Abstract
Epidemiological data of Bell’s palsy (BP) have been reported. For example, the annual incidence of BP is 15–30 per 100,000 persons, with equal numbers of men and women affected, and there is no predilection for either side of the face. However, details of the relationship between BP and morphometric aspects of the facial nerve have not been available in textbooks. We performed a morphometric analysis of human facial nerve fibers and estimated the total number of myelinated axons (TN) and average transverse area of myelinated axons (ATA). The facial nerve showed a significant decrease of TN with increasing age (r = −0.77; p < 0.01), but showed no significant changes of ATA with age (r = −0.01; p = 0.96). We supposed that the TN decrease with age was a factor in the delayed recovery from BP seen in the elderly. Moreover, the TN and ATA showed no significant differences between female and male specimens (p < 0.05), or between the right and left side specimens (p < 0.05). Our present results seem to explain the absence of significant sex and affected side differences in BP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bell’s palsy (BP) accounts for approximately 60% of all peripheral facial palsies, and the highest incidence of all facial paralyses. In the Japanese literature [1], the annual incidence of BP is indicated to be approximately 30 per 100,000 persons, with equal numbers of men and women affected. There is no predilection for either side of the face. Although epidemiological data of BP have been reported, no details of the relationship between BP and morphometric aspects of the facial nerve have been available in textbooks. This study closely investigated the human facial nerve to clarify these issues.
Materials and methods
Human facial nerves in a narrow sense (special visceral efferent nerves) were resected in the vicinity of the brainstem. The materials were obtained from 20 Japanese cadavers (10 females and 10 males) aged 43–89 years (average: 65.5). All the cadavers were donated with the individuals’ consent. We proceeded to perform this research in accordance with the law concerning autopsy and preservation of corpses, and concerning donation for medical and dental education. In no case was there a history of facial nerve disorders such as BP or facial schwannoma, or of treatment with toxic agents or irradiation therapy to the head. The causes of death did not directly or indirectly influence the nervous system, so the facial nerves were considered to be normal.
The methods of preparation of sections, also described in our previous report [2], were as follows:
Fixation. The fixation used was a two step process. For the first step, all the cadavers were fixed with a 10% solution of formalin (3.7% formaldehyde) within 24 h postmortem. After resecting the facial nerve, a 10% solution of formalin (3.7% formaldehyde) was used for immersion for at least a week. The solution was changed once in the first 30–60 min and again, later if desired.
The formalin-fixed materials were then transferred, without washing, to the secondary fixative (1:4 mixture of 5% K2Cr2O7 and 5% K2CrO4) and held at room temperature for 2 weeks. If the solution became turbid or precipitated it was changed. After this, the fixation was continued at 37°C for an additional week. The volume of fixative used was at least ten times the volume of the specimens.
Washing. The fixed materials were washed in running water for around 24 h. We used a siphon-operated automatic pipette washer with the materials packed in a small plastic basket.
Dehydration and celloidin embedding.
-
1.
50% ethanol, for several days
-
2.
70% ethanol, for several days
The alcohol in steps 1 and 2 was changed if it became yellow.
-
3.
90% ethanol, overnight
-
4.
95% ethanol, overnight
-
5.
Pure ethanol, one night or more
-
6.
Ether/ethanol, 1:1 overnight
-
7.
1% celloidin in ether/ethanol, several days
-
8.
7% celloidin, several days
-
9.
14% celloidin for embedding, several weeks
-
10.
Immerse hardened celloidin embedded blocks in 90–95% ethanol for several hours
-
11.
Keep celloidin blocks in 70% ethanol prior to sectioning
Staining procedures. Modified luxol fast blue-periodic acid Schiff-hematoxylin (LPH) triple stain.
-
1.
Cut sections 15 μm thick and place in 90% ethanol
-
2.
Rinse sections in 95% ethanol
-
3.
Keep at 58°C overnight in LFB solution (0.1% solution of luxol fast blue by dissolving 1.0 g of the substance in 1,000 ml of 95% ethanol) placed in a shallow sealed jar.
-
4.
Immerse in 95% ethanol and wash off excess stain
-
5.
Wash in distilled water
-
6.
Differentiate in 2% saturated lithium carbonate (= 0.03% Li2CO3) for 60 min
-
7.
Continue differentiation with one or two changes of 70% ethanol until myelin sheath can be distinguished. If necessary, repeat steps 5 through 7 until there is sharp contrast between myelin sheath and surrounding structure.
-
8.
Finish differentiation by rinsing in 95% ethanol
-
9.
Wash in distilled water (two changes)
-
10.
Oxidize for 5 min in 0.5% periodic acid
-
11.
Wash in distilled water (several changes)
-
12.
Immerse for 15 min in Schiff’s reagent
-
13.
Immediately transfer to 5% sodium hydrogen sulfite and leave for about 5 min, changing the solution three times
-
14.
Wash in distilled water (several changes)
-
15.
Immerse sections for around 5 min in Mayer’s hematoxylin solution
-
16.
Wash sections in distilled water (several changes) until sections turn bluish.
-
17.
Rinse in 90–95% ethanol
-
18.
Dehydrate sections in n-butyl alcohol (three changes)
-
19.
Clear sections in xylene (three changes)
-
20.
Mount in balsam
Morphometry
We observed the fascicles at low power (Fig. 1). We covered the entire area of the distributed myelinated axons in the facial nerve by moving the eyepiece grid vertically and horizontally. We confirmed that we could distinguish myelinated structures from vessels in the tissue with a computer or grouped unmyelinated axons with the naked eye in each grid. We counted the myelinated axons and measured the transverse area of the myelinated axons in a square eyepiece grid (the area covered was 0.004 mm2) at high power (Fig. 2). To avoid duplicate counts, we counted and measured all axons on the side of the grid that did not come into contact with the other grids. In the case of grids adjacent to the other grids, we counted and measured only the axons on the lower right side of the grid, not those on the upper left side. We employed a microscope in transmitted light mode (BX50, Olympus, Tokyo, Japan) equipped with a high-resolution digital camera (ColorView12, Soft Imaging System, Münster, Germany), a motorized XYZ stage (Märzhäuser, Wetzlar-Steindorf, Germany), a stage controller (Märzhäuser, Wetzlar-Steindorf, Germany), and a computer (Precision 530, Dell, Austin, TX, USA) with analyzing system software (analySIS 3.0, Soft Imaging System, Münster, Germany) for storing data on-line, calculations, and statistical analyses.
Statistical analyses
All statistical analyses were performed using JMP statistical software version 8.0.2.2 (SAS Institute Inc. Cary, NC, USA) on a Macintosh personal computer.
Researchers have studied shrinkage of embedding materials, and found that celloidin and plastination embedding display smaller shrinkage (around 10%) than paraffin and other embeddings [3]. Therefore, although we measured every myelinated axon, we calculated the average transverse area of myelinated axons after excluding data far from the median (15%) due to shrinkage.
The specimens used were sampled randomly. Two variables (X: age and Y: total number or average transverse area of nerve fibers) indicated a bivariate normal distribution. Thus, we calculated the coefficient of correlation (Pearson product moment; hereafter abbreviated as “r”) between the total number, the average transverse area of myelinated axons, and the subject’s age.
Morphological differences between female and male, and between right and left side specimens were analyzed by applying a parametric unpaired t test (where data were normally distributed with equal variance) to the total number of myelinated axons and the average transverse area. A p value of <0.05 was considered to indicate a statistically significant difference.
Results
Aging process
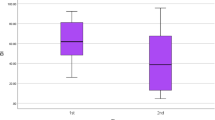
We estimated the total number of myelinated axons (TN) in the facial nerve to be 6,245 ± 860 (mean ± SD), and the average transverse area of myelinated axons (ATA) to be 6.31 ± 0.81 μm2 (mean ± SD). The facial nerve showed a significant decrease in TN with increasing age (r = −0.77; p < 0.01; Fig. 3), but showed no significant changes in ATA with age (r = −0.01; p = 0.96; Fig. 4). We then analyzed for significant differences in age between pairs of groups (female and male; right and left side) before comparing the TN and ATA between groups. The age of specimens showed no significant difference between female and male specimens (95% CI, −13.8 to 15.4; p = 0.91; Table 1), or between right and left side specimens (95% CI, −13.4 to 15.8; p = 0.86; Table 2). The data of the above pairs of groups were thus independent of the aging process.
Comparison of the facial nerve between females and males
Males had higher TN than did females, but the TN showed no statistically significant difference between the female and male specimens (95% CI, −357 to 1,244; p = 0.26; Table 1). Moreover, females indicated larger ATA than males, but the ATA showed no statistically significant difference between the female and male specimens (95% CI, −0.71 to 0.85; p = 0.86; Table 1).
Comparison of the facial nerve between right and left sides
Left side had greater numbers of TN and larger ATA than did right side, but neither TN nor ATA showed a statistically significant difference between right and left side specimens (TN: 95% CI, −741 to 917; p = 0.83; ATA: 95% CI, −0.58 to 0.97; p = 0.60; Table 2).
Discussion
Since Korczyn [4] first reported a high frequency (66%) of diabetes mellitus (DM) in patients presenting with BP, this association has been confirmed by several researchers, most recently by Savadi-Oskouei et al. [5] (prevalence of DM: 29%, the same as above). Researchers have also reported that the incidence of hypertension in patients with BP was higher than in control groups. For example, Abraham-Inpijn and co-workers [6] also found a statistically provable difference in the frequency of hypertension between BP patients and controls. Furthermore, Campbell and Brundage [7] stated that climate was associated with an increased risk of BP. Their results indicated that two physical stressors, residence in an arid climate (adjusted rate ratio for arid vs. nonarid = 1.34) and exposure to cold (adjusted rate ratio for cold vs. warm = 1.31), were independent predictors of BP. As the above-mentioned reports showed that DM, hypertension and climate influence the incidence of BP, we excluded cadavers that had past histories of DM and hypertension, or lived outside of the Tokyo metropolitan area. We initially examined the relationship between our data and the aging process. A Korean research group recently showed that BP recovery rates in adults and children were not significantly different [8]. (Due to the disposition of Korea, most adults were treated with acupuncture, compared with only 12% of children with BP). However, in general, an important factor in recovery from BP is the age of the patient. For example, Devriese and co-workers [9] reported that increased age was associated with a greater loss of function (paresis). In this study, the TN showed a significant decrease with age (r = −0.77; p < 0.01; Fig. 3), but the ATA showed no significant changes with age (r = −0.01; p = 0.96; Fig. 4). As axonal degeneration on the facial nerve fibers may be one of the significant etiologies of BP, these results indicated that the elderly facial nerve has weaker conduction of nerve impulses than younger facial nerves under the same nerve damage conditions. We supposed that the TN decrease with increasing age was a factor in the delayed recovery from BP among the elderly.
Next, we compared myelinated axons of the human facial nerve between female and male specimens. Campbell and Brundage [7] reported that the incidence rate of BP was slightly higher for females than for males (rate ratio = 1.16). But many researchers have reported finding no significant difference in BP incidence according to sex, as Tiemstra and Khatkhate [10] recently reported. In this study, males had higher TN than did females, but the TN showed no statistically significant difference between the female and male specimens (95% CI, −357 to 1,244; p = 0.26; Table 1). With regard to the ATA, females had larger ATA than did males, but the ATA showed no statistically significant difference between the female and male specimens (95% CI, −0.71 to 0.85; p = 0.86; Table 1).
We also compared myelinated axons of the human facial nerve between the right and left side specimens. Sridharan and co-workers [11] reported that the right side was involved slightly more often than the left side (128:115), but other investigators reported no significant difference in BP incidence according to affected side, for example, Tiemstra and Khatkhate [10]. In this study, left side had higher TN and larger ATA than right side, but neither TN nor ATA showed a statistically significant difference between right and left side specimens (TN: 95% CI, −741 to 917; p = 0.83; ATA: 95% CI, −0.58 to 0.97; p = 0.60; Table 2).
We previously conducted morphometric nerve fiber analyses and studied both the sexual dimorphism and lateral asymmetry of various human peripheral nerves [12–14]. These data excluding the human vestibular nerve showed similar results to the present study on the facial nerve. Our study regarding the human vestibular nerve indicated that a lower TN, but not ATA, of myelinated axons in the female vestibular nerve might be one of the reasons why vestibular disorders have a female preponderance [13]. We supposed that our present results could be a factor in the lack of significant sex and affected side differences in the incidence of BP. With regard to the relationships between incidence of BP and anatomical factors, there were reports on the vascularity of the facial nerve, the spatial relationship between the facial nerve and the facial canal, and the ratio of the facial nerve and fallopian canal [15, 16]. We conclude that our present study indicated the relationship between incidence of BP and anatomical factors of the facial nerve fiber itself.
Conclusion
We supposed that the TN decrease with age was a factor in the delayed recovery from BP seen in the elderly. Our present results seem to explain the absence of significant sex and affected side differences in BP.
References
Yanagihara N (1988) Incidence of Bell’s palsy. Ann Otol Rhinol Laryngol Suppl 137:3–4
Moriyama H, Shimada K, Goto N (1995) Morphometric analysis of neurons in ganglia: geniculate, submandibular, cervical spinal and superior cervical. Okajimas Folia Anat Jpn 72:185–190
Eckel HE, Sittel C, Walger M, Sprinzl G, Koebke J (1993) Plastination: a new approach to morphological research and instruction with excised larynges. Ann Otol Rhinol Laryngol 102:660–665
Korczyn AD (1971) Bell’s palsy and diabetes mellitus. Lancet 297:108–110
Savadi-Oskouei D, Abedi A, Sadeghi-Bazargani H (2008) Independent role of hypertension in Bell’s palsy: a case-control study. Eur Neurol 60:253–257
Abraham-Inpijn L, Devriese PP, Hart AAM (1982) Predisposing factors in Bell’s palsy: a clinical study with reference to diabetes mellitus, hypertension, clotting mechanism and lipid disturbance. Clin Otolaryngol 7:99–105
Campbell KE, Brundage JF (2002) Effects of climate, latitude, and season on the incidence of Bell’s palsy in the US armed forces, October 1997 to September 1999. Am J Epidemiol 156:32–39
Cha CI, Hong CK, Park MS, Yeo SG (2008) Comparison of facial nerve paralysis in adults and children. Yonsei Med J 49:725–734
Devriese PP, Schumacher T, Scheide A, de Jongh RH, Houtkooper JM (1990) Incidence, prognosis and recovery of Bell’s palsy. A survey of about 1,000 patients (1974–1983). Clin Otolaryngol Allied Sci 15:15–27
Tiemstra JD, Khatkhate N (2007) Bell’s palsy: diagnosis and management. Am Fam Physician 76:997–1002
Sridharan R, Radhakrishnan K, Ashok PP, Mousa ME (1988) Clinical and epidemiological study of Bell’s palsy in Benghazi, Libya. Afr J Med Med Sci 17:141–144
Miyauchi Y, Moriyama H, Goto N, Goto J, Ezure H (2002) Morphometric nerve fiber analysis of the human inferior alveolar nerve: lateral asymmetry. Okajimas Folia Anat Jpn 79:11–14
Moriyama H, Itoh M, Shimada K, Otsuka N (2007) Morphometric analysis of fibers of the human vestibular nerve: sex differences. Eur Arch Otorhinolaryngol 264:471–475
Moriyama H, Shimada K, Itoh M, Takahashi T, Otsuka N (2007) Morphometric analysis of the inferior alveolar nerve fails to demonstrate sexual dimorphism. J Oral Maxillofac Surg 65:1555–1561
Ogawa A, Sando I (1982) Spatial occupancy of vessels and facial nerve in the facial canal. Ann Otol Rhinol Laryngol 91:14–19
Eicher SA, Coker NJ, Alford BR, Igarashi M, Smith RJ (1990) A comparative study of the fallopian canal at the meatal foramen and labyrinthine segment in young children and adults. Arch Otolaryngol Head Neck Surg 116:1030–1035
Acknowledgments
We thank Ms. Ikuko Moriyama for assistance in preparing the manuscript. This work was supported by a Grant-in-aid for Scientific Research B14370007 from the Ministry of Education, Culture, Sports, Science and Technology of Japan. We certify that any affiliations with or involvement (either competitive or amiable) in any organization or entity with a direct financial interest in the subject matter or materials discussed in the manuscript (e.g., employment, consultancies, stockownership, honoraria, expert testimony, etc.). Otherwise, we have no such financial interest. All financial research or project support is identified in an acknowledgment in the manuscript.
Conflict of interest
The authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondo, Y., Moriyama, H., Hirai, S. et al. The relationship between Bell’s palsy and morphometric aspects of the facial nerve. Eur Arch Otorhinolaryngol 269, 1691–1695 (2012). https://doi.org/10.1007/s00405-011-1835-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-011-1835-0