindin 1 modulates brain activation in anterior cingulate cortex and right temporal gyrus during language production in healthy individuals. Neuroimage 47:2016–2022
Matsuo K, Kato T, Fukuda M, Kato N (2000) Alteration of hemoglobin oxygenation in the frontal region in elderly depressed patients as measured with near-infrared spectroscopy. J Neuropsychiatry Clin Neurosci 12:465–471
Matsuo K, Kato N, Kato T (2002) Decreased cerebral haemodynamic response to cognitive and physiological tasks in mood disorders as shown by near-infrared spectroscopy. Psychol Med 32:1029–1037
Mechelli A, Price CJ, Friston KJ, Ishai A (2004) Where bottom-up meets top-down: neuronal interactions during perception and imagery. Cereb Cortex 14:1256–1265
Millan MJ, Agid Y, Brüne M, Bullmore ET, Carter CS, Clayton NS, Connor R, Davis S, Deakin B, DeRubeis RJ, Dubois B, Geyer MA, Goodwin GM, Gorwood P, Jay TM, Joëls M, Mansuy IM, Meyer-Lindenberg A, Murphy D, Rolls E, Saletu B, Spedding M, Sweeney J, Whittington M, Young LJ (2012) Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11:141–168
Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV (2011) Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem 96:553–563
Nachev P, Kennard C, Husain M (2008) Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9:856–869
Nagels A, Kirner-Veselinovic A, Krach S, Kircher T (2011) Neural correlates of S-ketamine induced psychosis during overt continuous verbal fluency. Neuroimage 54:1307–1314
Nagels A, Kircher T, Dietsche B, Backes H, Marquetand J, Krug A (2012) Neural processing of overt word generation in healthy individuals: the effect of age and word knowledge. Neuroimage 61:832–840
Naismith SL, Lagopoulos J, Ward PB, Davey CG, Little C, Hickie IB (2010) Fronto-striatal correlates of impaired implicit sequence learning in major depression: an fMRI study. J Affect Disord 125:256–261
Neu P, Kiesslinger U, Schlattmann P, Reischies FM (2001) Time-related cognitive deficiency in four different types of depression. Psychiatry Res 103:237–247
Neu P, Bajbouj M, Schilling A, Godemann F, Berman RM, Schlattmann P (2005) Cognitive function over the treatment course of depression in middle-aged patients: correlation with brain MRI signal hyperintensities. J Psychiatr Res 39:129–135
Noda T, Yoshida S, Matsuda T, Okamoto N, Sakamoto K, Koseki S, Numachi Y, Matsushima E, Kunugi H, Higuchi T (2012) Frontal and right temporal activations correlate negatively with depression severity during verbal fluency task: a multi-channel near-infrared spectroscopy study. J Psychiatr Res 46:905–912
O’Craven KM, Kanwisher N (2000) Mental imagery of faces and places activates corresponding stimulus-specific brain regions. J Cogn Neurosci 12:1013–1023
O’Halloran CJ, Kinsella GJ, Storey E (2012) The cerebellum and neuropsychological functioning: a critical review. J Clin Exp Neuropsychol 34:35–56
Ohta H, Yamagata B, Tomioka H, Takahashi T, Yano M, Nakagome K, Mimura M (2008) Hypofrontality in panic disorder and major depressive disorder assessed by multi-channel near-infrared spectroscopy. Depress Anxiety 25:1053–1059
Okada G, Okamoto Y, Morinobu S, Yamawaki S, Yokota N (2003) Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology 47:21–26
Okada G, Okamoto Y, Yamashita H, Ueda K, Takami H, Yamawaki S (2009) Attenuated prefrontal activation during a verbal fluency task in remitted major depression. Psychiatry Clin Neurosci 63:423–425
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Philpot MP, Banerjee S, Needham-Bennett H, Costa DC, Ell PJ (1993) 99mTc-HMPAO single photon emission tomography in late life depression: a pilot study of regional cerebral blood flow at rest and during a verbal fluency task. J Affect Disord 28:233–240
Pomarol-Clotet E, Salvador R, Sarró S, Gomar J, Vila F, Martínez Á, Guerrero A, Ortiz-Gil J, Sans-Sansa B, Capdevila A, Cebamanos JM, McKenna PJ (2008) Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med 38:1185–1193
Price CJ (2010) The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 1191:62–88
Price JL, Drevets WC (2012) Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci 16:61–71
Pu S, Matsumura H, Yamada T, Ikezawa S, Mitani H, Adachi A, Nakagome K (2008) Reduced frontopolar activation during verbal fluency task associated with poor functioning in late-onset major depression: multi-channel near-infrared spectroscopy study. Psychiatry Clin Neurosci 62:728–737
Pu S, Nakagome K, Yamada T, Yokoyama K, Matsumura H, Mitani H, Adachi A, Nagata I, Kaneko K (2012) The relationship between the prefrontal activation during a verbal fluency task and stress-coping style in major depressive disorder: a near-infrared spectroscopy study. J Psychiatr Res 46:1427–1434
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci USA 98:676–682
Ravnkilde B, Videbech P, Clemmensen K, Egander A, Rasmussen NA, Rosenberg R (2002) Cognitive deficits in major depression. Scand J Psychol 43:239–251
Reitan RM (1955) The relation of the trail making test to organic brain damage. J Consult Psychol 19:393–394
Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H (2005) fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology 64:700–706
Rosenblau G, Sterzer P, Stoy M, Park S, Friedel E, Heinz A, Pilhatsch M, Bauer M, Ströhle A (2012) Functional neuroanatomy of emotion processing in major depressive disorder is altered after successful antidepressant therapy. J Psychopharmacol 26:1424–1433
Ruff RM, Light RH, Parker SB, Levin HS (1997) The psychological construct of word fluency. Brain Lang 57:394–405
Ryan L, Cox C, Hayes SM, Nadel L (2008) Hippocampal activation during episodic and semantic memory retrieval: comparing category production and category cued recall. Neuropsychologia 46:2109–2121
Sacher J, Neumann J, Fünfstuck T, Soliman A, Villringer A, Schroeter ML (2012) Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord 140:142–148
Schmid M, Strand M, Ardal G, Lund A, Hammar A (2011) Prolonged impairment in inhibition and semantic fluency in a follow-up study of recurrent major depression. Arch Clin Neuropsychol 26:677–686
Sheldon S, Moscovitch M (2012) The nature and time-course of medial temporal lobe contributions to semantic retrieval: an MRI study on verbal fluency. Hippocampus 22:1451–1466
Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME (2009) The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA 106:1942–1947
Sinha R, Lacadie C, Skudlarski P, Wexler BE (2004) Neural circuits underlying emotional distress in humans. Ann N Y Acad Sci 1032:254–257
Slotnick SD, Schacter DL (2004) A sensory signature that distinguishes true from false memories. Nat Neurosci 7:664–672
Slotnick SD, Moo LR, Segal JB, Hart J Jr (2003) Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cogn Brain Res 17:75–82
Snyder HR (2013) Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull 139:81–132
Sonuga-Barke EJ, Castellanos FX (2007) Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev 31:977–986
Sörös P, Sokoloff LG, Bose A, McIntosh AR, Graham SJ, Stuss DT (2006) Clustered functional MRI of overt speech production. Neuroimage 32:376–387
Stephens GJ, Silbert LJ, Hasson U (2010) Speaker-listener neural coupling underlies successful communication. Proc Natl Acad Sci USA 7:14425–14430
Stoodley CJ (2012) The cerebellum and cognition: evidence from functional imaging studies. Cerebellum 11:352–365
Stoodley CJ, Schmahmann JD (2009) Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44:489–501
Stoodley CJ, Schmahmann JD (2009) The cerebellum and language: evidence from patients with cerebellar degeneration. Brain Lang 110:149–153
Stoodley CJ, Schmahmann JD (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–844
Stordal KI, Lundervold AJ, Egeland J, Mykletun A, Asbjørnsen A, Landrø NI, Roness A, Rund BR, Sundet K, Oedegaard KJ, Lund A (2004) Impairment across executive functions in recurrent major depression. Nord J Psychiatry 58:41–47
Straube B, Green A, Weis S, Kircher T (2012) A supramodal neural network for speech and gesture semantics: an fMRI study. PLoS One 7:e51207
Stuhrmann A, Suslow T, Dannlowski U (2011) Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord 1:10
Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML (2005) A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 57:201–209
Suto T, Fukuda M, Ito M, Uehara T, Mikuni M (2004) Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. Biol Psychiatry 55:501–511
Swick D, Ashley V, Turken U (2011) Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 56:1655–1665
Takami H, Okamoto Y, Yamashita H, Okada G, Yamawaki S (2007) Attenuated anterior cingulate activation during a verbal fluency task in elderly patients with a history of multiple-episode depression. Am J Geriatr Psychiatry 15:594–603
Thomas EJ, Elliott R (2009) Brain imaging correlates of cognitive impairment in depression. Front Hum Neurosci 3:1–9
Tyler LK, Chiu S, Zhuang J, Randall B, Devereux BJ, Wright P, Clarke A, Taylor KI (2013) Objects and categories: feature statistics and object processing in the ventral stream. J Cogn Neurosci 25:1723–1735
Unsworth N, Spillers GJ, Brewer GA (2011) Variation in verbal fluency: a latent variable analysis of clustering, switching, and overall performance. Q J Exp Psychol 64:447–466
Videbech P, Ravnkilde B, Kristensen S, Egander A, Clemmensen K, Rasmussen NA, Gjedde A, Rosenberg R (2003) The Danish PET/depression project: poor verbal fluency performance despite normal prefrontal activation in patients with major depression. Psychiatry Res 123:49–63
Wagner S, Doering B, Helmreich I, Lieb K, Tadíc A (2012) A meta-analysis of executive dysfunctions in unipolar major depressive disorder without psychotic symptoms and their changes during antidepressant treatment. Acta Psychiatr Scand 125:281–292
Walther S, Höfle O, Federspiel A, Horn H, Hügli S, Wiest R, Strik W, Müller TJ (2012) Neural correlates of disbalanced motor control in major depression. J Affect Disord 136:124–133
Wang L, Hermens DF, Hickie IB, Lagopoulos J (2012) A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord 142:6–12
Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA (2013) The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. doi:10.1002/hbm.22234
Werner NS, Meindl T, Materne J, Engel RR, Huber D, Riedel M, Reiser M, Hennig-Fast K (2009) Functional MRI study of memory-related brain regions in patients with depressive disorder. J Affect Disord 119:124–131
Whitfield-Gabrieli S, Ford JM (2012) Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76
Wildgruber D, Ackermann H, Grodd W (2001) Differential contributions of motor cortex, basal ganglia, and cerebellum to speech motor control: effects of syllable repetition rate evaluated by fMRI. Neuroimage 13:101–109
Wittchen H-U, Wunderlich U, Gruschwitz S, Zaudig M (1997) SKID-I. Strukturiertes klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Interviewheft. Hogrefe, Göttingen
Xu G, Lin K, Rao D, Dang Y, Ouyang H, Guo Y, Ma J, Chen J (2012) Neuropsychological performance in bipolar I, bipolar II and unipolar depression patients: a longitudinal, naturalistic study. J Affect Disord 136:328–339
Xue G, Aron AR, Poldrack RA (2008) Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex 18:1923–1932
Zakzanis KK, Leach L, Kaplan E (1998) On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol 11:111–119
Zeng LL, Shen H, Liu L, Wang L, Li B, Fang P, Zhou Z, Li Y, Hu D (2012) Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain 135:1498–1507
Zhu X, Wang X, Xiao J, Liao J, Zhong M, Wang W, Yao S (2012) Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry 71:611–617
Zvyagintsev M, Clemens B, Chechko N, Mathiak KA, Sack AT, Mathiak K (2013) Brain networks underlying mental imagery of auditory and visual information. Eur J Neurosci 37:1421–1434
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (German Research Foundation) (Grant No. KR 3822/2-1) and by a Research Grant of the University Medical Center Giessen and Marburg (UKGM) (Grant No. 11/2010 MR).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Backes, H., Dietsche, B., Nagels, A. et al. Increased neural activity during overt and continuous semantic verbal fluency in major depression: mainly a failure to deactivate. Eur Arch Psychiatry Clin Neurosci 264, 631–645 (2014). https://doi.org/10.1007/s00406-014-0491-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-014-0491-y