Abstract
Introduction
The modern availability in daily practice of different DICOM viewers allows physicians to routinely evaluate computed tomography (CT) and magnetic resonance (MR) scans of patients in the pre-, intra-, and postoperative settings. Their systematic use, together with a close surgeon–radiologist cooperation, may greatly improve outcomes of patients to be treated by transoral microsurgery for laryngeal cancer.
Materials and methods
We herein propose guidelines for systematic evaluation of CT/MR images taken from patients affected by supraglottic and glottic cancer to be treated by transoral microsurgery.
Results
A methodical, step-by-step approach focused on laryngeal anatomy, systematically looking at each true and false vocal folds, anterior commissure, laryngeal ventricle, subglottic area, epiglottis, thyroid, cricoid, and arytenoid cartilages, posterior commissure, crico-arytenoid unit, paraglottic and pre-epiglottic spaces, and possible extra-laryngeal extension is proposed. This checklist may be useful before imaging performance (to focus on specific issues to be detailed by the radiologist), as well before and during surgery for the specific evaluation of details to be cleared during transoral microsurgery.
Conclusion
Detailed preoperative evaluation of supraglottic and glottic anatomy is essential prior to any transoral approach for neoplastic disease. The proposed imaging checklist described herein represents a step-by-step guide to surgeons performing this kind of interventions and an aid in achieving a meticulous approach from a surgical perspective.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Digital Imaging and COmmunications in Medicine (DICOM) is an international standardized file format and network communications protocol developed by the American College of Radiologists specifically for medical activity. The availability in daily practice of different DICOM viewers allows physicians to routinely evaluate computed tomography (CT) and magnetic resonance (MR) scans of patients in the office, during multidisciplinary tumor boards, and in the operating room, before, during, and after a given surgical procedure. In laryngeal cancer surgery, strict cooperation and understanding between head and neck surgeons and dedicated radiologists is mandatory and should be considered of paramount importance for an appropriate improvement of knowledge, skills and, ultimately, outcomes.
In particular, adequate planning of transoral surgical resection of laryngeal tumors is strictly related to their scrupulous preoperative three-dimensional (3D) assessment, implying a state-of-the-art endoscopic evaluation, complemented by a detailed description of deep tumor extension towards the paraglottic (PGS) and pre-epiglottic (PES) visceral spaces. Nevertheless, surgeons may be frequently prompted to pre- and/or intraoperatively re-evaluate some elements such as anatomical variations and tumor characteristics in the coronal, axial, and sagittal planes, possibly changing their therapeutic attitudes according to new elements emerging from the in-depth examination of a given clinical scenario. In this sense, multiplanar-reformatted (MPR) images in coronal and sagittal planes allow for more confident evaluation of the anatomic relationships between the tumor and surrounding structures [1].
The glottis is the most common laryngeal site of origin of squamous cell carcinoma, accounting for approximately 60% of new cases, followed by the supraglottis, whose tumors, at diagnosis, frequently extend also to the adjacent oropharynx and hypopharynx. Detailed knowledge of the patient's anatomy, as well as the precise 3D appreciation of tumor extent before surgery, increase therapeutic success, as already demonstrated in the sinonasal surgical field [2]. This application can support transfer of knowledge to a field in recent, constant evolution, such as modern transoral laryngeal surgery.
The aim of this paper is to present a proposal for an easy and practical checklist made by the European Laryngological Society (ELS) to be systematically applied in the preoperative setting to CT and MR evaluation of laryngeal tumors, whenever a transoral surgical approach is an option. Therefore, different subsites relevant to the adequate planning of a transoral organ preservation surgical strategy were included herein and selected to underline the relevant structures that can affect the pre- and intraoperative decision-making processes.
Proposal of an imaging checklist for glottic and supraglottic tumors to be treated by transoral microsurgery
Different DICOM viewers are widely available, some of them for free and usable on a personal laptop such as Osirix® (Pixmeo SARL, Geneva, Switzerland) or Horos Project® (Pixmeo SARL, Geneva, Switzerland), while others are available only for hospital environments such as Sectra—PACS® (Sectra AB, Linköping, Sweden). The authors, however, have no special recommendations in terms of software, and detailed explanations of the different characteristics of the DICOM viewers available, as well as information related to the acquisition technique or radiological settings, are beyond the scope of this paper. In fact, this checklist may be implemented using all available DICOM viewers and is not influenced by the choice of one over another.
The authors propose a methodical, step-by-step approach focused on laryngeal anatomy, evaluated first by looking at each true and false vocal folds, anterior commissure (AC), laryngeal ventricle, subglottic area, epiglottis, thyroid, cricoid, and arytenoid cartilages, posterior commissure, crico-arytenoid unit, PGS and PES, and possible extra-laryngeal extension. Careful scrolling in the axial, coronal and sagittal planes should be routinely performed. The direction of scrolling is not as important as the need to perform this evaluation, in the same manner, and each time (Table 1).
Even if a detailed description of imaging techniques is beyond the purpose of this document, some preliminary observations are essential to deal with images in a proper way. First of all, CT considered the working horse in evaluation of laryngeal cancer. In a few seconds, modern scanners acquire an entire volume that can be reconstructed in different planes, and the inherent technique is well standardized. On the other hand, MR can produce anatomical images with a high spatial and contrast resolution, with better visualization of cartilages (in particular their non-ossified portions) and muscular structures. Except for 3D sequences, the acquisition planes must be decided a priori and cannot be changed in the post-processing phase. Moreover, MR has longer acquisition times than CT and image quality is strictly related to a wide range of variables that should be carefully handled by expert users.
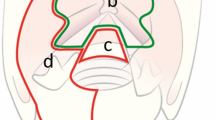
When reconstructing CT volumes or setting MR acquisitions, the choice of the correct planes is of paramount importance. The first step is to orientate the axial plane parallel to the glottis. If, for example, a purely horizontal plane is opted for, the consequences on image interpretation could be catastrophic, with vocal processes of arytenoids not on the same plane of AC but on the supracommissural region, ventricle or false vocal folds. The orientation of the axial plane in laryngeal evaluation must be, therefore, established as in Fig. 1a. As a consequence, the coronal plane is perpendicular to the axial, and the sagittal one cuts vertically the larynx from posterior to anterior. Moreover, some maneuvers may be beneficial during CT or MR images acquisition, being Valsalva and apnea two of the most frequently applied for modifying the adduction/abduction position of the vocal folds. In this way, fine details of the free margins of the true vocal cords (in abduction) or the PGS and piriform sinuses (in adduction) may be readily appreciated (Fig. 1b).
a The right axial orientation of the glottic plane is indicated by the continuous white line, going from the superior border of the cricoid lamina (C) to the inferior border of the thyroid lamina (T) on the midline (AC, anterior commissure; pvA, arytenoid vocal process). b Top left: MR, T2 sequence showing right vocal cord tumor (white arrow). Top right: same lesion post-contrast VIBE. Bottom left: CT with adducted vocal cords. Bottom right: abducted vocal folds during inspiration. The asterisk indicates the edematous changes in the vocal muscle and posterior PGS. Vocal cords abduction makes the detection of the lesion and its surface easier, while adduction enlarges the PGS and allows better evaluation of tumor deep extension
If this caveats have been properly observed by the radiologist during laryngeal imaging, the surgeon should start scrolling by noticing the following elements of the checklist:
True vocal fold(s) This is the first step, during which both vocal cords are evaluated in the axial plane. Identification of the vocal processes of arytenoid cartilages facilitates recognition of the true vocal cords which, differently from the false vocal cords, have a low fat content. Next, the surgeon needs to acknowledge the exact location of the lesion in relation to the anterior, middle and/or posterior thirds of the vocal folds, underlying composite involvement when more than one subsite is affected (Fig. 2a).
Fig. 2 a CT scan, axial plane, showing a glottic tumor (dotted line) involving the anterior two-thirds of the left vocal cord, up to the AC. b CT scan, axial plane, showing a glottic tumor (T) penetrating the AC (arrowhead). Arytenoid sclerosis (a), not specific for infiltration, but suggesting at least a close relationship of the tumor with the cartilage itself. Edema in the posterior PGS (asterisk), widened. On the sagittal plane, an anterior transcommissural tumor (T) infiltrating the thyroid cartilage just above the AC (arrowhead). c CT scan, axial plane, showing a tumor with subglottic median extent (arrowhead). On sagittal plane, the tumor presents evident subcommissural extension (arrowhead). d CT scan, axial plane, showing a tumor (T) in the central part of the PES. Normal fat fills the lateral part of the PES (arrows); a small secondary laryngocele (arrowhead, la) developed in the left PGS. On sagittal plane, the tumor (T) infiltrates the inferior portion of the PES
Anterior commissure Such a subsite plays a role with a special relevance in surgical planning of transoral surgery and its careful evaluation (both endoscopically as well as radiologically) is essential since this can be a pathway for tumor extension through the laryngeal framework, outside the organ (Fig. 2b). The axial plane is the best to evaluate its integrity: if the AC is thicker than 1 mm, this means it is abnormal, or one should wonder if not looking at something different (i.e. axial plane not properly oriented during image reconstruction). When anterior transcommissural extension is suspected (i.e. tumor spreads in the vertical direction from the AC to the petiole of the epiglottis and/or subglottis), then evaluation of MPR images in the sagittal plane is best suited to pinpoint subtle submucosal and/or cartilaginous neoplastic extension (Fig. 2c,d).
False vocal cord(s)/laryngeal ventricle Both need to be evaluated in the coronal plane (Fig. 3a, b ), paying attention to possible asymmetries or occupation of the ventricle by growing masses coming from adjacent structures as seen in transglottic tumors (Fig. 3c).
Fig. 3 a CT scan, anatomical images on the coronal plane. Top left: hyoid bone (H), thyro-arytenoid muscle (white dotted line), subtle fat pad indicating the PGS (arrows). Top right: central PES, lateral epiglottic space (L), lateral crico-arytenoid muscle (LCA), cricothyroid muscle (CT), cricoid lamina (C), inferior PGS (iPGS), way for extra-laryngeal tumor spread (curved arrow). Bottom left: false vocal cords (FVC), ventricle (arrowhead), true vocal cords (TVC). Bottom right: aryepiglottic folds (AEF), piriform sinus (PS), arytenoids (A), crico-arytenoid junction (white arrows), posterior PGS (pPGS), and inferior PGS (iPGS). b CT scan, coronal plane, through the mid-portion of the ventricle (dotted line), whose shape and contour must be compared to the contralateral one in search of possible asymmetry. c Top: tumor (T) infiltrating the anterior and posterior (arrow) commissures, sclerotic arytenoid (a), and tissue in the posterior PGS (asterisks) to be put into differential diagnosis between tumor invasion and peritumoral edema. Bottom: transglottic tumor (T) extending from the vocal fold to the ventricle and false vocal cord, involving the PGS
Subglottis Limited tumor invasion of this region does not represent a contraindication for transoral laryngeal surgery in expert hands. However, special considerations are necessary due to the difficulties usually related to the target exposure in this region, whose resection most often requires a peace-meal (or multi-bloc) approach to adequately assess caudal neoplastic extension. For these reasons, careful evaluation in both axial (Fig. 4a) and coronal (if the subglottic extent is lateral), or sagittal plane (if it is anteriorly located) is necessary.
Fig. 4 a MR after gadolinium injection, axial plane, showing a tumor (T) in the anterior and posterior (asterisk) PGS. The black dotted line indicates the M-plane separating the anterior from the posterior PGS according to Succo et al. [5] and Del Bon et al. [6] Subtle complete infiltration of the thyroid lamina with a thin layer of extra-laryngeal tissue (arrows). b MR showing tumor of the infrahyoid epiglottis (ihe), with no infiltration of the PES (Hb, hyoid bone; arrowhead, glossoepiglottic ligament). c MR and CT scan showing a supraglottic tumor with minimal infiltration of the median (mPES) and lateral pre-epiglottic space (lPES) at the level of the caudal portion of the epiglottic petiole on the left. Infiltration of the left piriform sinus (ps) is also evident. Hyoid bone is normal (arrow), while the glossoepiglottic ligament is visible posterior to the hyoid bone (sagittal images). The tumor does not reach the tongue base (TB). d MR showing a supraglottic tumor with massive invasion of the PES. On the left, thyrohyoid ligament (thl) is not transgressed. On the right, acquired on a parasagittal plane orthogonal to the thyroid lamina, the tumor abuts through the thyroid notch (black arrowhead), with possible infiltration of the thyrohyoid membrane, without invasion of the thyroid cartilage (tc). e Top left: MR, T2 sequence, axial plane showing a tumor (T) invading the anterior part of the thyroid laminae and extending into laryngeal soft tissues. Bottom left: CT scan of the same case, axial plane. The consistency of tumor borders is inferior, even if the large defect in the cartilage is clearly evident. Top right: MR, T2 sequence, axial plane showing a tumor (T) in the anterior supracommissural area and infiltrating the thyroid cartilage. Bottom right: CT scan of the same case, axial plane, where the tumor (approximately indicated by a question mark) is not well-visible
Laryngeal cartilages Evaluation of the laryngeal framework should be performed by taking advantage of all possible planes (axial, coronal, and sagittal). It is essential to evaluate the contour and integrity of each cartilage, remembering the different patterns of ossification among the various laryngeal cartilages and being aware of them to avoid confusion with tumor invasion. For example, the elastic cartilage forming the epiglottis never ossifies and its proper evaluation in case of supraglottic cancer, especially when considering the infrahyoid portion, poses problems of discrimination toward a possible PES involvement. This, indeed, represents a detail of paramount importance to be carefully addressed by targeted imaging (Fig. 4b, c). In fact, if PES is not infiltrated or just minimally involved, with preservation of even a subtle layer of normal fat in front of the tumor, between this and the thyroid cartilage, thyrohyoid membrane and hyoid bone, a transoral resection can be safely performed. In contrast, when the tumor reaches the above-mentioned structures, with no apparent cleavage from them, other surgical or non-surgical approaches should be considered, due to the objective risk of obtaining unsafe surgical margins by a purely transoral route (Fig. 4d) [3]. The non-ossified tracts of ossifying cartilages (especially thyroid and cricoid) are more frequently, but not always, symmetric. Use of a bone window on CT is useful to better discriminate contact from invasion and, when doubts still persist, MR may definitively give more information. Even subtle changes such as inner thyroid cortical interruption (Fig. 4e) or arytenoid sclerosis (sensitive even if poorly specific) must be diligently considered as possible sources of tumor upstaging. In fact, a target-directed transoral procedure aimed at getting histopathologic confirmation of such radiologic findings may lead to the subsequent choice of different therapeutic options, such as open partial horizontal laryngectomies (OPHLs) or non-surgical treatments (Fig. 5a).
Fig. 5 a Top: tumor (T) infiltrating the petiole of the epiglottis (p), the false vocal cord, and encasing the right arytenoid (curved arrow). Normal fat tissue in the contralateral posterior PGS. Bottom: tumor (T) infiltrating the inferior PGS (curved arrow and arrowhead), while on the left side normal fat in the inferior PGS is visible (arrowhead). b CT scan, on axial (A), coronal (B), and sagittal (C) planes for appropriate 3D evaluation of the crico-arytenoid joint (the articular facet, indicated by the arrow, is always easily visible). c Supraglottic tumor (T) invading laterally and posteriorly the left piriform sinus (ps). The tumor cranially infiltrates the left glossoepiglottic vallecula (v), as demonstrated also in the PET image. On the coronal sequence, the thyrohyoid membrane (thm, black arrowheads) and hyoid bone (hb) are not infiltrated. d Supraglottic tumor invading the aryepiglottic folds, medial walls of the piriform sinuses (ps), epiglottis, and base of the tongue (arrowheads). The PES is not massively invaded. The hyoid bone (hb) is reached by the tumor but not infiltrated. The white line indicates the glottic plane
Crico-arytenoid unit Its assessment needs to be performed preferentially in the axial plane (Fig. 5b). Its involvement should be considered an absolute contraindication to transoral surgical approaches and, when involvement of the cricoid facet of the joint (corresponding to hemilarynx fixation at endoscopy) has been histologically confirmed, it can also represent a contraindication to the “classic” forms of supracricoid open partial laryngectomies (Type IIa and IIb according to the ELS classification) [4], leading to the need for an entire crico-arytenoid unit resection through a Type IIIa or IIIb OPHL, when not a total laryngectomy.
Posterior commissure Its assessment on the axial plane gives valuable information to the surgeon given that its bilateral deep invasion contraindicates any type of transoral or open partial surgical approach (Fig. 3c).
Visceral spaces and extra-laryngeal extension PGS and PES (Figs. 2d, 3a, 4c, d) represent important avenues for tumor spread. This is the reason why careful exploration of these fat-containing visceral spaces is essential for proper T staging and consequent N treatment (since cT3 tumors with massive PGS and/or PES involvement should receive special consideration for potential lymph node metastases). The PGS should be divided into three sub-compartments: (a) the anterior PGS, mainly filled by the thyro-arytenoid muscle leaving just a very thin lateral fat pad, difficult to assess on axial images; (b) the posterior PGS, lying behind the “M-plane” [5, 6] traced between the thyroid lamina and the vocal process of the arytenoids (also called the “thyro-arytenoid space”, which is a fat area opened medially towards the crico-arytenoid unit and posteriorly to the piriform sinus [Fig. 4a]); (c) the inferior PGS, i.e. the continuation of the posterior PGS below the glottic level, which is a fatty space between the inferior border of the thyroid lamina and the upper margin of the cricoid cartilage (Fig. 3a). Anterior and posterior PGS should be assessed on the axial plane, while the inferior PGS in the axial and coronal planes. For supraglottic lesions, special attention should be also paid to properly exclude tumor extension toward the piriform sinus (through the aryepiglottic and pharyngoepiglottic folds), glossoepiglottic valleculae, and base of tongue (Fig. 5c, d).
Discussion
Transoral surgery for laryngeal tumors is one of the most common procedures performed by modern laryngologists and head and neck surgeons. The most popular technique is represented by transoral carbon dioxide laser microsurgery (defined as CO2 TOLMS according to Remacle et al. [7]) due to its well-known advantages such as magnification generated by the microscope and possibility to perform a clear-cut and blood-less dissection within deep laryngeal tissues. However, other techniques are now available such as microdissection electrodes [8] or robotic surgery [9], with objective difficulties, at least to date, in distinguishing the pros and cons of each approach compared to the gold standard (still represented by CO2 TOLMS). Whatever technical tools are applied for transoral removal of laryngeal tumors, this surgical procedure has passed the test of time and its success rate, when properly indicated and performed, is almost always comparable, if not superior, to those obtained by other therapeutic approaches. Although safely accomplished in the majority of instances, errors during planning and performance of this kind of surgery due to under-appreciation of subtle endoscopic and radiologic details may lead to a number of possible complications, incomplete resections with positive surgical margins, and consequent worsening of local control and overall survival.
One possible way to try to reduce the risk of inadequate planning is to formalize a number of items using a checklist that every radiologist and surgeon embracing TOLMS for laryngeal tumors should focus on. Experienced surgeons can argue that this tool is not necessary. However, every beginner at his/her first stages of the steep learning curve in this kind of surgery can find a step-by-step guideline to perform detailed imaging analysis of the relevant subsites in this checklist, thus building a 3D-map of tumor extension in his/her mind, and reasoning about the following steps needed for its safe removal.
Previous guidelines related to the classification of extent of transoral laryngeal resections as proposed by the ELS demonstrate its relevance in the clinic and as a nomenclature system for publications and presentations [4, 10,11,12]. In the same way, we consider this material to be a useful tool for otolaryngologists starting TOLMS for removal of glottic and supraglottic tumors to help them in standardizing methodic and systematic imaging evaluation of the larynx before all procedures.
On the other hand, such a checklist should be also considered as a list of questions that every radiologist evaluating a laryngeal tumor to be surgically treated should answer to maximize the preoperative imaging value. Moreover, the need for a proper pre- and intraoperative endoscopic work-up by white light as well as biologic endoscopy tools (such as Narrow Band Imaging or others) cannot be overemphasized [13]. After an exhaustive preoperative endoscopy, the head and neck surgeon should be able to direct specific questions to the radiologist and, whenever the checklist is discussed together, any potential doubt should be resolved by scrupulous intraoperative endoscopy, with possible surgical exploration of given targets. Outcomes from such an intraoperative search, as well as those deriving from definitive histopathological examination, should form the basis for subsequent feedback to the radiologist carrying out preoperative imaging. Such a mutual exchange of information, helped by sharing the imaging checklist proposed herein, have been confirmed in several experiences to be of great help in developing a common multidisciplinary treatment strategy and therapeutic know-how.
Conclusions
Detailed preoperative evaluation of laryngeal anatomy is essential before any TOLMS for neoplastic disease. The available DICOM viewers represent a unique opportunity for surgeons performing transoral minimally invasive glottic and supraglottic surgery to improve oncological and functional outcomes. The ELS proposal of imaging checklist described herein represents a step-by-step guide to those performing this kind of surgery and a meticulous approach from a surgical perspective. However, it is important to underline that it is not intended to replace in any way proper evaluation of imaging by an expert and dedicated head and neck radiologist, with whom the surgeon is prompted to build and maintain a constant pre- and postoperative exchange of information and feedback.
References
Agnello F, Cupido F, Sparacia G, Midiri F, Miroddi M, Grassedonio E et al (2017) Computerised tomography and magnetic resonance imaging of laryngeal squamous cell carcinoma: a practical approach. Neuroradiol J 30:197–204
Becker SS (2010) Preoperative computed tomography evaluation in sinus surgery: a template-driven approach. Otolaryngol Clin North Am 43:731–751
Peretti G, Piazza C, Mora F, Garofolo S, Guastini L (2016) Reasonable limits for transoral laser microsurgery in laryngeal cancer. Curr Opin Otolaryngol Head Neck Surg 24:135–139
Succo G, Peretti G, Piazza C, Remacle M, Eckel HE, Chevalier D et al (2014) Open partial horizontal laryngectomies: a proposal for classification by the working committee on nomenclature of the European Laryngological Society. Eur Arch Otorhinolaryngol 271:2489–2496
Succo G, Crosetti E, Bertolin A, Piazza C, Molteni G, Cirillo S et al (2018) Treatment for T3 to T4a laryngeal cancer by open partial horizontal laryngectomies: Prognostic impact of different pathologic tumor subcategories. Head Neck 40:1897–1908
Bon F, Piazza C, Lancini D, Paderno A, Bosio P, Taboni S et al (2019) Open partial horizontal laryngectomies for T3–T4 laryngeal cancer: prognostic impact of anterior vs posterior laryngeal compartmentalization. Cancers (Basel). 11.pii:E289
Remacle M, Arens C, Eldin MB, Campos G, Estomba CC, Dulguerov P et al (2017) Laser-assisted surgery of the upper aero-digestive tract: a clarification of nomenclature. A consensus statement of the European Laryngological Society. Eur Arch Otorhinolaryngol 274:3723–3727
Basterra J, Frias S, Alba JR, Zapater E (2006) A new device for treating laryngeal carcinoma using microdissection electrodes. Laryngoscope 116:2232–2234
Remacle M, Prasad VMN (2018) Preliminary experience in transoral laryngeal surgery with a flexible robotic system for benign lesions of the vocal folds. Eur Arch Otorhinolaryngol 275:761–765
Remacle M, Eckel HE, Antonelli A, Brasnu D, Chevalier D, Friedrich G et al (2000) Endoscopic cordectomy. A proposal for a classification by the Working Committee, European Laryngological Society. Eur Arch Otorhinolaryngol 257:227–231
Remacle M, Van Haverbeke C, Eckel H, Bradley P, Chevalier D, Djukic V et al (2007) Proposal for revision of the European Laryngological Society classification of endoscopic cordectomies. Eur Arch Otorhinolaryngol 264:499–504
Remacle M, Hantzakos A, Eckel H, Evrard AS, Bradley PJ, Chevalier D et al (2009) Endoscopic supraglottic laryngectomy: a proposal for a classification by the working committee on nomenclature, European Laryngological Society. Eur Arch Otorhinolaryngol 266:993–998
Piazza C, Del Bon F, Peretti G, Nicolai P (2011) “Biologic endoscopy”: optimization of upper aerodigestive tract cancer evaluation. Curr Opin Otolaryngol Head Neck Surg 19:67–76
Funding
No funding was used for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare they do not have any conflict of interest.
Ethical approval
No ethical approval was required.
Informed consent
Consent for the use of medical images were obtained from patients included.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chiesa-Estomba, C.M., Ravanelli, M., Farina, D. et al. Imaging checklist for preoperative evaluation of laryngeal tumors to be treated by transoral microsurgery: guidelines from the European Laryngological Society. Eur Arch Otorhinolaryngol 277, 1707–1714 (2020). https://doi.org/10.1007/s00405-020-05869-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-020-05869-0